Natural Products for Liver Cancer Treatment: From Traditional Medicine to Modern Drug Discovery
Abstract
1. Introduction
2. Methods
3. Results
3.1. In Vivo Mice Studies—Mixtures
3.2. In Vivo Mice Studies—Single Compounds
3.2.1. Alkaloids
3.2.2. Fatty Acids
3.2.3. Hydrocarbons
3.2.4. Polyphenols
3.2.5. Polysaccharides
3.2.6. Quinones
3.3. In Vivo Rat Studies—Mixtures
3.4. In Vivo Rat Studies—Single compounds
3.4.1. Fatty Acids
3.4.2. Hydrocarbons
3.4.3. Polysaccharides
3.4.4. Polyphenols
3.4.5. Quinones
3.5. Clinical Trials
4. Discussion
4.1. Apoptosis
4.2. Metastasis
4.3. Angiogenesis
4.4. Limitations and Prospects of This Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Chen, K.F.; Chen, P.J. Treatment of Liver Cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a021535. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Aouissi, H.; Gourine, N.; Wang, H.; Chen, X.; Bombarda, I.; Boudjeniba, M.; Yousfi, M. Chemical composition, antioxidative, antimicrobial and anti-cancer activities of Asteriscus graveolens (Forssk) essential oil. Orient. Pharm. Exp. Med. 2018, 18, 217–223. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Stud. Nat. Prod. Chem. 2017, 54, 21–86. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Gaston, T.E.; Mendrick, D.L.; Paine, M.F.; Roe, A.L.; Yeung, C.K. “Natural” is not synonymous with “Safe”: Toxicity of natural products alone and in combination with pharmaceutical agents. Regul. Toxicol. Pharmacol. 2020, 113, 104642. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Chen, H.W.; Shen, A.L.; Liu, L.Y.; Peng, J.; Chu, J.F. Bear Bile Powder Inhibits Growth of Hepatocellular Carcinoma via Suppressing STAT3 Signaling Pathway in Mice. Chin. J. Integr. Med. 2020, 26, 370–374. [Google Scholar] [CrossRef]
- Wasonga, C.; Omwandho, C. Inhibitory effects of mushroom extracts on progression of carcinogenesis in mice. J. Exp. Oncol. 2018, 12, 231–237. [Google Scholar]
- Lee, J.D.; Choi, M.A.; Ro, S.W.; Yang, W.I.; Cho, A.E.; Ju, H.L.; Baek, S.; Chung, S.I.; Kang, W.J.; Yun, M.; et al. Synergic chemoprevention with dietary carbohydrate restriction and supplementation of AMPK-activating phytochemicals: The role of SIRT1. Eur. J. Cancer Prev. 2016, 25, 54–64. [Google Scholar] [CrossRef]
- He, Z.; Jiang, C.; Zhang, J.; Yin, Z.; Yin, Z.; Zhu, Y.; Fu, J. Neem tree (Azadirachta indica) extract specifically suppresses the growth of tumors in H22-bearing Kunming mice. Z Naturforsch. C 2016, 71, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Man, S.; Yang, H.; Fan, W.; Yu, P.; Gao, W. Utilization of metabonomics to identify serum biomarkers in murine H22 hepatocarcinoma and deduce antitumor mechanism of Rhizoma Paridis saponins. Chem. Biol. Interact. 2016, 256, 55–63. [Google Scholar] [CrossRef]
- Sun, Y.W.; Qiu, H.C.; Ou, M.C.; Chen, R.L.; Liang, G. Saponins isolated from Schizocapsa plantaginea inhibit human hepatocellular carcinoma cell growth in vivo and in vitro via mitogen-activated protein kinase signaling. Chin. J. Nat. Med. 2018, 16, 29–40. [Google Scholar] [CrossRef]
- Xia, H.; Liu, C.; Li, C.C.; Fu, M.; Takahashi, S.; Hu, K.Q.; Aizawa, K.; Hiroyuki, S.; Wu, G.; Zhao, L.; et al. Dietary Tomato Powder Inhibits High-Fat Diet-Promoted Hepatocellular Carcinoma with Alteration of Gut Microbiota in Mice Lacking Carotenoid Cleavage Enzymes. Cancer Prev. Res. 2018, 11, 797–810. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, F.; Shao, D.; Zhang, Z.; Cui, L.; Zhang, J.; Dawulieti, J.; Meng, Z.; Zhang, M.; Chen, L. Gram-scale production of carrier-free fluorescent berberine microrods for selective liver cancer therapy. BioFactors 2018, 44, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, G.; Yang, L.; Pang, L.; Zhan, Y. The Antitumor Effects of Britanin on Hepatocellular Carcinoma Cells and its Real-Time Evaluation by In Vivo Bioluminescence Imaging. Anticancer Agents Med. Chem. 2020, 20, 1147–1156. [Google Scholar] [CrossRef]
