Association between High-Fat Diet during Pregnancy and Heart Weight of the Offspring: A Multivariate and Mediation Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Extraction of the Placenta and Heart Tissue
2.3. Fetal Sex Determination
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
3.1. Procedure for the Construction of Animal Models and General Condition of Offspring
3.2. Analysis of Factors Influencing the Effect of a High-Fat Diet on the Heart Weight of Fetal Mice
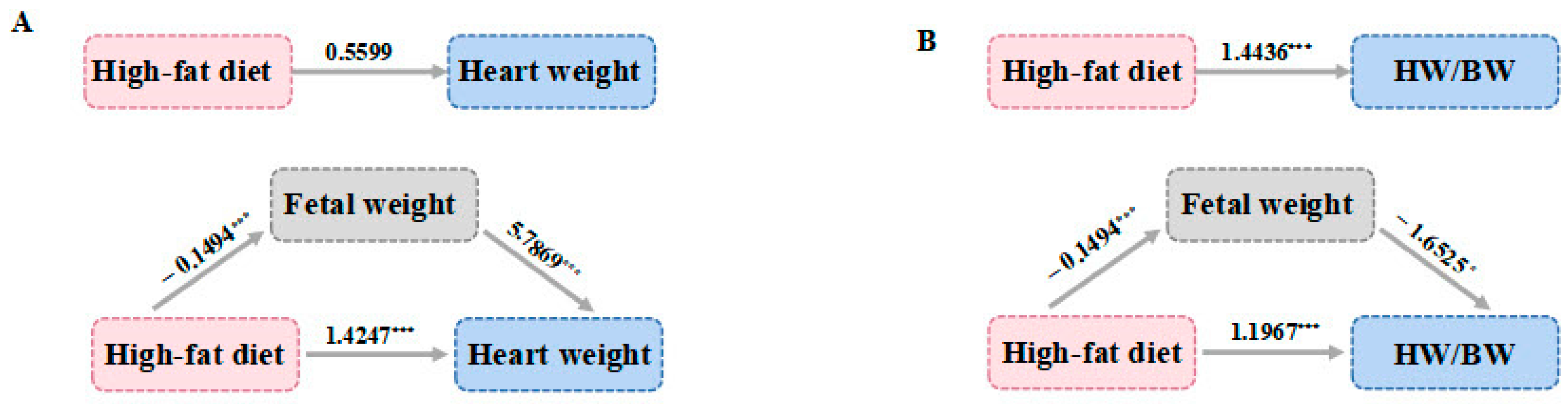
3.3. Analysis of the Mediating Effect of a High-Fat Diet during Pregnancy on the Heart Weight of the Offspring
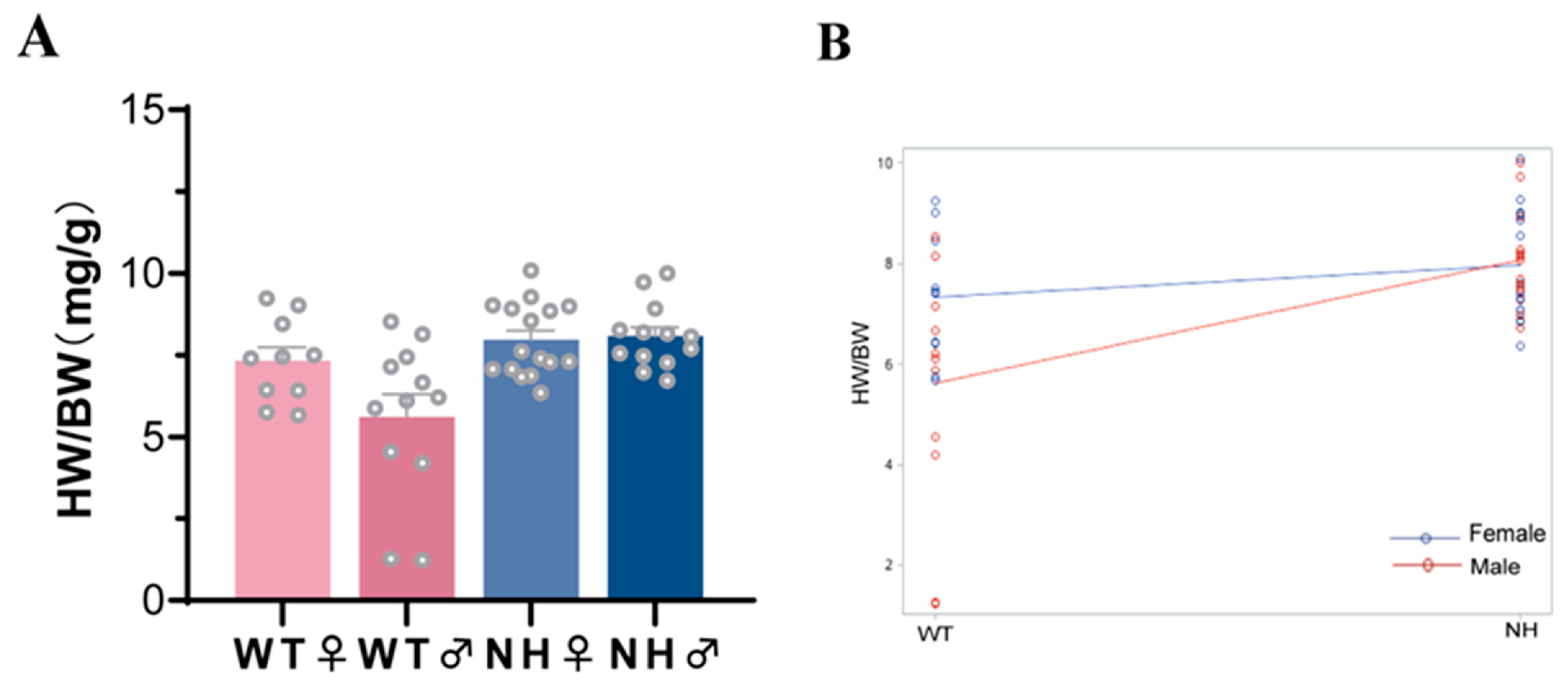
3.4. Subgroup Analysis of the Effect of Sex on the Effect of a High-Fat Diet during Pregnancy on HW/BW in the Offspring
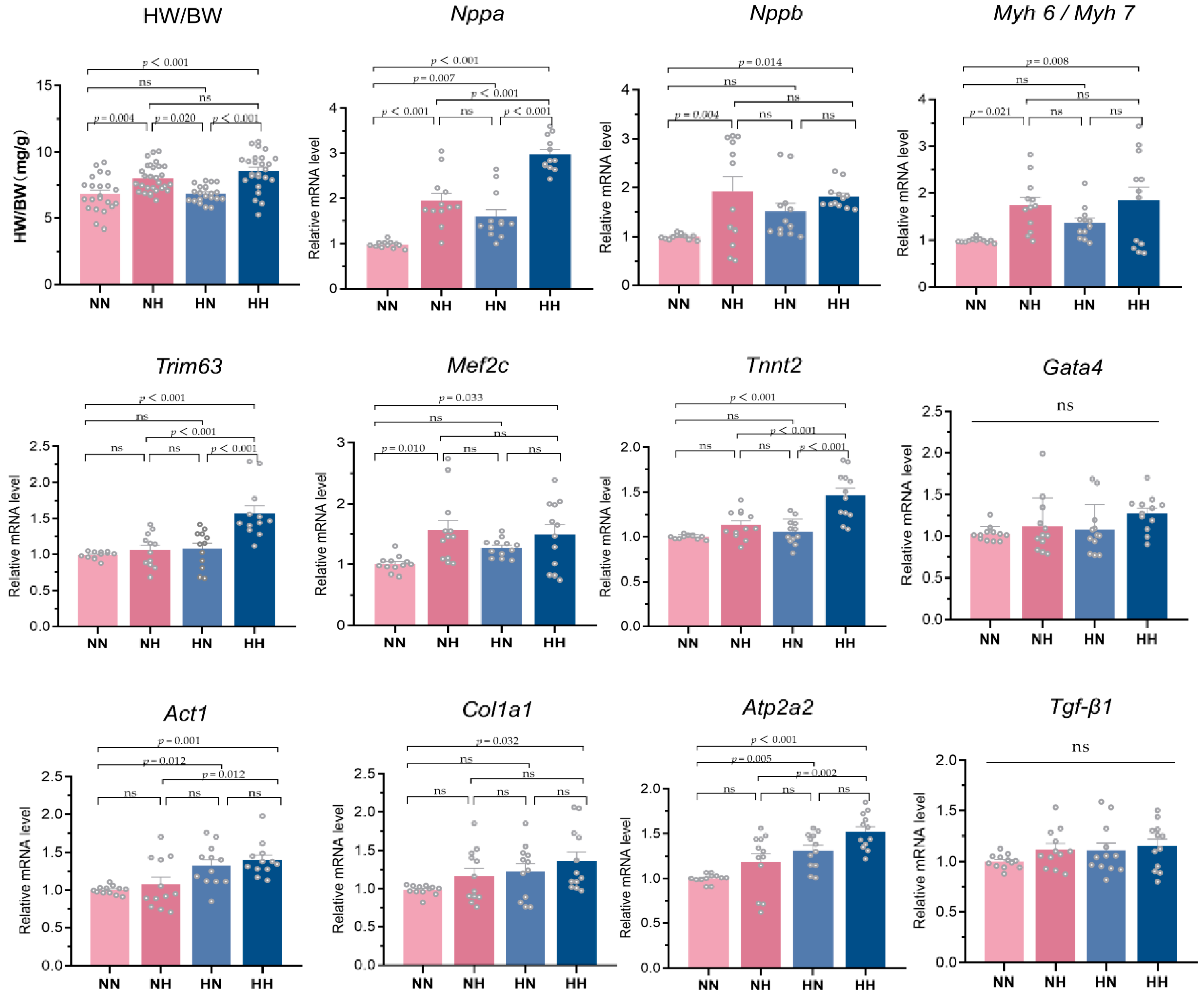
3.5. Effect of a High-Fat Diet on the Expression of Key Indicators of the Fetal Heart in the Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, D.J. Fetal origins of coronary heart disease. BMJ (Clin. Res. ed.) 1995, 311, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.C.; Lawlor, D.A.; Richmond, R.C.; Fraser, A.; Simpkin, A.; Suderman, M.; Shihab, H.A.; Lyttleton, O.; McArdle, W.; Ring, S.M.; et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: Findings from the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2015, 44, 1288–1304. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.L.; Moura, E.G.; Lisboa, P.C. Litter size reduction as a model of overfeeding during lactation and its consequences for the development of metabolic diseases in the offspring. Nutrients 2022, 14, 2045. [Google Scholar] [CrossRef]
- Zhao, L.; Law, N.C.; Gomez, N.A.; Son, J.; Gao, Y.; Liu, X.; de Avila, J.M.; Zhu, M.J.; Du, M. Obesity impairs embryonic myogenesis by enhancing BMP signaling within the Dermomyotome. Adv. Sci. 2021, 8, e2102157. [Google Scholar] [CrossRef]
- Chen, F.; Cao, K.; Zhang, H.; Yu, H.; Liu, Y.; Xue, Q. Maternal high-fat diet increases vascular contractility in adult offspring in a sex-dependent manner. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2021, 44, 36–46. [Google Scholar] [CrossRef]
