Preoperative High Visceral Fat Increases Severe Complications but Improves Long-Term Prognosis after Gastrectomy for Patients with Advanced Gastric Cancer: A Propensity Score Matching Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Postoperative Chemotherapy
2.3. Body Composition Analysis
2.4. Outcomes
2.5. Statistical Analyses
3. Results
3.1. Patient Background
3.2. Comparison of Postoperative Outcomes after Matching
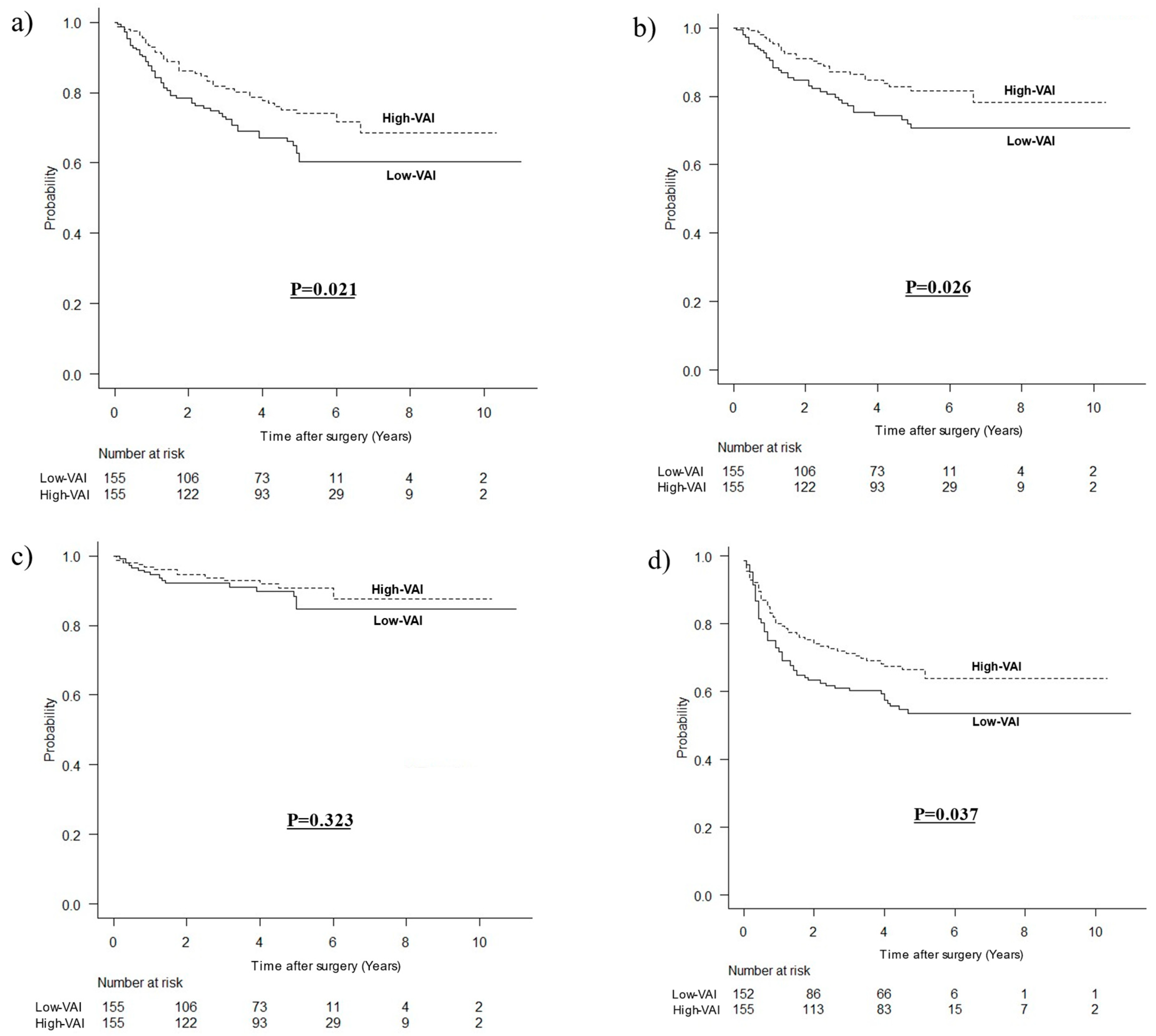
3.3. Long-Term Outcomes According to VAI after Matching
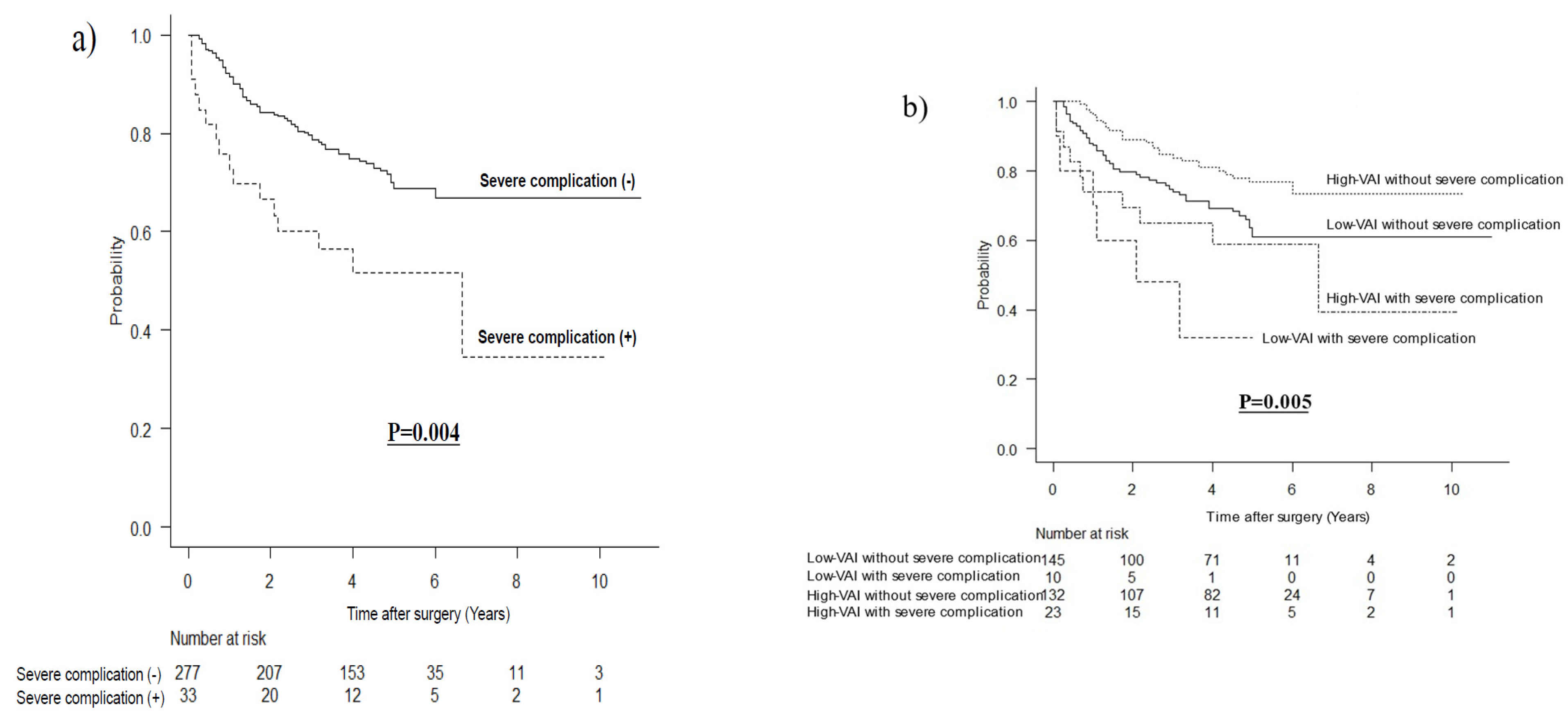
3.4. Long-Term Outcomes According to Severe Complications after Matching
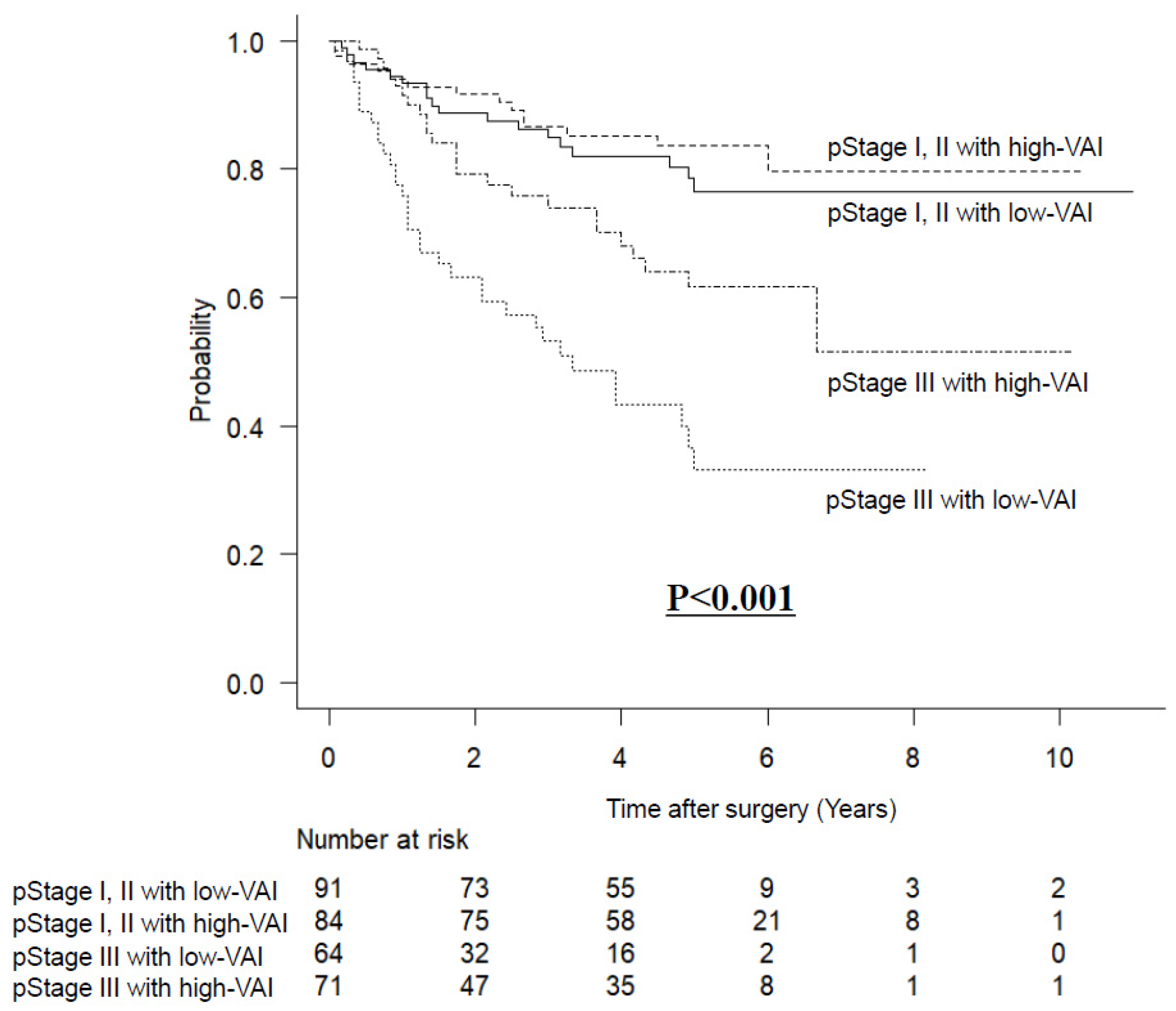
3.5. Long-Term Outcomes Stratified by VAI and pStage after Matching
3.6. Prognostic Factors for OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, J.P.; Wang, X.L.; Tian, H.; Gao, T.T.; Tang, L.M.; Tian, F.; Wang, J.W.; Zheng, H.J.; Zhang, L.; et al. Computed tomography-quantified body composition predicts short-term outcomes after gastrectomy in gastric cancer. Curr. Oncol. 2018, 25, e411–e422. [Google Scholar] [CrossRef]
- Yang, S.-J.; Li, H.-R.; Zhang, W.-H.; Liu, K.; Zhang, D.-Y.; Sun, L.-F.; Chen, X.-L.; Zhao, L.-Y.; Chen, X.-Z.; Yang, K.; et al. Visceral fat area (VFA) superior to BMI for predicting postoperative complications after radical gastrectomy: A prospective cohort study. J. Gastrointest. Surg. 2020, 24, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, N.; Tokunaga, M.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Intra-abdominal infectious complications following gastrectomy in patients with excessive visceral fat. Gastric Cancer 2012, 15, 206–212. [Google Scholar] [CrossRef]
- Sato, Y.; Inokuchi, M.; Otsuki, S.; Fujimori, Y.; Kojima, K. Risk factor of pancreatic fistula after radical gastrectomy from the viewpoint of fatty pancreas. Dig. Surg. 2017, 34, 455–461. [Google Scholar] [CrossRef]
- Tokunaga, M.; Hiki, N.; Fukunaga, T.; Ogura, T.; Miyata, S.; Yamaguchi, T. Effect of individual fat areas on early surgical outcomes after open gastrectomy for gastric cancer. Br. J. Surg. 2009, 96, 496–500. [Google Scholar] [CrossRef]