- Wang, C.; Wei, X.; Shao, G. Functional Doxorubicin-Loaded Omega-3 Unsaturated Fatty Acids Nanoparticles in Reversing Hepatocellular Carcinoma Multidrug Resistance. Med. Sci. Monit. 2021, 27, e927727. [Google Scholar] [CrossRef]
- Ou, W.; Mulik, R.S.; Anwar, A.; McDonald, J.G.; He, X.; Corbin, I.R. Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic. Biol. Med. 2017, 112, 597–607. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, T.; Yang, X.; Xue, J.; Chen, J. Atractylon induces apoptosis and suppresses metastasis in hepatic cancer cells and inhibits growth in vivo. Cancer Manag. Res. 2019, 11, 5883–5894. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, K.; Kuang, S.; Cao, R.; Bao, L.; Liu, R.; Liu, H.; Sun, C. The natural compound GL22, isolated from Ganoderma mushrooms, suppresses tumor growth by altering lipid metabolism and triggering cell death. Cell Death Dis. 2018, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Roy, G.; Guan, S.; Liu, H.; Zhang, L. Rotundic Acid Induces DNA Damage and Cell Death in Hepatocellular Carcinoma Through AKT/mTOR and MAPK Pathways. Front. Oncol. 2019, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ma, H.; Shi, W.; Duan, J.; Wang, Y.; Zhang, C.; Li, C.; Lin, J.; Li, S.; Lv, J.; et al. Inhibition of STAT3 signaling pathway by ursolic acid suppresses growth of hepatocellular carcinoma. Int. J. Oncol. 2017, 51, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A.; Zhang, B.; Teng, K.Y.; Barajas, J.M.; Motiwala, T.; Hu, P.; Yu, L.; Brüschweiler, R.; Ghoshal, K.; Jacob, S.T. Reprograming of Glucose Metabolism by Zerumbone Suppresses Hepatocarcinogenesis. Mol. Cancer Res. 2018, 16, 256–268. [Google Scholar] [CrossRef]
- Yao, W.; Ba, Q.; Li, X.; Li, H.; Zhang, S.; Yuan, Y.; Wang, F.; Duan, X.; Li, J.; Zhang, W.; et al. A Natural CCR2 Antagonist Relieves Tumor-associated Macrophage-mediated Immunosuppression to Produce a Therapeutic Effect for Liver Cancer. EBioMedicine 2017, 22, 58–67. [Google Scholar] [CrossRef]
- Xiao, Y.; Gong, Q.; Wang, W.; Liu, F.; Kong, Q.; Pan, F.; Zhang, X.; Yu, C.; Hu, S.; Fan, F.; et al. The combination of Biochanin A and SB590885 potentiates the inhibition of tumour progression in hepatocellular carcinoma. Cancer Cell Int. 2020, 20, 371. [Google Scholar] [CrossRef]
- Wieczorek, A.; Lysek-Gladysinska, M.; Krol, T.; Kordos, K.; Kosińska, K.; Atanasov, A.G.; Strzalkowska, N.; Jozwik, A. Biochemical and morphological changes in mouse liver induced by mistletoe toxins. Food Chem. Toxicol. 2019, 129, 229–238. [Google Scholar] [CrossRef]
- Shi, F.; Li, J.; Ye, Z.; Yang, L.; Chen, T.; Chen, X.; Ye, M. Antitumor effects of melanin from Lachnum YM226 and its derivative in H22 tumor-bearing mice. MedChemComm 2018, 9, 1059–1068. [Google Scholar] [CrossRef]
- Li, J.; Fu, Y.; Hu, X.; Xiong, Y. Psoralidin inhibits the proliferation of human liver cancer cells by triggering cell cycle arrest, apoptosis and autophagy and inhibits tumor growth in vivo. J. BUON 2019, 24, 1950–1955. [Google Scholar]
