Red Pepper Seeds Inhibit Hepatic Lipid Accumulation by Inducing Autophagy via AMPK Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. Animals
2.3. Determination of Serum AST and ALT levels
2.4. Measurement of Hepatic TG Level
2.5. Cell Culture and Cell Viability Assay
2.6. Establishment of an Oleic Acid-Induced Hepatic Steatosis HepG2 Cell Model
2.7. Cell Transfection with siRNA
2.8. Western Blot Analysis
2.9. Oil Red O (ORO) Staining
2.10. Statistical Analysis
3. Results
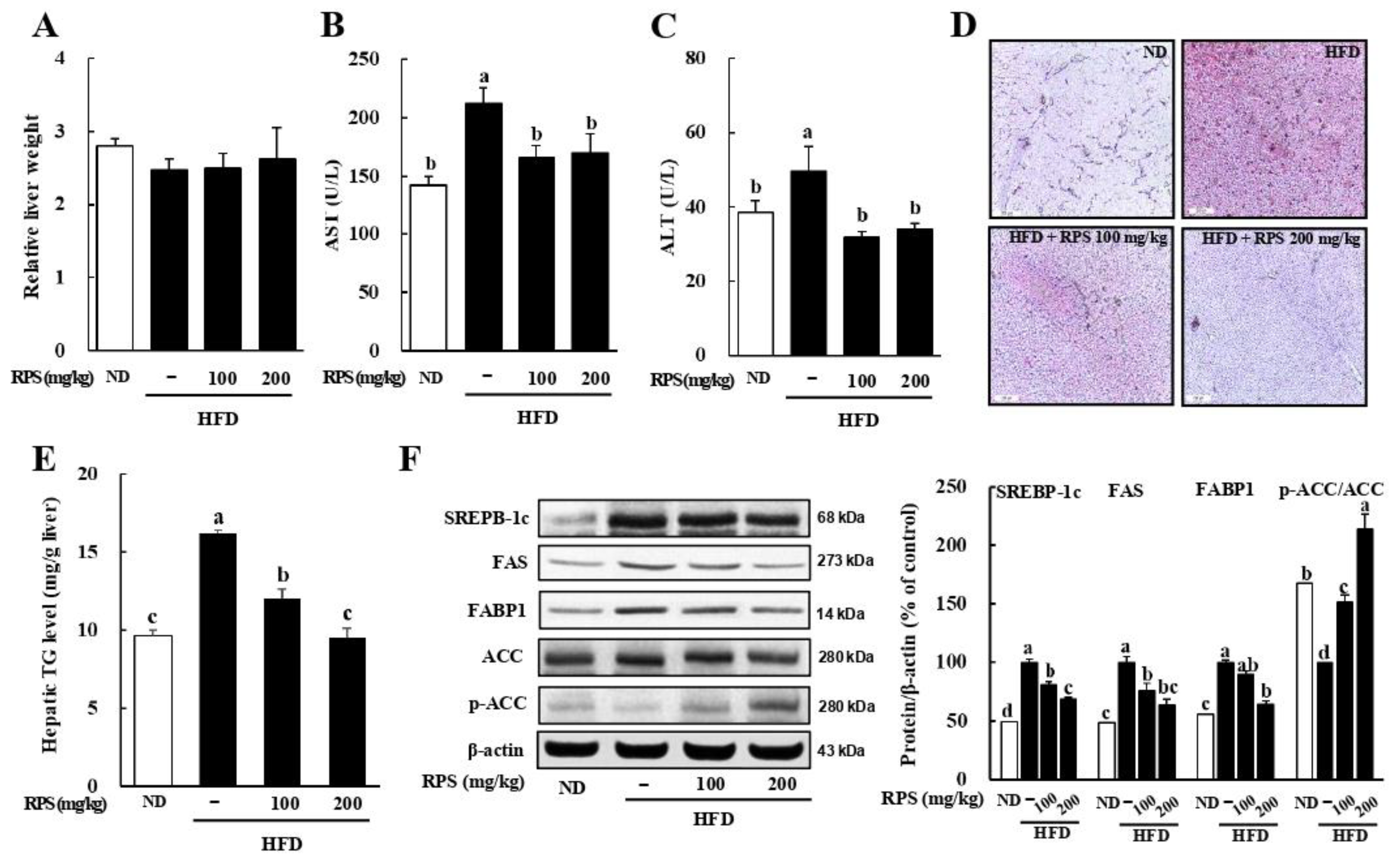
3.1. RPS Decreased Hepatic Toxicity, Lipid Droplet Accumulation, and Lipogenic-Associated Protein Expression in HFD-Fed Mice
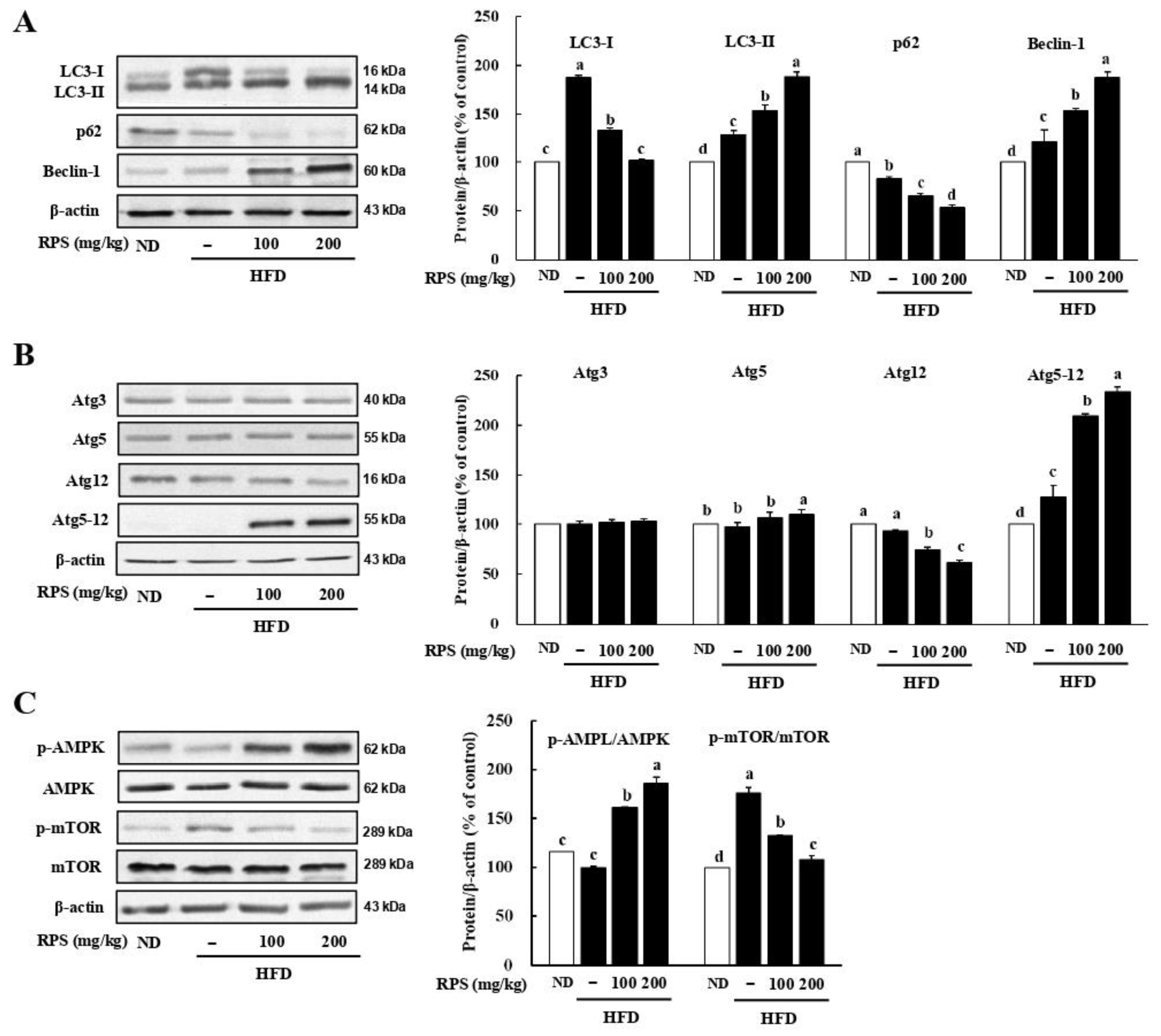
3.2. RPS Treatment Induced Autophagic Flux and AMPK Activation in the Livers of HFD-Fed Mice
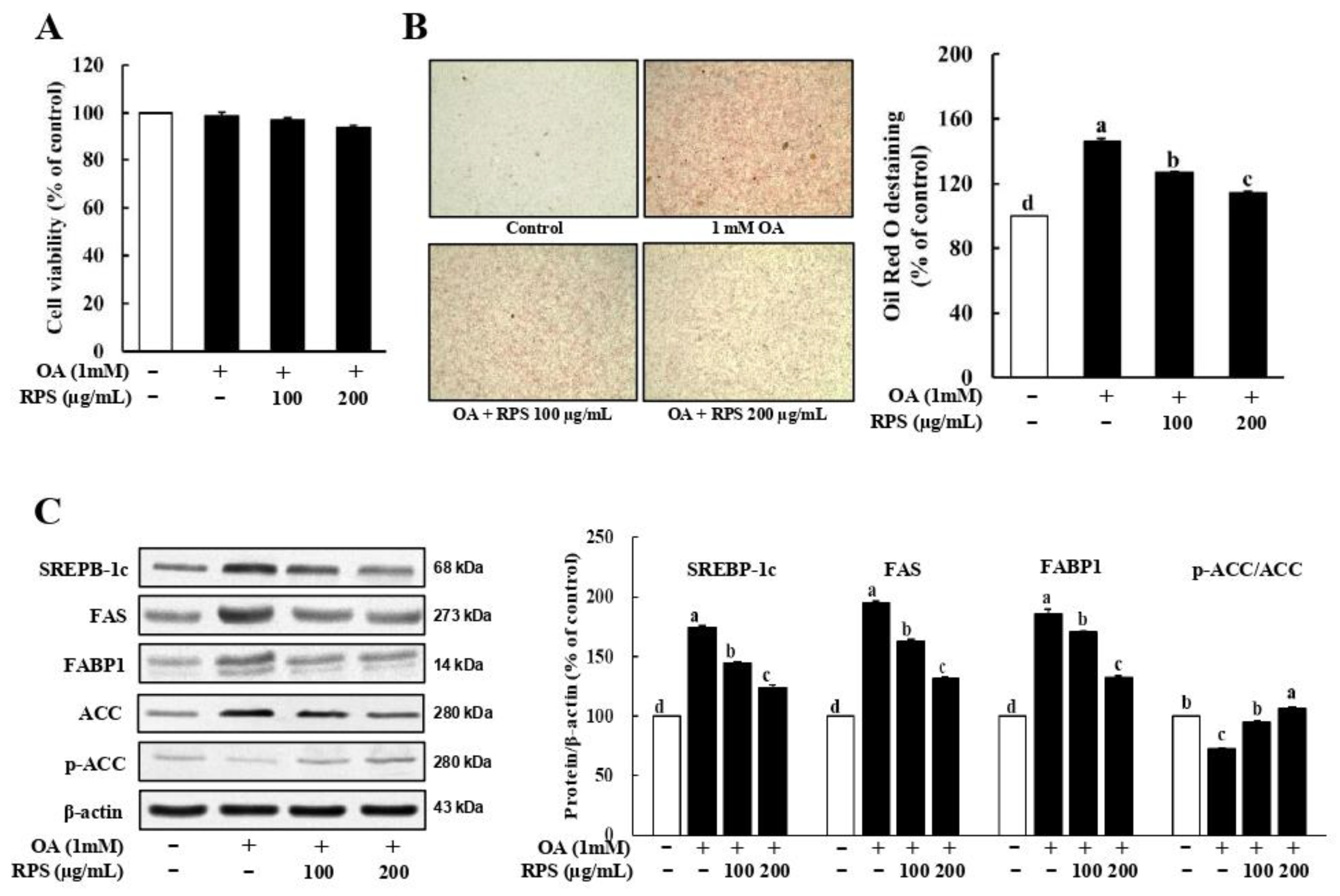
3.3. RPS Down-Regulated the Expression of Lipogenesis-Related Proteins in a Cell Model of OA-Induced Steatosis
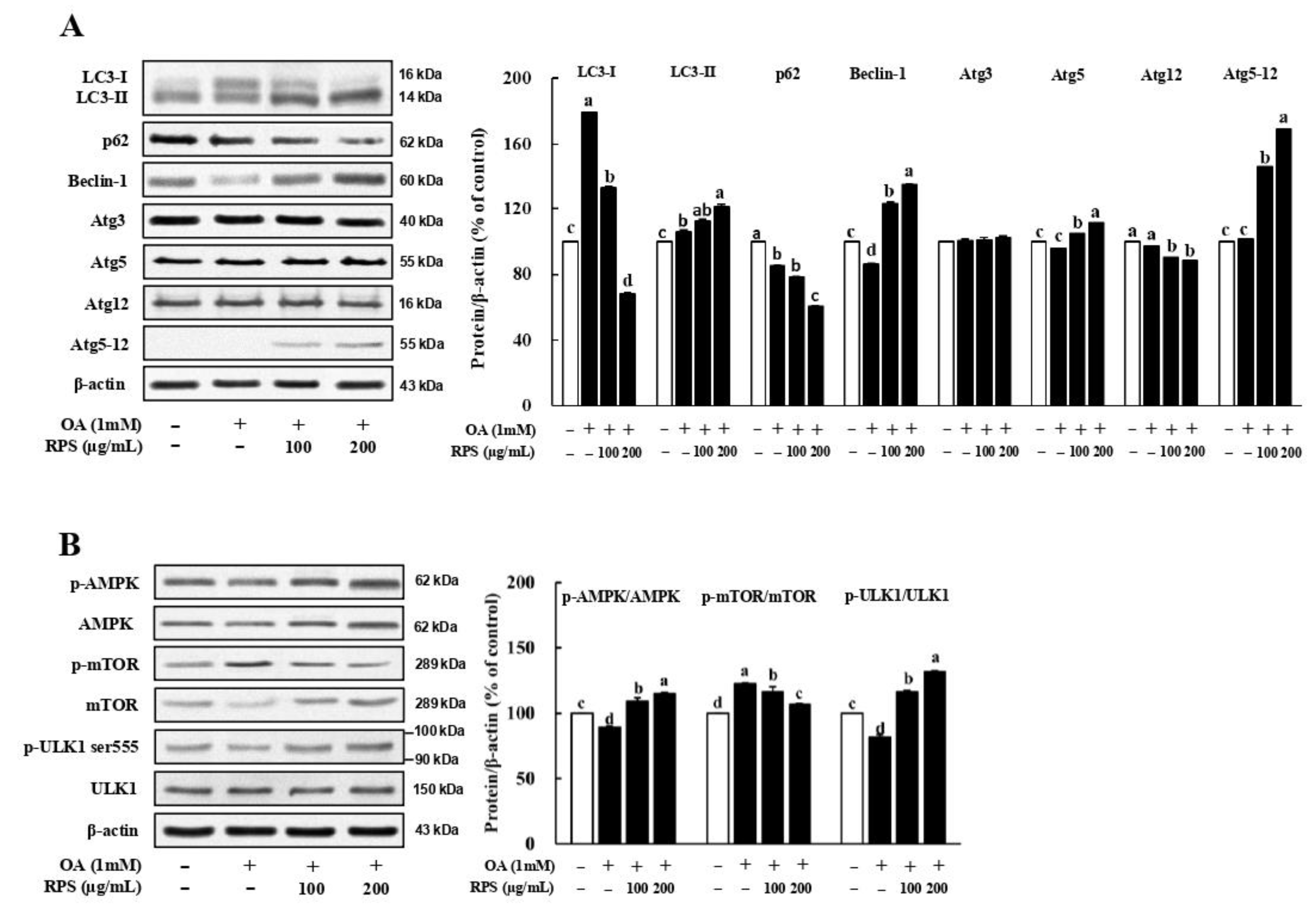
3.4. RPS Up-Regulated Autophagic Flux in a Cell Model of OA-Induced Steatosis
3.5. Inhibition of Autophagy Abolished the RPS-Induced Suppression of Lipogenesis in a Cell Model of OA-Induced Steatosis
3.6. RPS-Induced Autophagy Activation Is Associated with the AMPK/mTOR Signaling in a Cell Model of OA-Induced Steatosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kamikubo, R.; Kai, K.; Tsuji-Naito, K.; Akagawa, M. β-Caryophyllene attenuates palmitate-induced lipid accumulation through AMPK signaling by activating CB2 receptor in human HepG2 hepatocytes. Mol. Nutr. Food Res. 2016, 60, 2228–2242. [Google Scholar] [CrossRef]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 2017, 179, 142–157. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Reccia, I.; Kumar, J.; Akladios, C.; Virdis, F.; Pai, M.; Habib, N.; Spalding, D. Non-alcoholic fatty liver disease: A sign of systemic disease. Metabolism 2017, 72, 94–108. [Google Scholar] [CrossRef]
- Negi, C.K.; Babica, P.; Bajard, L.; Bienertova-Vasku, J.; Tarantino, G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism 2022, 126, 154925. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Hicks, S.B.; Mara, K.C.; Larson, J.J.; Therneau, T.M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity–a longitudinal cohort study. J. Hepatol. 2019, 71, 1229–1236. [Google Scholar] [CrossRef]
- Bertot, L.C.; Adams, L.A. Trends in hepatocellular carcinoma due to non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Huber, Y.; Labenz, C.; Michel, M.; Wörns, M.-A.; Galle, P.R.; Kostev, K.; Schattenberg, J.M. Tumor incidence in patients with non-alcoholic fatty liver disease. Dtsch. Ärzteblatt Int. 2020, 117, 719. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, P.; Fu, S.; Calay, E.S.; Hotamisligil, G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010, 11, 467–478. [Google Scholar] [CrossRef]
- Ueno, T.; Komatsu, M. Autophagy in the liver: Functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 170–184. [Google Scholar] [CrossRef]
- Cahova, M.; Dankova, H.; Palenickova, E.; Papackova, Z.; Kazdova, L. The autophagy-lysosomal pathway is involved in TAG degradation in the liver: The effect of high-sucrose and high-fat diet. Folia Biol. 2010, 56, 173–182. [Google Scholar]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Czaja, M.J. Autophagy in nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 159–166. [Google Scholar] [CrossRef]