- Gillman, M.W. Interrupting intergenerational cycles of maternal obesity. Nestlé Nutr. Inst. Workshop Ser. 2016, 85, 59–69. [Google Scholar]
- Persson, M.; Razaz, N.; Edstedt Bonamy, A.K.; Villamor, E.; Cnattingius, S. Maternal overweight and obesity and risk of congenital heart defects. J. Am. Coll. Cardiol. 2019, 73, 44–53. [Google Scholar] [CrossRef]
- Razaz, N.; Villamor, E.; Muraca, G.M.; Bonamy, A.E.; Cnattingius, S. Maternal obesity and risk of cardiovascular diseases in offspring: A population-based cohort and sibling-controlled study. Lancet. Diabetes Endocrinol. 2020, 8, 572–581. [Google Scholar] [CrossRef]
- Kandadi, M.R.; Hua, Y.; Zhu, M.; Turdi, S.; Nathanielsz, P.W.; Ford, S.P.; Nair, S.; Ren, J. Influence of gestational overfeeding on myocardial proinflammatory mediators in fetal sheep heart. J. Nutr. Biochem. 2013, 24, 1982–1990. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Blackmore, H.L.; Siggens, L.; Giussani, D.A.; Cross, C.M.; Foo, R.; Ozanne, S.E. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology 2012, 153, 5961–5971. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, X.; Zhao, J.X.; Zhu, M.J.; McCormick, R.J.; Ford, S.P.; Nathanielsz, P.W.; Ren, J.; Du, M. Maternal obesity induces fibrosis in fetal myocardium of sheep. Am. J. Physiology. Endocrinol. Metab. 2010, 299, E968–E975. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Kanai, Y.; Powell, T.L.; Jansson, T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity (Silver Spring Md.) 2015, 23, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.; Rosario, F.J.; Powell, T.L.; Jansson, T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc. Natl. Acad. Sci. USA 2015, 112, 12858–12863. [Google Scholar] [CrossRef] [PubMed]
- Frihauf, J.B.; Fekete, É.M.; Nagy, T.R.; Levin, B.E.; Zorrilla, E.P. Maternal Western diet increases adiposity even in male offspring of obesity-resistant rat dams: Early endocrine risk markers. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2016, 311, R1045–R1059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribaroff, G.A.; Wastnedge, E.; Drake, A.J.; Sharpe, R.M.; Chambers, T. Animal models of maternal high fat diet exposure and effects on metabolism in offspring: A meta-regression analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Pankey, C.L.; Walton, M.W.; Odhiambo, J.F.; Smith, A.M.; Ghnenis, A.B.; Nathanielsz, P.W.; Ford, S.P. Intergenerational impact of maternal overnutrition and obesity throughout pregnancy in sheep on metabolic syndrome in grandsons and granddaughters. Domest. Anim. Endocrinol. 2017, 60, 67–74. [Google Scholar] [CrossRef]
- Garaulet, M.; Ortega, F.B.; Ruiz, J.R.; Rey-López, J.P.; Béghin, L.; Manios, Y.; Cuenca-García, M.; Plada, M.; Diethelm, K.; Kafatos, A.; et al. Short sleep duration is associated with increased obesity markers in European adolescents: Effect of physical activity and dietary habits. The HELENA study. Int. J. Obes. 2011, 35, 1308–1317. [Google Scholar] [CrossRef]
- Wolff, C.B.; Wolff, H.K. Maternal eating patterns and birth weight of Mexican American infants. Nutr. Health 1995, 10, 121–134. [Google Scholar] [CrossRef]
- Kaijser, M.; Bonamy, A.K.; Akre, O.; Cnattingius, S.; Granath, F.; Norman, M.; Ekbom, A. Perinatal risk factors for ischemic heart disease: Disentangling the roles of birth weight and preterm birth. Circulation 2008, 117, 405–410. [Google Scholar] [CrossRef]
- Mdaki, K.S.; Larsen, T.D.; Wachal, A.L.; Schimelpfenig, M.D.; Weaver, L.J.; Dooyema, S.D.; Louwagie, E.J.; Baack, M.L. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. Am. J. Physiology. Heart Circ. Physiol. 2016, 310, H681–H692. [Google Scholar] [CrossRef]
- Thornburg, K.L.; O’tierney, P.F.; Louey, S. Review: The placenta is a programming agent for cardiovascular disease. Placenta 2010, 31 (Suppl. S54–S59), S54–S59). [Google Scholar] [CrossRef]
- Forsén, T.; Eriksson, J.G.; Tuomilehto, J.; Teramo, K.; Osmond, C.; Barker, D.J. Mothers weight in pregnancy and coronary heart disease in a cohort of Finnish men: Follow up study. BMJ (Clin. Res. Ed.) 1997, 315, 837–840. [Google Scholar] [CrossRef]