- Kunisaki, C.; Makino, H.; Takagawa, R.; Sato, K.; Kawamata, M.; Kanazawa, A.; Yamamoto, N.; Nagano, Y.; Fujii, S.; Ono, H.A.; et al. Predictive factors for surgical complications of laparoscopy-assisted distal gastrectomy for gastric cancer. Surg. Endosc. 2009, 23, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, A.; Imamura, K.; Kobayashi, R.; Takami, M.; Matsumoto, J. Impact of visceral fat on laparoscopy-assisted distal gastrectomy. Surgeon 2013, 11, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Shimada, M.; Kurita, N.; Iwata, T.; Nishioka, M.; Morimoto, S.; Miyatani, T.; Komatsu, M.; Mikami, C.; Kashihara, H. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy-assisted gastrectomy for gastric cancer. Surg. Endosc. 2011, 25, 3825–3830. [Google Scholar] [CrossRef]
- Takeuchi, M.; Ishii, K.; Seki, H.; Yasui, N.; Sakata, M.; Shimada, A.; Matsumoto, H. Excessive visceral fat area as a risk factor for early postoperative complications of total gastrectomy for gastric cancer: A retrospective cohort study. BMC Surg. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Wang, S.-L.; Ma, L.-L.; Chen, X.-Y.; Zhou, N.-L.; Li, B.; Huang, D.-D.; Yu, Z.; Shen, X.; Zhuang, C.-L. Impact of visceral fat on surgical complications and long-term survival of patients with gastric cancer after radical gastrectomy. Eur. J. Clin. Nutr. 2018, 72, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Son, S.Y.; Cui, L.H.; Byun, C.; Hur, H.; Lee, J.H.; Kim, Y.C.; Han, S.U.; Cho, Y.K. Is there any role of visceral fat area for predicting difficulty of laparoscopic gastrectomy for gastric cancer? J. Gastric Cancer 2015, 15, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Ichimiya, H.; Okido, M.; Kato, M. The impact of visceral fat accumulation on laparoscopy-assisted distal gastrectomy for early gastric cancer. J. Laparoendosc. Adv. Surg. Tech. 2009, 19, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Yoshikawa, T.; Oba, M.S.; Hirabayashi, N.; Shirai, J.; Aoyama, T.; Hayashi, T.; Yamada, T.; Oba, K.; Morita, S.; et al. Matched pair analysis to examine the effects of a planned preoperative exercise program in early gastric cancer patients with metabolic syndrome to reduce operative risk: The Adjuvant Exercise for General Elective Surgery (AEGES) study group. Ann. Surg. Oncol. 