- Iorizzi, M.; Lanzotti, V.; Ranalli, G.; De Marino, S.; Zollo, F. Antimicrobial furostanol saponins from the seeds of Capsicum annuum L. var. acuminatum. J. Agric. Food Chem. 2002, 50, 4310–4316. [Google Scholar] [CrossRef]
- Sim, K.-H.; Han, Y.-S. The antimutagenic and antioxidant effects of red pepper seed and red pepper pericarp (Capsicum annuum L.). Prev. Nutr. Food Sci. 2007, 12, 273–278. [Google Scholar] [CrossRef][Green Version]
- Sim, K.H.; Sil, H.Y. Antioxidant activities of red pepper (Capsicum annuum) pericarp and seed extracts. Int. J. Food Sci. Technol. 2008, 43, 1813–1823. [Google Scholar] [CrossRef]
- Song, W.-Y.; Ku, K.-H.; Choi, J.-H. Effect of ethanol extracts from red pepper seeds on antioxidative defense system and oxidative stress in rats fed high-fat·high-cholesterol diet. Nutr. Res. Pract. 2010, 4, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, C.T.; Kim, I.H.; Kim, Y. Effects of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytother. Res. 2011, 25, 935–939. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Wang, F.; Chen, J.; Zhao, Y.; Wang, P.; Nilius, B.; Liu, D.; Zhu, Z. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor δ activation. Pflügers Arch. Eur. J. Physiol. 2013, 465, 1303–1316. [Google Scholar] [CrossRef]
- Shin, M.K.; Yang, S.-M.; Han, I.-S. Capsaicin suppresses liver fat accumulation in high-fat diet-induced NAFLD mice. Anim. Cells Syst. 2020, 24, 214–219. [Google Scholar] [CrossRef]