- Xiao, B.; Lin, D.; Zhang, X.; Zhang, M.; Zhang, X. TTF1, in the Form of Nanoparticles, Inhibits Angiogenesis, Cell Migration and Cell Invasion In Vitro and In Vivo in Human Hepatoma through STAT3 Regulation. Molecules 2016, 21, 1507. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Pan, J.; Liu, Y.; Chen, L.; Ren, Y. Anti-tumour effects of polysaccharide extracted from Acanthopanax senticosus and cell-mediated immunity. Exp. Ther. Med. 2018, 15, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wei, J.; Chen, S.; Wang, H.; Ning, L.; Luo, S.H.; Liu, C.L.; Song, G.; Yao, Q. Fucoidan Inhibits the Progression of Hepatocellular Carcinoma via Causing lncRNA LINC00261 Overexpression. Front. Oncol. 2021, 11, 653902. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, Z.; Fan, X.; Chen, M.; Cheng, X.; Zhang, D.; Wu, F.; Ying, X.; Ji, J. Shikonin potentiates the effect of arsenic trioxide against human hepatocellular carcinoma in vitro and in vivo. Oncotarget 2016, 7, 70504–70515. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.; Amaral, R.G.; Dória, G.A.; Fonseca, C.S.; da Silva, T.K.; Albuquerque Júnior, R.L.; Thomazzi, S.M.; do Nascimento, L.G.; Carvalho, A.A.; de Sousa, D.P. In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol. Int. J. Mol. Sci. 2016, 17, 32. [Google Scholar] [CrossRef]
- Khan, F.; Khan, T.J.; Kalamegam, G.; Pushparaj, P.N.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Al-Qahtani, M. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement. Altern. Med. 2017, 17, 418. [Google Scholar] [CrossRef]
- Yang, X.; Ren, L.; Zhang, S.; Zhao, L.; Wang, J. Antitumor Effects of Purified Protosappanin B Extracted From Lignum Sappan. Integr. Cancer Ther. 2016, 15, 87–95. [Google Scholar] [CrossRef]
- Samad, N.; Abdul, A.; Rahman, H.; Abdullah, R.; Ibrahim, T.; Othman, H. Antiproliferative and antiangiogenic effects of zerumbone from Zingiber zerumbet L. Smith in sprague dawley rat model of hepatocellular carcinoma. Pharmacogn. Mag. 2019, 15, 277. [Google Scholar] [CrossRef]
- Shahin, Y.R.; Elguindy, N.M.; Abdel Bary, A.; Balbaa, M. The protective mechanism of Nigella sativa against diethylnitrosamine-induced hepatocellular carcinoma through its antioxidant effect and EGFR/ERK1/2 signaling. Environ. Toxicol. 2018, 33, 885–898. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, Y.F.; Huang, J.H.; Wang, Z.Y.; Chen, Q.Y.; Su, L.T.; Liu, Z.T.; Xiong, C.M.; Tao, Z.; Guo, R.P. The Chinese medicine, Jianpi Huayu Decoction, inhibits the epithelial mesenchymal transition via the regulation of the Smad3/Smad7 cascade. Am. J. Transl. Res. 2017, 9, 2694–2711. [Google Scholar]
- Ahmed, H.; Rady, H.; Kotob, S. Evidences for the antitumor potentiality of Hemimycale arabica and Negombata magnifica mesohyls in hepatocellular carcinoma rat model. Med. Chem. Res. 2018, 27, 1538–1548. [Google Scholar] [CrossRef]
- Dokkaew, A.; Punvittayagul, C.; Insuan, O.; Limtrakul Dejkriengkraikul, P.; Wongpoomchai, R. Protective Effects of Defatted Sticky Rice Bran Extracts on the Early Stages of Hepatocarcinogenesis in Rats. Molecules 2019, 24, 2142. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Reynolds, L.; Mulik, R.S.; Kim, S.Y.; Van Treuren, T.; Nguyen, L.H.; Zhu, H.; Corbin, I.R. Hepatic Arterial Infusion of Low-Density Lipoprotein Docosahexaenoic Acid Nanoparticles Selectively Disrupts Redox Balance in Hepatoma Cells and Reduces Growth of Orthotopic Liver Tumors in Rats. Gastroenterology 2016, 150, 488–498. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Zhu, F.; Li, Z.; Zhang, L.; Xie, Y.; Chinnathambi, A.; Alahmadi, T.A.; Lu, B. Phyllanthin prevents diethylnitrosamine (DEN) induced liver carcinogenesis in rats and induces apoptotic cell death in HepG2 cells. Biomed. Pharmacother. 2021, 137, 111335. [Google Scholar] [CrossRef]