2014, 21, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, L.; Wang, Q.; Li, J.; Bai, B.; Li, Z.; Wu, X.; Yu, P.; Li, X.; Yin, J. Postoperative complications and prognosis after radical gastrectomy for gastric cancer: A systematic review and meta-analysis of observational studies. World J. Surg. Oncol. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Jiang, N.; Deng, J.Y.; Ding, X.W.; Zhang, L.; Liu, H.G.; Liang, Y.X.; Liang, H. Effect of complication grade on survival following curative gastrectomy for carcinoma. World J. Gastroenterol. 2014, 20, 8244–8252. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Peterson, L.L. Obesity and cancer: Evidence, impact, and future directions. Clin. Chem. 2018, 64, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The obesity paradox in cancer: A Review. Curr. Oncol. Rep. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Huang, J.; Fan, J.; Du, H.; Liu, L.; Che, G. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: Does the ‘obesity paradox’ really exist? Eur. J. Cardiothorac. Surg. 2017, 51, 817–828. [Google Scholar] [CrossRef]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J.; Weltzien, E.; Feliciano, E.C.; Castillo, A.L.; Quesenberry, C.P.; Kwan, M.L.; et al. Explaining the obesity paradox: The association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol. Biomark. Prev. 2017, 26, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Jiralerspong, S.; Goodwin, P.J. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. J. Clin. Oncol. 2016, 34, 4203–4216. [Google Scholar] [CrossRef]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Hatano, E.; Okajima, H.; et al. Visceral adiposity and sarcopenic visceral obesity are associated with poor prognosis after resection of pancreatic cancer. Ann. Surg. Oncol. 2017, 24, 3732–3740. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Bickenbach, K.A.; Denton, B.; Gonen, M.; Brennan, M.F.; Coit, D.G.; Strong, V.E. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann. Surg. Oncol. 2013, 20, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sui, W. Influence of obesity on short- and long-term outcomes after laparoscopic distal gastrectomy for gastric cancer. J. BUON 2017, 22, 417–423. [Google Scholar] [PubMed]