- Kang, J.-H.; Tsuyoshi, G.; Le Ngoc, H.; Kim, H.-M.; Tu, T.H.; Noh, H.-J.; Kim, C.-S.; Choe, S.-Y.; Kawada, T.; Yoo, H. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J. Med. Food 2011, 14, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Yen, G.-C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J. Agric. Food Chem. 2007, 55, 1730–1736. [Google Scholar] [CrossRef]
- Kim, H.-J.; You, M.-K.; Wang, Z.; Kim, H.-A. Red pepper seed inhibits differentiation of 3T3-L1 cells during the early phase of adipogenesis via the activation of AMPK. Am. J. Chin. Med. 2018, 46, 107–118. [Google Scholar] [CrossRef]
- Kim, H.-J.; You, M.-K.; Wang, Z.; Lee, Y.-H.; Kim, H.-A. Red pepper seed water extract suppresses high-fat diet-induced obesity in C57BL/6 mice. Food Sci. Biotechnol. 2020, 29, 275–281. [Google Scholar] [CrossRef]
- Youn, S.H.; Yin, J.; Ahn, H.S.; Tam, L.T.; Kwon, S.H.; Min, B.; Yun, S.H.; Kim, H.Y.; Lee, M.W. Quantitative analysis of Icariside E 5 and vanilloyl Icariside E 5 from the seed of Capsicum annuum L. Korean J. Pharmacogn. 2017, 48, 160–165. [Google Scholar]
- Lee, S.R.; Kwon, S.W.; Kaya, P.; Lee, Y.H.; Lee, J.G.; Kim, G.; Lee, G.-S.; Baek, I.-J.; Hong, E.-J. Loss of progesterone receptor membrane component 1 promotes hepatic steatosis via the induced de novo lipogenesis. Sci. Rep. 2018, 8, 15711. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef]
- Lystad, A.H.; Carlsson, S.R.; Laura, R.; Kauffman, K.J.; Nag, S.; Yoshimori, T.; Melia, T.J.; Simonsen, A. Distinct functions of ATG16L1 isoforms in membrane binding and LC3B lipidation in autophagy-related processes. Nat. Cell Biol. 2019, 21, 372–383. [Google Scholar] [CrossRef]
- Mizushima, N.; Yamamoto, A.; Hatano, M.; Kobayashi, Y.; Kabeya, Y.; Suzuki, K.; Tokuhisa, T.; Ohsumi, Y.; Yoshimori, T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 2001, 152, 657–668. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic control of autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef]