- Badr El-Din, N.K.; Ali, D.A.; Othman, R.; French, S.W.; Ghoneum, M. Chemopreventive role of arabinoxylan rice bran, MGN-3/Biobran, on liver carcinogenesis in rats. Biomed. Pharmacother. 2020, 126, 110064. [Google Scholar] [CrossRef] [PubMed]
- Sadeeshkumar, V.; Duraikannu, A.; Ravichandran, S.; Kodisundaram, P.; Fredrick, W.S.; Gobalakrishnan, R. Modulatory efficacy of dieckol on xenobiotic-metabolizing enzymes, cell proliferation, apoptosis, invasion and angiogenesis during NDEA-induced rat hepatocarcinogenesis. Mol. Cell. Biochem. 2017, 433, 195–204. [Google Scholar] [CrossRef]
- Krishnan, G.; Subramaniyan, J.; Chengalvarayan Subramani, P.; Muralidharan, B.; Thiruvengadam, D. Hesperetin conjugated PEGylated gold nanoparticles exploring the potential role in anti-inflammation and anti-proliferation during diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian J. Pharm. Sci. 2017, 12, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.; Saidan, S.; Morsy, B.M. Coenzyme Q10 attenuates rat hepatocarcinogenesis via the reduction of CD59 expression and phospholipase D activity. Cell Biochem. Funct. 2020, 38, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Kumar, M.; Kaur, P.; Kaur, S.; Singh, A.P.; Kaur, S. Hepatoprotective activity of Butea monosperma bark against thioacetamide-induced liver injury in rats. Biomed. Pharmacother. 2017, 89, 332–341. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; Liu, Y.; Ye, X.; Ji, X.; Sun, L.; Gao, F.; Zhang, Q.; Li, Y.; Zhu, B.; et al. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine 2021, 87, 153575. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Yin, X.; Huang, W.; Cao, H.; Wang, G.; Wang, J.; Zhou, J. Jiedu Granule Combined with Transcatheter Arterial Chemoembolization and Gamma Knife Radiosurgery in Treating Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Biomed. Res. Int. 2019, 2019, 4696843. [Google Scholar] [CrossRef] [PubMed]
- Changou, C.A.; Shiah, H.S.; Chen, L.T.; Liu, S.; Luh, F.; Liu, S.H.; Cheng, Y.C.; Yen, Y. A Phase II Clinical Trial on the Combination Therapy of PHY906 Plus Capecitabine in Hepatocellular Carcinoma. Oncologist 2021, 26, e367–e373. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.F.; Liu, X.L.; Shen, F.; Fan, J.; Ling, C.Q. Traditional herbal medicine prevents postoperative recurrence of small hepatocellular carcinoma: A randomized controlled study. Cancer 2018, 124, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.S.; Liu, Y.; Zhang, Q.; Li, C.; Zhang, Y.W.; Ren, Z.Z.; Zhou, J.; Zhang, M. Transarterial chemoembolization combined with Huaier granule for the treatment of primary hepatic carcinoma: Safety and efficacy. Medicine 2017, 96, e7589. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Zhuang, L.; Lin, J.; Chen, H.; Zhu, X.; Bei, W.; Zhao, Q.; Wu, H.; Meng, Z. Jian Pi Li Qi Decoction Alleviated Postembolization Syndrome Following Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma: A Randomized, Double-Blind, Placebo-Controlled Trial. Integr. Cancer Ther. 2016, 15, 349–357. [Google Scholar] [CrossRef]
- Ye, X.; Lu, D.; Chen, X.; Li, S.; Chen, Y.; Deng, L. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Shuangbai San for Treating Primary Liver Cancer Patients with Cancer Pain. J. Pain Symptom Manag. 2016, 51, 979–986. [Google Scholar] [CrossRef]
- Chay, W.Y.; Tham, C.K.; Toh, H.C.; Lim, H.Y.; Tan, C.K.; Lim, C.; Wang, W.W.; Choo, S.P. Coriolus versicolor (Yunzhi) Use as Therapy in Advanced Hepatocellular Carcinoma Patients with Poor Liver Function or Who Are Unfit for Standard Therapy. J. Altern. Complement. Med. 2017, 23, 648–652. [Google Scholar] [CrossRef]