- Struecker, B.; Biebl, M.; Dadras, M.; Chopra, S.; Denecke, C.; Spenke, J.; Heilmann, A.-C.; Bahra, M.; Sauer, I.; Pratschke, J.; et al. The impact of obesity on outcomes following resection for gastric cancer. Dig. Surg. 2017, 34, 133–141. [Google Scholar] [CrossRef]
- Kambara, Y.; Yuasa, N.; Takeuchi, E.; Miyake, H.; Nagai, H.; Yoshioka, Y.; Okuno, M.; Miyata, K. Overweight or obesity is an unfavorable long-term prognostic factor for patients who underwent gastrectomy for stage II/III gastric cancer. World J. Surg. 2019, 43, 1766–1776. [Google Scholar] [CrossRef]
- Shimada, S.; Sawada, N.; Ishiyama, Y.; Nakahara, K.; Maeda, C.; Mukai, S.; Hidaka, E.; Ishida, F.; Kudo, S.-E. Impact of obesity on short- and long-term outcomes of laparoscopy assisted distal gastrectomy for gastric cancer. Surg. Endosc. 2018, 32, 358–366. [Google Scholar] [CrossRef]
- Harada, K.; Baba, Y.; Ishimoto, T.; Kosumi, K.; Tokunaga, R.; Izumi, D.; Ida, S.; Imamura, Y.; Iwagami, S.; Miyamoto, Y.; et al. Low visceral fat content is associated with poor prognosis in a database of 507 upper gastrointestinal cancers. Ann. Surg. Oncol. 2015, 22, 3946–3953. [Google Scholar] [CrossRef]
- Abdiev, S.; Kodera, Y.; Fujiwara, M.; Koike, M.; Nakayama, G.; Ohashi, N.; Tanaka, C.; Sakamoto, J.; Nakao, A. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer 2011, 14, 144–149. [Google Scholar] [CrossRef]
- Kiyama, T.; Mizutani, T.; Okuda, T.; Fujita, I.; Tokunaga, A.; Tajiri, T.; Barbul, A. Postoperative changes in body composition after gastrectomy. J. Gastrointest. Surg. 2005, 9, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Yoshikawa, T.; Maezawa, Y.; Kano, K.; Hara, K.; Sato, T.; Hayashi, T.; Yamada, T.; Cho, H.; Ogata, T.; et al. A Comparison of the body composition changes between laparoscopy-assisted and open total gastrectomy for gastric cancer. Vivo 2018, 32, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Kawabe, T.; Hirohito, F.; Hayashi, T.; Yamada, T.; Tsuchida, K.; Sato, T.; Oshima, T.; Rino, Y.; Masuda, M.; et al. Body composition analysis within 1 month after gastrectomy for gastric cancer. Gastric Cancer 2016, 19, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.Y.; Kim, H.K.; Kim, J.A.; Choi, C.S.; Yun, E.J.; Chang, S.K.; Park, C.H.; Lee, Y.-J. Changes in the abdominal fat distribution after gastrectomy: Computed tomography assessment. ANZ J. Surg. 2007, 77, 121–125. [Google Scholar] [CrossRef]
- Aoyama, T.; Yoshikawa, T.; Shirai, J.; Hayashi, T.; Yamada, T.; Tsuchida, K.; Hasegawa, S.; Cho, H.; Yukawa, N.; Oshima, T.; et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann. Surg. Oncol. 2013, 20, 2000–2006. [Google Scholar] [CrossRef]
- Takayoshi, K.; Uchino, K.; Nakano, M.; Ikejiri, K.; Baba, E. Weight loss during initial chemotherapy predicts survival in patients with advanced gastric cancer. Nutr. Cancer 2017, 69, 408–415. [Google Scholar] [CrossRef]
- Aoyama, T.; Sato, T.; Maezawa, Y.; Kano, K.; Hayashi, T.; Yamada, T.; Yukawa, N.; Oshima, T.; Rino, Y.; Masuda, M.; et al. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int. J. Clin. Oncol. 2017, 22, 476–483. [Google Scholar] [CrossRef]
- Hynes, O.; Anandavadivelan, P.; Gossage, J.; Johar, A.M.; Lagergren, J.; Lagergren, P. The impact of pre- and post-operative weight loss and body mass index on prognosis in patients with oesophageal cancer. Eur. J. Surg. Oncol. 2017, 43, 1559–1565. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.S.; Beom, S.H.; Rha, S.Y.; Chung, H.C.; Kim, J.H.; Chun, Y.J.; Lee, S.W.; Choe, E.-A.; Heo, S.J.; et al. Marked loss of muscle, visceral fat, or subcutaneous fat after gastrectomy predicts poor survival in advanced gastric cancer: Single-center study from the CLASSIC trial. Ann. Surg. Oncol. 2018, 25, 3222–3230. [Google Scholar] [CrossRef]
- Seidell, J.C.; Bakker, C.J.; van der Kooy, K. Imaging techniques for measuring adipose-tissue distribution—A comparison between computed tomography and 1.5-T magnetic resonance. Am. J. Clin. Nutr. 1990, 51, 953–957. [Google Scholar] [CrossRef]