- Sung, J.; Jeong, H.S.; Lee, J. Effect of the capsicoside G-rich fraction from pepper (Capsicum annuum L.) seeds on high-fat diet-induced obesity in mice. Phytother. Res. 2016, 30, 1848–1855. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A Tale of Two “Hits”? Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.-P.; O’neill, H.M.; Ford, R.J.; Palanivel, R.; O’brien, M. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Assifi, M.M.; Suchankova, G.; Constant, S.; Prentki, M.; Saha, A.K.; Ruderman, N.B. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E794–E800. [Google Scholar] [CrossRef]
- Munday, M. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 2002, 30, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D. Banting lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [CrossRef]
- Lamming, D.W.; Sabatini, D.M. A central role for mTOR in lipid homeostasis. Cell Metab. 2013, 18, 465–469. [Google Scholar] [CrossRef]
- Tuohetahuntila, M.; Molenaar, M.R.; Spee, B.; Brouwers, J.F.; Wubbolts, R.; Houweling, M.; Yan, C.; Du, H.; VanderVen, B.C.; Vaandrager, A.B. Lysosome-mediated degradation of a distinct pool of lipid droplets during hepatic stellate cell activation. J. Biol. Chem. 2017, 292, 12436–12448. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, Z.; Ji, G. Herbal extracts and natural products in alleviating non-alcoholic fatty liver disease via activating autophagy. Front. Pharmacol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Liao, L.-Z.; Chen, Y.-L.; Lu, L.-H.; Zhao, Y.