- Kulhanek, D.; Weigel, R.; Paulsen, M.E. Maternal high-fat-high-carbohydrate diet-induced obesity is associated with increased appetite in peripubertal male but not female C57Bl/6J mice. Nutrients 2020, 12, 2919. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Huo, Y.; Zheng, Y.; Gui, Y. Effects of the leptin-mediated MAPK/ERK signaling pathway on collagen II expression in knee cartilage of newborn male mice from obese maternal offspring. Biomolecules 2022, 12, 477. [Google Scholar] [CrossRef]
- Rey, S.; Luo, W.; Shimoda, L.A.; Semenza, G.L. Metabolic reprogramming by HIF-1 promotes the survival of bone marrow-derived angiogenic cells in ischemic tissue. Blood 2011, 117, 4988–4998. [Google Scholar] [CrossRef] [PubMed]
- Helle, E.; Priest, J.R. Maternal obesity and diabetes mellitus as risk factors for congenital heart disease in the offspring. J. Am. Heart Assoc. 2020, 9, e011541. [Google Scholar] [CrossRef]
- Leddy, M.A.; Power, M.L.; Schulkin, J. The impact of maternal obesity on maternal and fetal health. Rev. Obstet. Gynecol. 2008, 1, 170–178. [Google Scholar] [PubMed]
- Gaillard, R.; Steegers, E.A.; Franco, O.H.; Hofman, A.; Jaddoe, V.W. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int. J. Obes. 2015, 39, 677–685. [Google Scholar] [CrossRef]
- Fraser, A.; Tilling, K.; Macdonald-Wallis, C.; Sattar, N.; Brion, M.J.; Benfield, L.; Ness, A.; Deanfield, J.; Hingorani, A.; Nelson, S.M.; et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 2010, 121, 2557–2564. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Q.; Tsai, H.J.; Wang, G.; Hong, X.; Zhou, Y.; Zhang, C.; Liu, C.; Liu, R.; Wang, H.; et al. Maternal preconception body mass index and offspring cord blood DNA methylation: Exploration of early life origins of disease. Environ. Mol. Mutagen. 2014, 55, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, K.; Mishra, J.S.; Ross, J.R.; Abbott, D.H.; Kumar, S. Hyperandrogenism diminishes maternal-fetal fatty acid transport by increasing FABP 4-mediated placental lipid accumulation. Biol. Reprod. 2022, 107, 514–528. [Google Scholar] [CrossRef]