- Kobayashi, J.; Tadokoro, N.; Watanabe, M.; Shinomiya, M. A novel method of measuring intra-abdominal fat volume using helical computed tomography. Int. J. Obes. 2002, 26, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Inaki, N.; Tsuji, T. Impact of preoperative muscle quality on postoperative severe complications after radical gastrectomy for gastric cancer patients. Ann. Gastroenterol. Surg. 2021, 5, 510–518. [Google Scholar] [CrossRef] [PubMed]
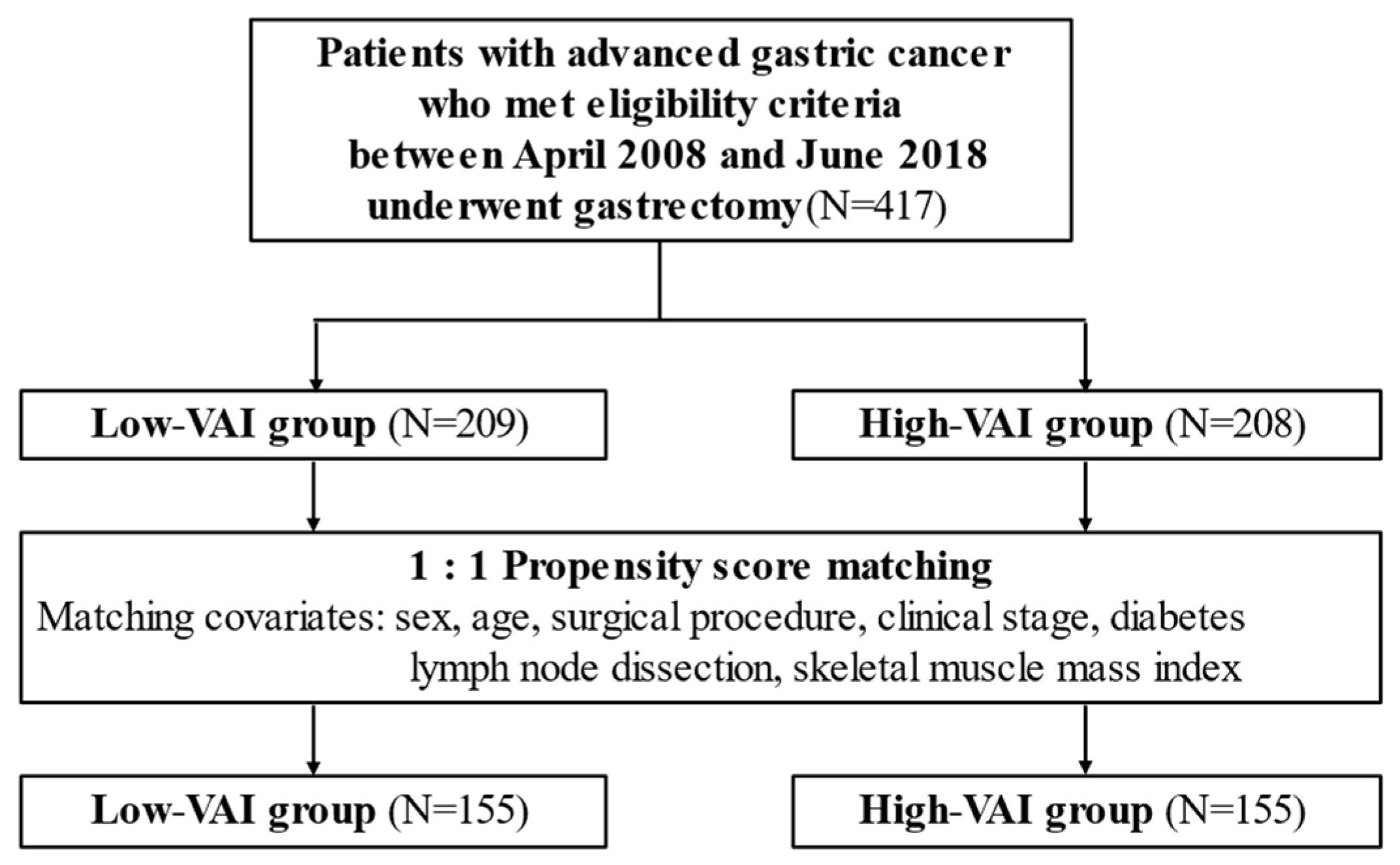
| All Patients | After Matching | |||||
|---|---|---|---|---|---|---|
| Low-VAI Group (N = 209) | High-VAI Group (N = 208) | p Value | Low-VAI Group (N = 155) | High-VAI Group (N = 155) | p Value | |
| Sex | ||||||
| Male Female | 140 (67.0%) 69 (33.0%) | 140 (67.3%) 68 (32.7%) | 1.000 | 104 (67.1%) 51 (32.9%) | 103 (66.5%) 52 (33.5%) | 1.000 |
| Age, mean ± SD | 67.48 ± 12.09 | 67.91 ± 9.73 | 0.689 | 67.99 ± 11.74 | 67.63 ± 9.91 | 0.766 |
| Body mass index, mean ± SD | 20.99 ± 2.55 | 25.06 ± 3.12 | <0.001 | 21.32 ± 2.50 | 24.62 ± 2.99 | <0.001 |
| Surgical approach | ||||||
| Laparoscopic surgery Open surgery | 110 (52.6%) 99 (47.6%) | 117 (56.2%) 91 (43.8%) | 0.492 | 87 (56.1%) 68 (43.9%) | 84 (54.2%) 71 (45.8%) | 0.819 |
| Surgical procedure | ||||||
| Distal gastrectomy Proximal gastrectomy Total gastrectomy | 112 (53.6%) 11 (5.3%) 86 (41.1%) | 122 (58.7%) 11 (5.3%) 75 (36.1%) | 0.551 | 85 (54.8%) 7 (4.5%) 63 (40.6%) | 87 (56.1%) 9 (5.8%) 59 (38.1%) | 0.812 |
| Lymph node dissection | ||||||
| D1+ D2 | 82 (39.2%) 127 (60.8%) | 101 (48.6%) 107 (51.4%) | 0.061 | 72 (46.5%) 83 (53.5%) | 63 (40.6%) 92 (59.4%) | 0.359 |
| Clinical stage | ||||||
| I II III | 32 (15.3%) 89 (42.6%) 88 (42.1%) | 50 (24.0%) 75 (36.1%) 83 (39.9%) | 0.069 | 29 (18.7%) 62 (40.0%) 64 (41.3%) | 32 (20.6%) 52 (33.5%) 71 (45.8%) | 0.513 |
| Comorbidity | ||||||
| CKD | 35 (16.7%) | 37 (17.8%) | 0.797 | 29 (18.7%) | 21 (13.5%) | 0.280 |
| COPD | 45 (21.5%) | 40 (19.2%) | 0.627 | 37 (23.9%) | 27 (17.4%) | 0.206 |
| Diabetes | 30 (14.4%) | 49 (23.6%) | 0.018 | 28 (18.1%) | 26 (16.8%) | 0.881 |
| CHF | 8 (3.8%) | 13 (6.2%) | 0.273 | 6 (3.9%) | 8 (5.2%) | 0.786 |