-H.; Guo, H.-L.; Wu, W.-K. Polysaccharide from Fuzi likely protects against starvation-induced cytotoxicity in H9c2 cells by increasing autophagy through activation of the AMPK/mTOR pathway. Am. J. Chin. Med. 2013, 41, 353–367. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, Z.-Q.; Wang, B.; Jiang, H.-X.; Cheng, L.; Shen, L.-M. Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int. 2014, 14, 49. [Google Scholar] [CrossRef]
- Mei, Y.; Hu, H.; Deng, L.; Sun, X.; Tan, W. Therapeutic effects of isosteviol sodium on non-alcoholic fatty liver disease by regulating autophagy via Sirt1/AMPK pathway. Sci. Rep. 2022, 12, 12857. [Google Scholar] [CrossRef]
- Dunlop, E.; Tee, A. mTOR and autophagy: A dynamic relationship governed by nutrients and energy. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2014; pp. 121–129. [Google Scholar]
- Zeng, J.; Zhu, B.; Su, M. Autophagy is involved in acetylshikonin ameliorating non-alcoholic steatohepatitis through AMPK/mTOR pathway. Biochem. Biophys. Res. Commun. 2018, 503, 1645–1650. [Google Scholar] [CrossRef]
- Yan, L.-S.; Zhang, S.-F.; Luo, G.; Cheng, B.C.-Y.; Zhang, C.; Wang, Y.-W.; Qiu, X.-Y.; Zhou, X.-H.; Wang, Q.-G.; Song, X.-L. Schisandrin B mitigates hepatic steatosis and promotes fatty acid oxidation by inducing autophagy through AMPK/mTOR signaling pathway. Metabolism 2022, 131, 155200. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, J.; Guo, Y.; Yu, Y.; Wu, X.; Xiao, H. Ursodeoxycholic acid alleviates nonalcoholic fatty liver disease by inhibiting apoptosis and improving autophagy via activating AMPK. Biochem. Biophys. Res. Commun. 2020, 529, 834–838. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, Y.; Fan, X. Inhibition of ghrelin o-acyltransferase attenuated lipotoxicity by inducing autophagy via AMPK–mTOR pathway. Drug Des. Dev. Ther. 2018, 12, 873. [Google Scholar] [CrossRef]