- Alves, M.L.; Warren, C.M.; Simon, J.N.; Gaffin, R.D.; Montminy, E.M.; Wieczorek, D.F.; Solaro, R.J.; Wolska, B.M. Early sensitization of myofilaments to Ca2+ prevents genetically linked dilated cardiomyopathy in mice. Cardiovasc. Res. 2017, 113, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Steuer, J.; Gillgren, P.; Hayderi, A.; Liu, A.; Frostegård, J. Induction of dendritic cell-mediated activation of T cells from atherosclerotic plaques by human heat shock protein 60. J. Am. Heart Assoc. 2017, 6, e006778. [Google Scholar] [CrossRef]
- Hennig, M.; Fiedler, S.; Jux, C.; Thierfelder, L.; Drenckhahn, J.D. Prenatal mechanistic target of rapamycin complex 1 (m TORC1) inhibition by rapamycin treatment of pregnant mice causes intrauterine growth restriction and alters postnatal cardiac growth, morphology, and function. J. Am. Heart Assoc. 2017, 6, e005506. [Google Scholar] [CrossRef] [PubMed]
- Olander, R.; Sundholm, J.; Ojala, T.H.; Andersson, S.; Sarkola, T. Differences in cardiac geometry in relation to body size among neonates with abnormal prenatal growth and body size at birth. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2020, 56, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, K.; Osmond, C.; Fall, C.H.D. Early Origins of Cardiometabolic Disease. In Cardiovascular, Respiratory, and Related Disorders, 3rd ed.; Prabhakaran, D., Anand, S., Gaziano, T.A., Mbanya, J.C., Wu, Y., Nugent, R., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Bolam, S.M.; Satokar, V.V.; Konar, S.; Coleman, B.; Monk, A.P.; Cornish, J.; Munro, J.T.; Vickers, M.H.; Albert, B.B.; Musson, D.S. A Maternal high fat diet leads to sex-specific programming of mechanical properties in supraspinatus tendons of adult rat offspring. Front. Nutr. 2021, 8, 729427. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, L.; Tan, Y.; Zheng, Y.; Gui, Y. N-acetylcysteine protects neonatal mice from ventricular hypertrophy induced by maternal obesity in a sex-specific manner. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 133, 110989. [Google Scholar] [CrossRef]