- Keino, D.; Yokosuka, T.; Hirose, A.; Sakurai, Y.; Nakamura, W.; Fujita, S.; Hayashi, A.; Miyagawa, N.; Iwasaki, F.; Hamanoue, S.; et al. Pilot study of the combination of sorafenib and fractionated irinotecan in pediatric relapse/refractory hepatic cancer (FINEX pilot study). Pediatr. Blood Cancer 2020, 67, e28655. [Google Scholar] [CrossRef]
- Deng, Z.; Xu, X.Y.; Yunita, F.; Zhou, Q.; Wu, Y.R.; Hu, Y.X.; Wang, Z.Q.; Tian, X.F. Synergistic anti-liver cancer effects of curcumin and total ginsenosides. World J. Gastrointest. Oncol. 2020, 12, 1091–1103. [Google Scholar] [CrossRef]
- Man, S.; Luo, C.; Yan, M.; Zhao, G.; Ma, L.; Gao, W. Treatment for liver cancer: From sorafenib to natural products. Eur. J. Med. Chem. 2021, 224, 113690. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacol. Res. 2019, 144, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Liu, Z.; Ci, L.; Shuai, C.; Lv, X.; Li, J. Research progress on the anti-hepatic fibrosis action and mechanism of natural products. Int. Immunopharmacol. 2019, 75, 105765. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Frew, A.J.; Smyth, M.J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer 2008, 8, 782–798. [Google Scholar] [CrossRef]
- Heldin, C.H.; Vanlandewijck, M.; Moustakas, A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012, 586, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Koh, W.; Lee, E.O.; Lee, H.J.; Lee, H.J.; Bae, H.; Lü, J.; Kim, S.H. Antiangiogenic phytochemicals and medicinal herbs. Phytother. Res. 2011, 25, 1–10. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- He, S.; Zhang, X.; Lu, S.; Zhu, T.; Sun, G.; Sun, X. A Computational Toxicology Approach to Screen the Hepatotoxic Ingredients in Traditional Chinese Medicines: Polygonum multiflorum Thunb as a Case Study. Biomolecules 2019, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.H.; Kil, J.H.; Ahn, Y.C.; Son, C.G. Systematic review of published data on herb induced liver injury. J. Ethnopharmacol. 2019, 233, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Amadi, C.N.; Orisakwe, O.E. Herb-Induced Liver Injuries in Developing Nations: An Update. Toxics 2018, 6, 24. [Google Scholar] [CrossRef]
- Zhai, X.R.; Zou, Z.S.; Wang, J.B.; Xiao, X.H. Herb-Induced Liver Injury Related to Reynoutria multiflora (Thunb.) Moldenke: Risk Factors, Molecular and Mechanistic Specifics. Front. Pharmacol. 2021, 12, 738577. [Google Scholar] [CrossRef]
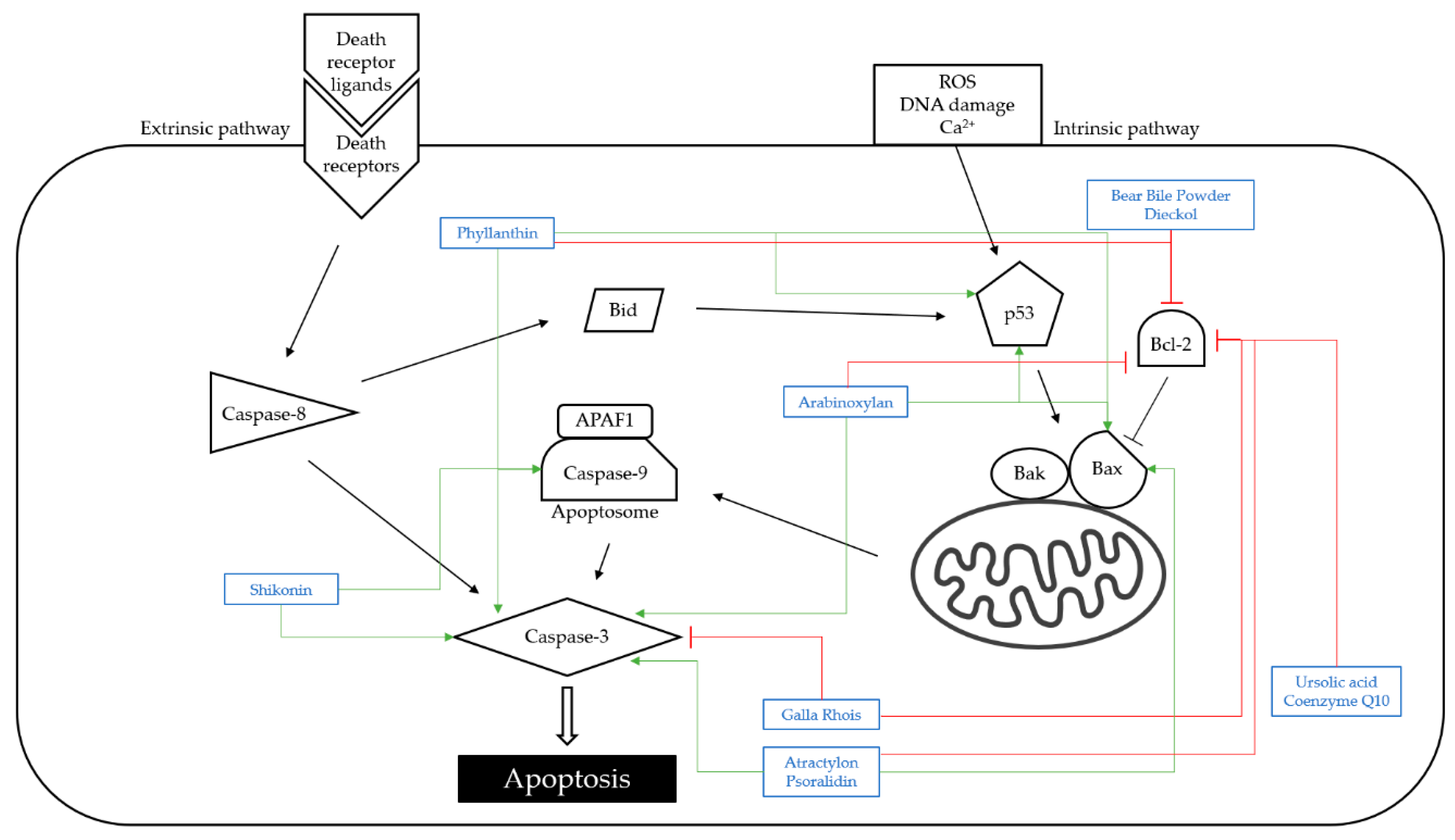
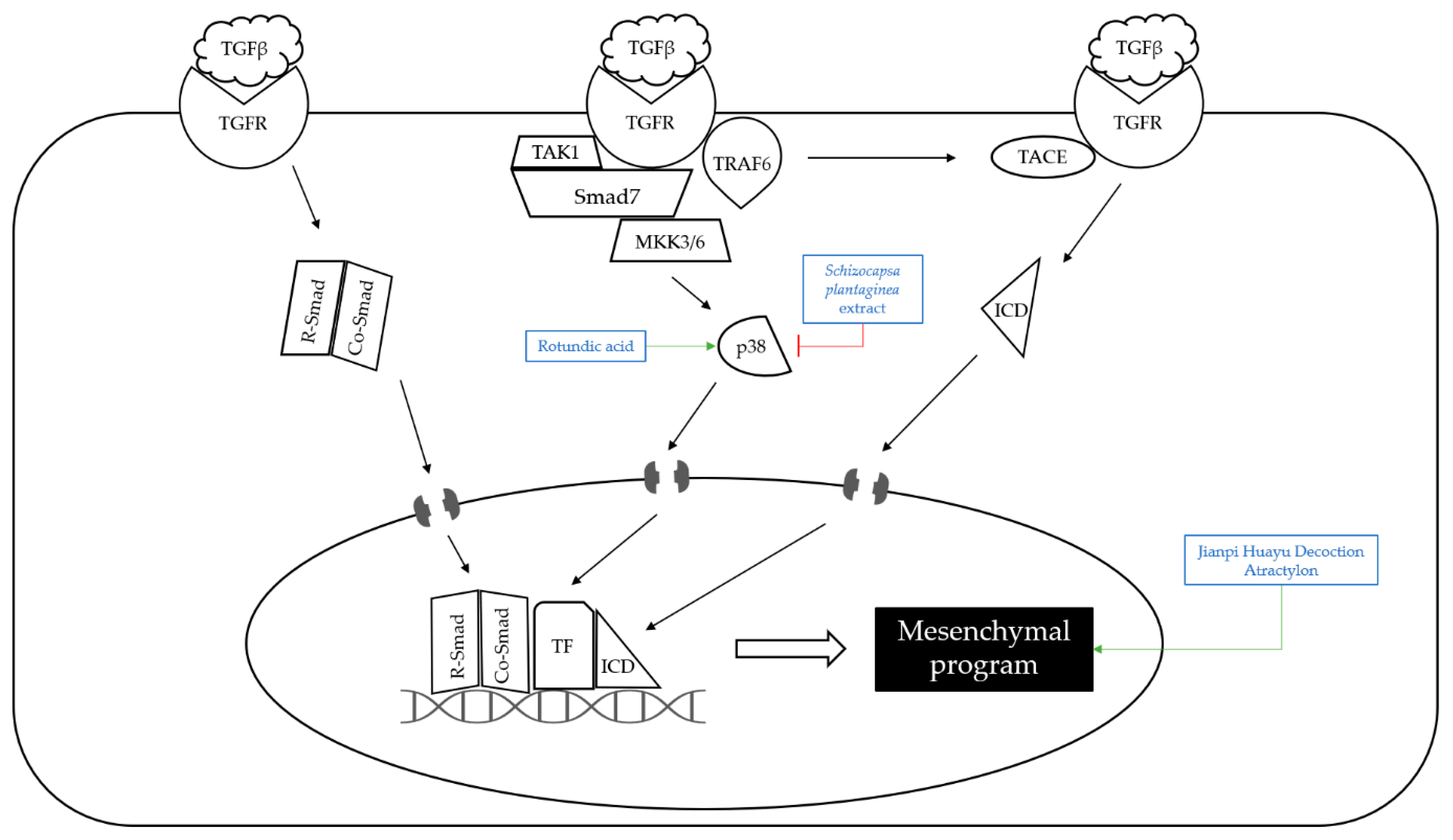
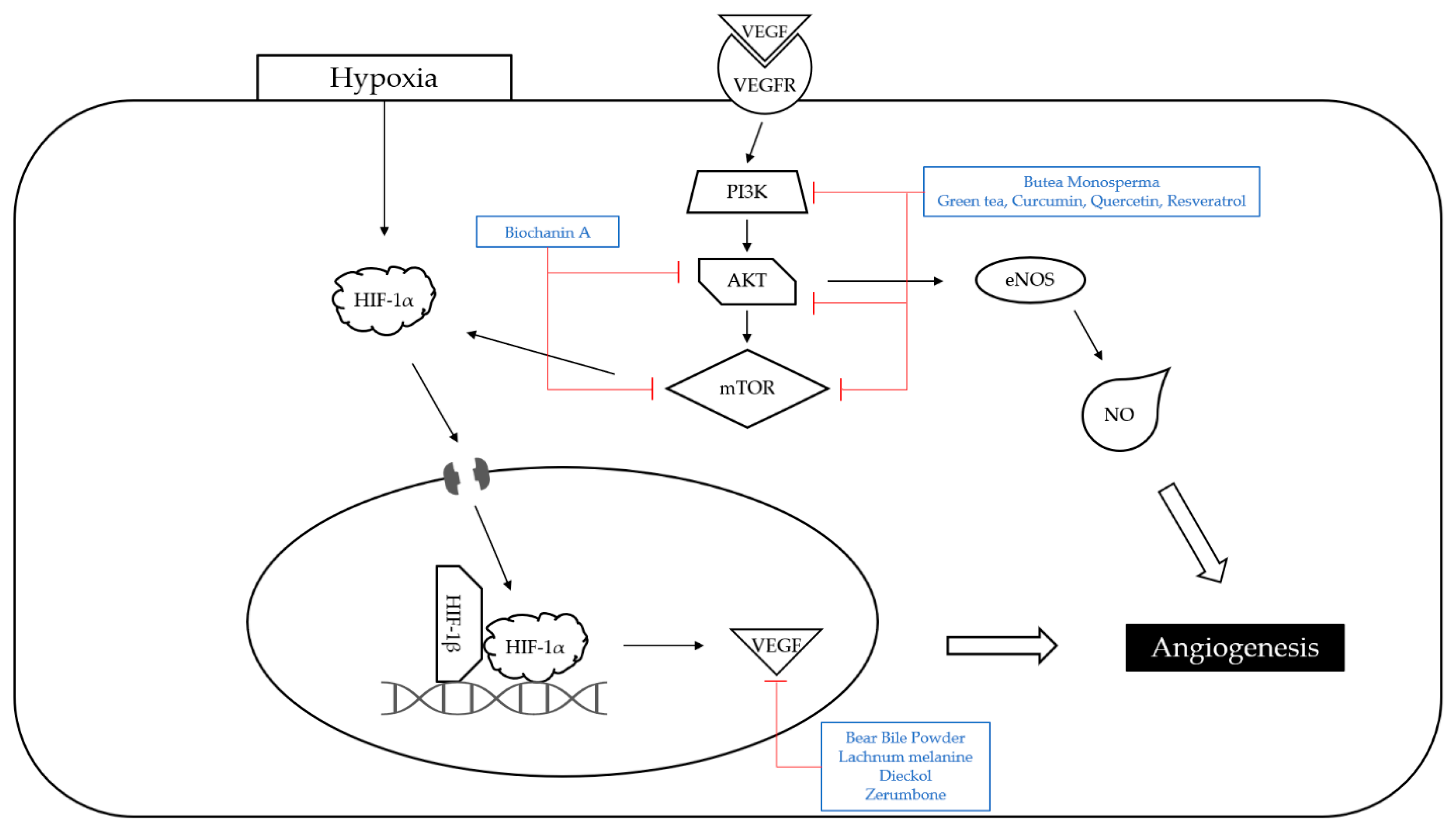
| Classification | Compound/Extract | Source | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Animal | Bear Bile Powder | Fel Ursi | 10 mg/kg; 3 weeks | Induction of cell apoptosis Inhibition of tumor growth | ↓Bcl-2, Cyclin D1, CDK4, VEGF-A ↑Bax | [10] | |
| Fungi | Agaricus Bisporus extracts | Agaricus bisporus | CBA mice | 1 g; 12 weeks | Inhibition of the progression of the carcinogenesis | ↓Sialic acid | [11] |
| Plant | Green tea, curcumin, quercetin, resveratrol extracts | C57BL6 mice | 5 mg/g; 50 days | Increase in the survival time | ↑AMPK, SIRT1, LKB1 ↓ IGF-1R, PI3K, AKT, mTOR, NF-κB | [12] | |
| Plant | Neem tree extract | Azadirachta indica | Kunming mice | 150, 300, 600 mg/kg; 27 days | Increase in survival rate Inhibition of H22 tumor growth | [13] | |