| SMI (cm2/m2), median (IQR) | 38.00 (32.06–43.13) | 41.52 (36.73–48.23) | <0.001 | 39.18 (34.20–44.21) | 39.16 (35.46–44.54) | 0.572 |
| Low-SMI | 127 (60.8%) | 81 (38.9%) | <0.001 | 80 (51.6%) | 77 (49.7%) | 0.820 |
| VAI (cm2/m2), median (IQR) | 18.18 (8.95–25.10) | 51.86 (42.18–66.22) | <0.001 | 19.69 (9.62–25.84) | 50.17 (40.98–60.74) | <0.001 |
| Low-VAI Group (N = 155) | High-VAI Group (N = 155) | p Value | |
|---|---|---|---|
| Pathological stage | |||
| I II III | 29 (18.7%) 62 (40.0%) 64 (41.3%) | 32 (20.6%) 52 (33.5%) 71 (45.8%) | 0.513 |
| Lymph node metastasis | |||
| Absent Present | 46 (29.7%) 109 (70.3%) | 41 (26.5%) 114 (73.5%) | 0.613 |
| Histological type | |||
| Differentiated Undifferentiated | 63 (40.6%) 92 (59.4%) | 75 (48.4%) 80 (51.6%) | 0.209 |
| Operating time (min), median (IQR) | 245.0 (207.5–302.5) | 245.0 (197.5–325.0) | 0.734 |
| Intraoperative blood loss (g), median (IQR) | 30.0 (10.0–145.0) | 40.0 (17.5–165.0) | 0.286 |
| Postoperative complication | |||
| Total number of postoperative complications | 28 (18.1%) | 41 (26.5%) | 0.101 |
| Severe complications | 10 (6.5%) | 23 (14.8%) | 0.026 |
| Infectious complications | 17 (11.0%) | 29 (18.7%) | 0.078 |
| Abdominal abscess | 12 (7.7%) | 23 (14.8%) | 0.072 |
| Incisional surgical site infection | 3 (1.9%) | 5 (3.2%) | 0.723 |
| Anastomotic leakage | 6 (3.9%) | 9 (5.8%) | 0.598 |
| Pancreatic leakage | 5 (3.2%) | 14 (9.0%) | 0.056 |
| Pneumonia | 6 (3.9%) | 7 (4.5%) | 1.000 |
| Ileus | 5 (3.2%) | 5 (3.2%) | 1.000 |
| Cardiovascular complications | 1 (0.6%) | 2 (1.3%) | 1.000 |
| Bleeding complications | 1 (0.6%) | 5 (3.2%) | 0.214 |
| Postoperative chemotherapy Completion rate for one year | 99 (63.9%) 80 (76.9%) | 99 (63.9%) 94 (91.3%) | 1.000 0.007 |
| Postoperative body weight loss (%) | |||
| For 1 month, median (IQR) For 6 months, median (IQR) For 1 year, median (IQR) | 7.52 (5.40–11.42) 10.11 (6.09–15.75) 8.87 (5.02–14.77) | 8.30 (5.76–11.94) 14.33 (9.50–18.98) 15.79 (10.41–19.62) | 0.451 <0.001 <0.001 |
| Pathological stage | |||
| I II III | 29 (18.7%) 62 (40.0%) 64 (41.3%) | 32 (20.6%) 52 (33.5%) 71 (45.8%) | 0.513 |