- Colafella, K.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Reviews. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Reviews. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Omland, T.; Wolf, P.A.; Vasan, R.S. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N. Engl. J. Med. 2004, 350, 655–663. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Ma, G.; Liu, J.F.; Cai, Y.Y.; Zhang, J.Y.; Wei, T.T.; Pan, A.; Jiang, S.; Xiao, Y.; Xiao, P.; et al. Neuraminidase 1 is a driver of experimental cardiac hypertrophy. Eur. Heart J. 2021, 42, 3770–3782. [Google Scholar] [CrossRef]
- Lin, Q.; Schwarz, J.; Bucana, C.; Olson, E.N. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 1997, 276, 1404–1407. [Google Scholar] [CrossRef]
- Gordon, J.W.; Pagiatakis, C.; Salma, J.; Du, M.; Andreucci, J.J.; Zhao, J.; Hou, G.; Perry, R.L.; Dan, Q.; Courtman, D.; et al. Protein kinase A-regulated assembly of a MEF2·repressor complex controls c-Jun expression in vascular smooth muscle cells. J. Biol. Chem. 2009, 284, 19027–19042. [Google Scholar] [CrossRef]
- Karamboulas, C.; Dakubo, G.D.; Liu, J.; De Repentigny, Y.; Yutzey, K.; Wallace, V.A.; Kothary, R.; Skerjanc, I.S. Disruption of MEF2 activity in cardiomyoblasts inhibits cardiomyogenesis. J. Cell Sci. 2006, 119 Pt 20, 4315–4321. [Google Scholar] [CrossRef]
- Gupta, M.P. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2007, 43, 388–403. [Google Scholar] [CrossRef]
- Iwaki, H.; Sasaki, S.; Matsushita, A.; Ohba, K.; Matsunaga, H.; Misawa, H.; Oki, Y.; Ishizuka, K.; Nakamura, H.; Suda, T. Essential role of TEA domain transcription factors in the negative regulation of the MYH 7 gene by thyroid hormone and its receptors. PLoS ONE 2014, 9, e88610. [Google Scholar] [CrossRef]
- Nie, J.; Ngokana, L.D.; Kou, J.; Zhao, Y.; Tu, J.; Ji, H.; Tan, P.; Zhao, T.; Cao, Y.; Wu, Z.; et al. Low-dose ethanol intake prevents high-fat diet-induced adverse cardiovascular events in mice. Food Funct. 2020, 11, 3549–3562. [Google Scholar] [CrossRef]
- Niwano, K.; Arai, M.; Koitabashi, N.; Watanabe, A.; Ikeda, Y.; Miyoshi, H.; Kurabayashi, M. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 1026–1032. [Google Scholar] [CrossRef]
- Kim, M.S.; Fleres, B.; Lovett, J.; Anfinson, M.; Samudrala, S.; Kelly, L.J.; Teigen, L.E.; Cavanaugh, M.; Marquez, M.; Geurts, A.M.; et al. Contractility of induced pluripotent stem cell-cardiomyocytes with an MYH6 head domain variant associated with hypoplastic left heart syndrome. Front. Cell Dev. Biol. 2020, 8, 440. [Google Scholar] [CrossRef] [PubMed]