| Plant | Rhizoma Paridis saponins extract | Paris polyphylla var. yunnanensis | Kunming mice | 100 mg/kg; 14 days | Decrease in tumor weight | ↓Lactate, acetate, N-acetyl amino acid | [14] |
| Plant | Schizocapsa plantaginea extract | Schizocapsa plantaginea | 10, 25, 50 mg/kg; 15 days | Inhibition of tumor growth | ↑JNK, ERK1/2 ↓p38 | [15] | |
| Plant | Tomato powder | 41.9 g/kg; 24 weeks | Prevention of HFD-induced inflammation Inhibition of HFD-promoted HCC development | ↑SIRT1, NAMPT, NAD+ ↓IL1β, IL-6, IL12α, MCP1 | [16] |
| Classification | Compound/Extract | Source | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Alkaloids | Berberine (Berberine hydrochloride) | ICR mice | 50, 100 mg/kg; 15 days | Inhibition of H22 tumor growth | [17] | ||
| Alkaloids | Britanin | Inula Linariifolia Turcz | BALB/c mice | 5, 10 mg/kg; 30 days | Inhibition of tumor proliferation Induction of apoptosis | ↑Bax, ↓Bcl-2, NF-κB, p65 | [18] |
| Fatty Acids | DOX-nano | BALB/c mice | 2 mg/kg; 10 days | Inhibition of tumor growth | ↓MRP, LRP, BCRP, Bcl-2, PKC-α | [19] | |
| Fatty Acids | LDL–DHA | BALB/c mice | 2.5 mg/kg; 3 days | Induction of ferroptotic cell death | ↑Lipid hydroperoxides ↓GPx-4 | [20] | |
| Hydrocarbons | Atractylon | Atractylodes lancea Atractylodes chinensis | NOD/SCID mice | 5, 10 mg/kg; 15 days | Inhibition of the hepatic cancer growth Inhibition of the EMT process | ↑ E-cadherin, TIMP2, Bax, c- caspase-3 ↓ Ki-67, N-cadherin, Vimentin, α-SMA, MMP-2, MMP-9, Bcl-2 | [21] |
| Hydrocarbons | GL22 | Ganoderma leucocontextum | BALB/C nu-nu mice | 50 mg/ kg; 7 days | Decrease in tumor volume | ↓PPARα, PPARγ, FABP1, FABP4, FABP5 | [22] |
| Hydrocarbons | Rotundic acid | Ilex Rotunda | BALB/c mice | 50 mg/kg; 60 days | Inhibition of proliferation and angiogenesis Induction of apoptosis | ↑p38 ↓Ki-67, CD-31 | [23] |
| Hydrocarbons | Ursolic acid | 60 mg/kg; 15 days | Inhibition of HepG2 tumor growth | ↑c-caspase3 ↓p-STAT3, Bcl-2 | [24] | ||
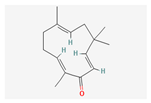
| Hydrocarbons | Zerumbone | Zingiber zerumbet | NSG mice | 20 mg/kg; 21 days | Suppression of subcutaneous, orthotopic growth and lung metastasis | [25] | |
| Polyphenols | 747 | Abies georgei | BALB/c mice, C57BL/6 mice | 50, 100 mg/kg; 24 days | Enhancement of tumor immunosuppressive microenvironment Increase in the therapeutic effect of sorafenib | ↑CD8 T cells ↓TAMs | [26] |
| Polyphenols | Biochanin A | Athymic nude mice | 25 mg/kg; 5 weeks | Inhibition of tumor growth | ↓PCNA | [27] | |
| Polyphenols | Iscador P | Viscum album | Swiss Albino mice | 0.1, 1, 2 mg/kg; 24, 48 h | Induction of autophagy | ↑aminopeptidases, β-D-glucuronidase, hepatocyte mitochondria ↓lysosomal hydrolases | [28] |
| Polyphenols | Lachnum melanin | Lachnum | Kunming mice | 50, 200 mg/kg; 12 days | Improvement in the immune functions Inhibition of angiogenesis | ↑IL-2, IL-6, TNF-α, IFN-γ ↓VEGF, bFGF | [29] |
| Polyphenols | Psoralidin | Psoralea Corylifolia | 10, 20, 40 mg/kg; 6 weeks | Inhibition of tumor growth Induction of cell cycle arrest and apoptosis | ↑Bax, c-caspase-3, -9 ↓Bcl-2 | [30] | |
| Polyphenols | TTF1-NP | Sorbaria sorbifolia | BALB/c mice | 5, 10, 20 µmol/kg | Inhibition of tumor growth | ↓STAT3, p-STAT3 | [31] |
| Polysaccharides | Acanthopanax senticosus extract | Acanthopanax Senticosus | Kunming mice | 50, 100, 200 mg/kg; 10 days | Inhibition of tumor growth | ↑IL-2, IL-12, INF-γ | [32] |
| Polysaccharides | Fucoidan | BALB/c mice | 15 mg/kg, 3 weeks | Inhibition of motility and invasion of tumor | ↑LINC00261 ↓miR-522-3p | [33] | |