- Fujita, N.; Itoh, T.; Omori, H.; Fukuda, M.; Noda, T.; Yoshimori, T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 2008, 19, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Noda, N.N.; Satomi, Y.; Ichimura, Y.; Fujioka, Y.; Takao, T.; Inagaki, F.; Ohsumi, Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007, 282, 37298–37302. [Google Scholar] [CrossRef]
- Mizushima, N.; Noda, T.; Yoshimori, T.; Tanaka, Y.; Ishii, T.; George, M.D.; Klionsky, D.J.; Ohsumi, M.; Ohsumi, Y. A protein conjugation system essential for autophagy. Nature 1998, 395, 395–398. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Kim, H.-J.; You, M.; Kim, H.-A. Red Pepper Seeds Inhibit Hepatic Lipid Accumulation by Inducing Autophagy via AMPK Activation. Nutrients 2022, 14, 4247. https://doi.org/10.3390/nu14204247
Lee Y-H, Kim H-J, You M, Kim H-A. Red Pepper Seeds Inhibit Hepatic Lipid Accumulation by Inducing Autophagy via AMPK Activation. Nutrients. 2022; 14(20):4247. https://doi.org/10.3390/nu14204247
Chicago/Turabian StyleLee, Young-Hyun, Hwa-Jin Kim, Mikyoung You, and Hyeon-A Kim. 2022. "Red Pepper Seeds Inhibit Hepatic Lipid Accumulation by Inducing Autophagy via AMPK Activation" Nutrients 14, no. 20: 4247. https://doi.org/10.3390/nu14204247
APA StyleLee, Y.-H., Kim, H.-J., You, M., & Kim, H.-A. (2022). Red Pepper Seeds Inhibit Hepatic Lipid Accumulation by Inducing Autophagy via AMPK Activation. Nutrients, 14(20), 4247. https://doi.org/10.3390/nu14204247