| Group | ||||||
|---|---|---|---|---|---|---|
| NN (n = 22) | NH (n = 29) | HN (n = 21) | HH (n = 25) | χ2/F | p Value | |
| Gender Female Male | ||||||
| 10 (45.45%) | 16 (55.17%) | 9 (42.86%) | 11 (44.00%) | 1.03 | 0.7950 | |
| 12 (54.55%) | 13 (44.83%) | 12 (57.14%) | 14 (56.00%) | |||
| Body Weight (g) | 1.31 ± 0.09 | 1.12 ± 0.13 * | 1.08 ± 0.10 * | 0.96 ± 0.39 * | 40.77 | <0.0001 |
| Heart weight (mg) | 9.00 ± 1.00 | 9.00 ± 2.00 | 7.00 ± 1.00 *# | 8.00 ± 1.00 | 16.89 | 0.0007 |
| HW/BW (mg/g) | 6.83 ± 1.35 | 8.02 ± 1.04 *△ | 6.85 ± 0.63 | 8.58 ± 1.43 *△ | 13.42 | <0.0001 |
| Placenta weight (mg) | 93.50 ± 21.00 | 89.10 ± 9.79 | 101.00 ± 12.00 # | 89.28 ± 14.49 △ | 17.68 | 0.0005 |
| PW/BW (mg/g) | 73.66 ± 13.01 | 80.53 ± 6.75 | 97.68 ± 15.92 *# | 97.01 ± 25.57 *# | 26.68 | <0.0001 |
| Heart Weight (M ± Q) | χ2 | p-Value | HW/BW | t/F Value | p-Value | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 8.00 ± 3.00 | 0.01 | 0.9795 | 7.54 ± 1.46 | 0.79 | 0.4296 |
| Male | 8.00 ± 2.00 | 7.76 ± 1.26 | ||||
| High-Fat During Pregnancy | ||||||
| Yes | 8.00 ± 2.00 | 5.01 | 0.0251 | 8.28 ± 1.25 | −6.05 | <0.0001 |
| No | 8.00 ± 2.00 | 6.84 ± 1.05 | ||||
| Obesity | ||||||
| Yes | 8.00 ± 1.00 | 10.61 | 0.0011 | 7.79 ± 1.42 | −1.03 | 0.3054 |
| No | 9.00 ± 2.00 | 7.50 ± 1.31 | ||||
| High-fat diet time | ||||||
| None | 9.00 ± 1.00 | 16.88 | 0.0007 | 6.83 ± 1.35 | ||
| Short | 9.00 ± 2.00 | 8.02 ± 1.04 | 13.42 | <0.0001 | ||
| Medium | 7.00 ± 1.00 | 6.85 ± 0.63 | ||||
| Long | 8.00 ± 1.00 | 8.58 ± 1.43 | ||||
| Placenta weight | ||||||
| Light | 8.00 ± 2.00 | 0.51 | 0.4732 | 7.71 ± 1.41 | 0.52 | 0.6063 |
| Heavy | 8.00 ± 3.00 | 7.57 ± 1.34 | ||||
| PW/BW | ||||||
| Light | 9.00 ± 2.00 | 15.11 | 0.0001 | 7.49 ± 1.29 | −1.08 | 0.2844 |
| Heavy | 8.00 ± 1.00 | 7.79 ± 1.44 |
| Univariate Linear Regression Analysis | ||||
|---|---|---|---|---|
| β | S.E | t Value | p-Value | |
| Model 1 | ||||
| Obesity | 0.2863 | 0.2778 | 1.03 | 0.3054 |
| Model 2 | ||||
| High-fat diet time | 0.4094 | 0.1192 | 3.44 | 0.0009 |