| Quinones | Shikonin | Lithospermum erythrorhizon | nu/nu mice | 3 mg/kg; 30 days | Inhibition of ATO-induced tumor growth Inducement of ROS accumulation | ↑CHOP, caspase 3, caspase 9, MDA | [34] |
| Classification | Compound/Extract | Source | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Plant | Nigella Sativa extract | Nigella Sativa | Wistar Albino rats | 150, 250, 350 mg/kg; 12 days | Inhibition of proliferation | ↓EGFR, ERK1/2, PCNA, c-fos, Bcl-2 | [39] |
| Plant | Ajwa dates extract | Phoenix dactylifera | Wistar rats | 0.5, 1.0 g/kg; 10 weeks | Reversal of liver damage | ↑SOD, GR, GPx, ALT, AST, ALP, IL-2, IL-12 ↓ IL-1α, IL-1β, GM-CSF, AFP, IL-6 | [36] |
| Plant | Jianpi Huayu Decoction extract | Panax ginseng, Atractylodes macrocephala, Dioscorea opposita thumb, Wolfiporia extens, Cortex Moutan Radicis, Salvia miltiorrhiza bunge, Curcuma aromatica, Curcuma phaeocaulis val, Radix Bupleuri, Radix liquiritiae | Wistar rats | 5 mL/dose/kg; 7 days | Inhibition of epithelial—mesenchymal transition | ↑E-cadherin, EpCAM ↓N-cadherin, Vimentin | [40] |
| Plant | Mesohyl blue (Marine sponge extract) | Hemimycale arabica | Albino rats | 1 mL; 4 weeks | Induction of hepatoprotective, potency, apoptosis Inhibition of proliferation | ↓AFP, AFU, AST, ALT, bilirubin, CEA, GGT | [41] |
| Plant | Mesohyl red (Marine sponge extract) | Negombata magnifica | Albino rats | 1 mL; 4 weeks | Induction of hepatoprotective potency, apoptosis Inhibition of proliferation | ↓AFP, AFU, AST, ALT, bilirubin, CEA GGT | [41] |
| Plant | Purple rice bran methanol extract | Rats | 500 mg/kg; 15 weeks | Inhibition of hepatocarcinogenesis | ↓TNF-α, iNOS, NF- κB. | [42] |
| Classification | Compound/Extract | Source | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Fatty Acids | LDL–DHA | AxC-Irish rats | 2 mg/kg | Inhibition of redox reactions in tumor tissues | ↓GPx-4 | [43] | |
| Hydrocarbons | Phyllanthin | Wistar Albino rats | 30 mg/kg; 14 weeks | Inhibition of tumorigenesis Suppression of liver damage | ↑p53, Bax, c-caspase-3,9 ↓Bcl-2 | [44] | |
| Hydrocarbons | Zerumbone | Zingiber zerumbet | Sprague Dawley rats | 30, 60 mg/kg; 3 weeks | Induction of apoptosis Inhibition of angiogenesis | ↓Ki-67, VEGF, MMP-9 | [38] |
| Polysaccharides | Arabinoxylan | Oryza Sativa | Wistar rats | 25 mg/kg; 22 weeks | Induction of cell cycle arrest, DNA fragmentation in cancer cells, apoptosis Inhibition of hepatocarcinogenesis | ↑Bax, c-caspase-3, p53, IκB-α, NF-κB/p65 ↓Bcl-2 | [45] |
| Polyphenols | Dieckol | Ecklonia cava | Wistar rats | 40 mg/kg; 15 weeks | Regulation of apoptosis, inflammation, invasion, and angiogenesis | ↓XMEs, Bcl-2, MMP2/9, VEGF, NF- κB, COX2 ↑Bax, cytochrome c, caspase-3 | [46] |
| Polyphenols | Hesperetin | Wistar rats | 20 mg/kg; 16 weeks | Inhibition of cell inflammation and proliferation | ↓TNF-α, NF-κB, glycoconjugates, PCNA | [47] | |
| Quinones | Coenzyme Q10 | Albino rats | 0.4 mg/kg; 2 weeks | Inhibition of proliferation, histological alterations | ↑c-caspase-3 ↓CD59, Bcl-2, SRB1, PLD | [48] |
 Berberine | 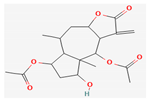 Britanin | 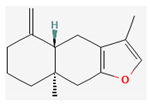 Atractylon | 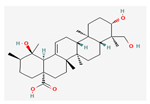 Rotundic acid | 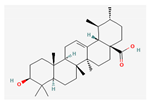 Ursolic acid |
 Zerumbone | 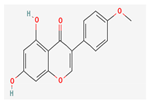 Biochanin A | 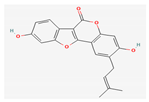 Psoralidin | 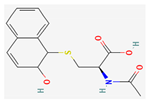 Acanthopanax senticosus extract | 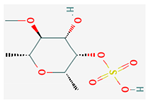 Fucoidan |