| Variables | Multivariate Analysis | |||
|---|---|---|---|---|
| HR | 95%CI | p Value | ||
| Age (years) | <70 | 1 2.101 | 1.399–3.155 | <0.001 |
| ≥70 | ||||
| Surgical approach | Laparoscopic surgery | 1 2.091 | 1.411–3.098 | <0.001 |
| Open surgery | ||||
| Pathological stage | <III | 1 4.110 | 2.675–6.316 | <0.001 |
| ≥III | ||||
| Postoperative chemotherapy | Absent | 1 0.492 | 0.315–0.767 | 0.002 |
| Present | ||||
| Diabetes | Absent | 1 1.580 | 1.013–2.465 | 0.044 |
| Present | ||||
| Postoperative complications | Absent | 1 1.791 | 1.085–2.958 | 0.023 |
| Clavien-Dindo ≥ 3 | ||||
| SMI (cm2/m2) | High-SMI | 1 1.352 | 0.915–1.999 | 0.129 |
| Low-SMI | ||||
| VAI (cm2/m2) | Low-VAI | 1 0.457 | 0.307–0.680 | <0.001 |
| High-VAI | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsui, R.; Inaki, N.; Tsuji, T.; Kokura, Y.; Momosaki, R. Preoperative High Visceral Fat Increases Severe Complications but Improves Long-Term Prognosis after Gastrectomy for Patients with Advanced Gastric Cancer: A Propensity Score Matching Analysis. Nutrients 2022, 14, 4236. https://doi.org/10.3390/nu14204236
Matsui R, Inaki N, Tsuji T, Kokura Y, Momosaki R. Preoperative High Visceral Fat Increases Severe Complications but Improves Long-Term Prognosis after Gastrectomy for Patients with Advanced Gastric Cancer: A Propensity Score Matching Analysis. Nutrients. 2022; 14(20):4236. https://doi.org/10.3390/nu14204236
Chicago/Turabian StyleMatsui, Ryota, Noriyuki Inaki, Toshikatsu Tsuji, Yoji Kokura, and Ryo Momosaki. 2022. "Preoperative High Visceral Fat Increases Severe Complications but Improves Long-Term Prognosis after Gastrectomy for Patients with Advanced Gastric Cancer: A Propensity Score Matching Analysis" Nutrients 14, no. 20: 4236. https://doi.org/10.3390/nu14204236
APA StyleMatsui, R., Inaki, N., Tsuji, T., Kokura, Y., & Momosaki, R. (2022). Preoperative High Visceral Fat Increases Severe Complications but Improves Long-Term Prognosis after Gastrectomy for Patients with Advanced Gastric Cancer: A Propensity Score Matching Analysis. Nutrients, 14(20), 4236. https://doi.org/10.3390/nu14204236