| Model 3 | ||||
| High fat during pregnancy | 1.4436 | 0.2386 | 6.05 | <0.0001 |
| Model 4 | ||||
| Placenta weight | −0.0177 | 0.0090 | −1.96 | 0.0525 |
| Model 5 | ||||
| Fetal weight | −2.9170 | 0.6841 | −4.26 | <0.0001 |
| Stepwise linear regression model | ||||
| Model Total | β | S.E | F | p value |
| Fetal weight | −1.6525 | 0.6738 | 6.02 | 0.0160 |
| High fat during pregnancy | 1.1967 | 0.2534 | 22.30 | <0.0001 |
| Model 1 X (High-Fat Diet) M (Fetal Weight) Y(Heart Weight) | Model 2 X(High-Fat Diet) M (Fetal Weight) Y(HW/BW) | |||
|---|---|---|---|---|
| Effect Coefficient | 95% CI | Effect Coefficient | 95% CI | |
| Indirect effect | −0.8648 | (−1.3583, −0.4318) | 0.2469 | (0.0155, 0.5125) |
| Direct effect | 1.4247 | (0.8560, 1.9933) | 1.1967 | (0.6936, 1.6997) |
| Total effect | 0.5599 | (−0.0995, 1.2193) | 1.4436 | (0.9700, 1.9172) |
| Proportion of suppression/mediated effect | 60.70% | 17.10% | ||
| Factor | Mean Square | F Value | p Value |
|---|---|---|---|
| NH | 29.88 | 12.97 | 0.0008 |
| Gender | 8.02 | 3.48 | 0.0683 |
| NH*Gender | 10.36 | 4.49 | 0.0393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Huo, Y.; Zhang, J.; Xu, D.; Bai, F.; Gui, Y. Association between High-Fat Diet during Pregnancy and Heart Weight of the Offspring: A Multivariate and Mediation Analysis. Nutrients 2022, 14, 4237. https://doi.org/10.3390/nu14204237
Wang W, Huo Y, Zhang J, Xu D, Bai F, Gui Y. Association between High-Fat Diet during Pregnancy and Heart Weight of the Offspring: A Multivariate and Mediation Analysis. Nutrients. 2022; 14(20):4237. https://doi.org/10.3390/nu14204237
Chicago/Turabian StyleWang, Wenji, Yu Huo, Jialing Zhang, Da Xu, Fan Bai, and Yonghao Gui. 2022. "Association between High-Fat Diet during Pregnancy and Heart Weight of the Offspring: A Multivariate and Mediation Analysis" Nutrients 14, no. 20: 4237. https://doi.org/10.3390/nu14204237
APA StyleWang, W., Huo, Y., Zhang, J., Xu, D., Bai, F., & Gui, Y. (2022). Association between High-Fat Diet during Pregnancy and Heart Weight of the Offspring: A Multivariate and Mediation Analysis. Nutrients, 14(20), 4237. https://doi.org/10.3390/nu14204237