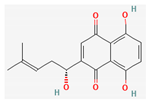 Shikonin | 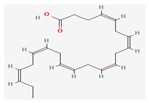 LDL–DHA |  Phyllanthin | 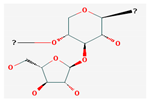 Arabinoxylan |  Dieckol |
 Hesperetin |  Coenzyme Q10 |
| Compound/Extract | Source | Patients | Efficacy | Results of the Treatment Groups | Results of the Control Groups | p-Value | Registration Number | Reference |
|---|---|---|---|---|---|---|---|---|
| Fuzheng Jiedu Xiaoji formulation | Codonopsis pilosula Astragalus mongolicus Atractylodes macrocephala Angelica sinensis Poria cocos Adenophora stricta Ophiopogon japonicus Rehmannia glutinosa Paris polyphylla Curcuma phaeocaulis Pinellia ternata | 291 | Inhibition of the proliferation and migration of liver cancer cells Extension of one-year OS and PFS | One-year OS rate was 80.6%, and PFS rate was 48.6% | One-year OS rate was 68%, and PFS rate was 27.9% | 0.0233 (OS) 0.0064 (PFS) | [50] | |
| Jiedu Granule | Salvia chinensis Actinidia valvata Gallus gallus domesticus Cremastra appendiculata | 190 | Extension of median overall survival rates | The median OS was 15.8 months | The median OS was 11.3 months | 0.00047 | [51] | |
| PHY906 | Scutelleria baicalensis Paeonia lactiflora Glycyrrhiza uralensis Ziziphus jujuba | 39 | Extension of median overall survival rates | The median PFS was 1.5 months, and median OS was 6 months with a 51.3% 6-month survival rate. | N/A | N/A | NCT00076609 | [52] |
| THM | Bufo gargarizans Actinidia valvata Salvia chinensis Pseudobulbus cremastrae Gallus gallus domesticus | 364 | Prevention of small hepatocellular carcinoma recurrence | The median RFS was 85.83 months and the 5-year OS rates was 71.11% | The median RFS was 26.00 months and the 5-year OS rates was 63.04% | <0.001 (RFS) 0.0076 (OS) | [53] | |
| Huaier granule | Trametes robiniophila | 62 | Improvement in treatment response Extension of median survival rates | The 6- and 12-month OS were 100% and 93.5%, respectively | The 6- and 12-month OS were 90.3% and 80.6%, respectively | <0.05 (6 month) >0.05 (12 month) | [54] | |
| Jian Pi Li Qi decoction | Poria cocos, Atractylodes macrocephala Codonopsis pilosula Fructus aurantii Akebia fruit Citrus Chirocarpus | 140 | Improvement in liver function | N/A | N/A | N/A | [55] | |
| Shuangbai San | Radix et Rhizoma Rhei Platycladus orientalis Phellodendron amurense Lycopus lucidus M. haplocalyx | 134 | Relief of cancer pain and improvement in quality of life | N/A | N/A | N/A | [56] | |
| Yunzhi | Coriolus Versicolor | 15 | Extension of median survival rates | The median PFS was 2.5 months, and the median OS was 6.5 months | The median PFS was 1.1 months, and the median OS was 2.2 months | 0.144 (PFS) 0.105 (OS) | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.B.; Lee, D.K.; Cheon, C.; Ribeiro, R.I.M.A.; Kim, B. Natural Products for Liver Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients 2022, 14, 4252. https://doi.org/10.3390/nu14204252
Kim DB, Lee DK, Cheon C, Ribeiro RIMA, Kim B. Natural Products for Liver Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients. 2022; 14(20):4252. https://doi.org/10.3390/nu14204252
Chicago/Turabian StyleKim, Da Bin, Do Kyeong Lee, Chunhoo Cheon, Rosy Iara Maciel A. Ribeiro, and Bonglee Kim. 2022. "Natural Products for Liver Cancer Treatment: From Traditional Medicine to Modern Drug Discovery" Nutrients 14, no. 20: 4252. https://doi.org/10.3390/nu14204252
APA StyleKim, D. B., Lee, D. K., Cheon, C., Ribeiro, R. I. M. A., & Kim, B. (2022). Natural Products for Liver Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients, 14(20), 4252. https://doi.org/10.3390/nu14204252









