Infant Red Blood Cell Arachidonic to Docosahexaenoic Acid Ratio Inversely Associates with Fat-Free Mass Independent of Breastfeeding Exclusivity
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of the Breastfed and FF Mother and Infant Dyads
3.2. Infant RBC and Breastmilk AA/DHA Ratios Are Related Only in Breastfeeding Dyads
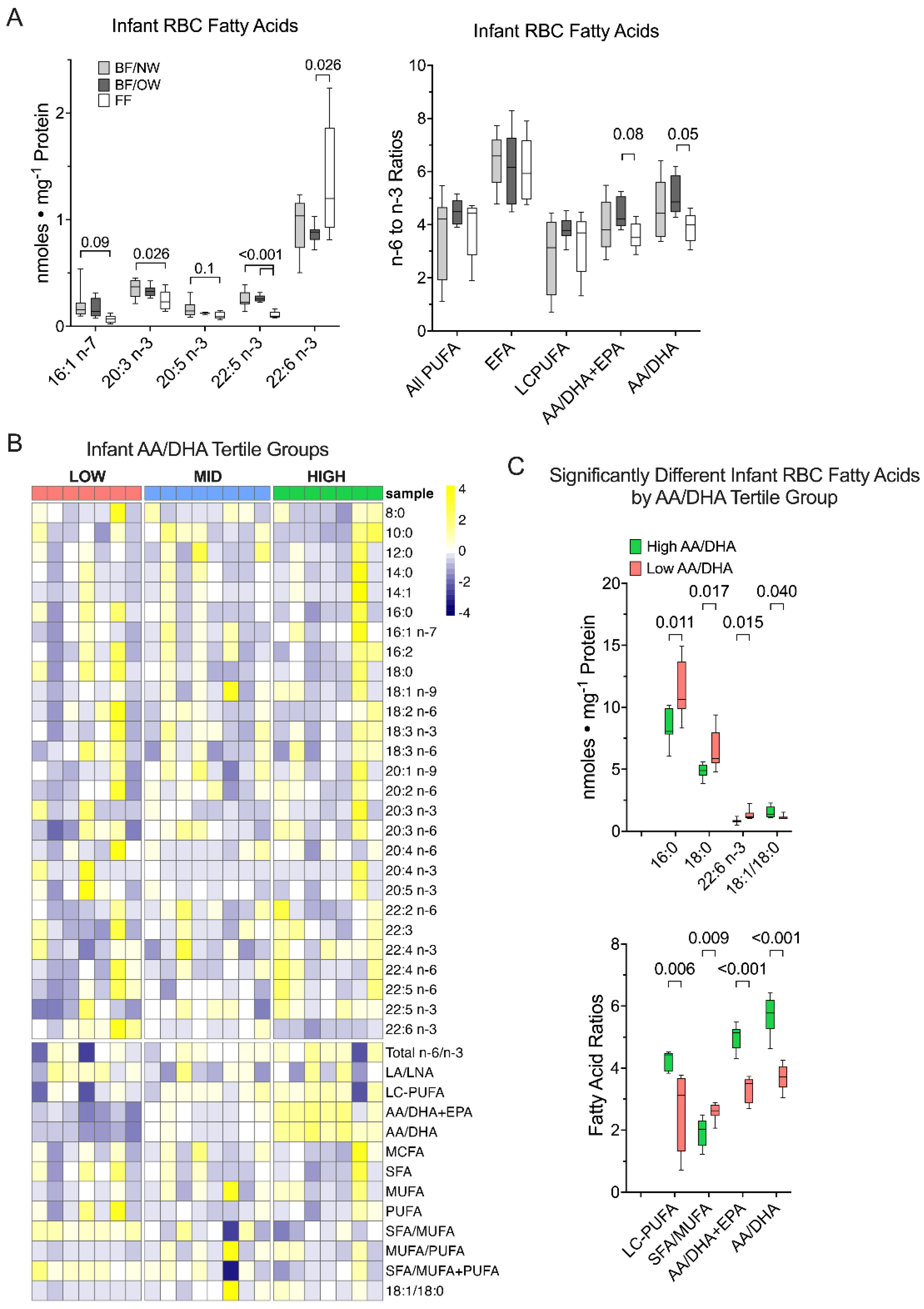
3.3. Docosahexaenoic Acid, but Not Other n-3 PUFA, Is Greater in Formula-Fed Infants
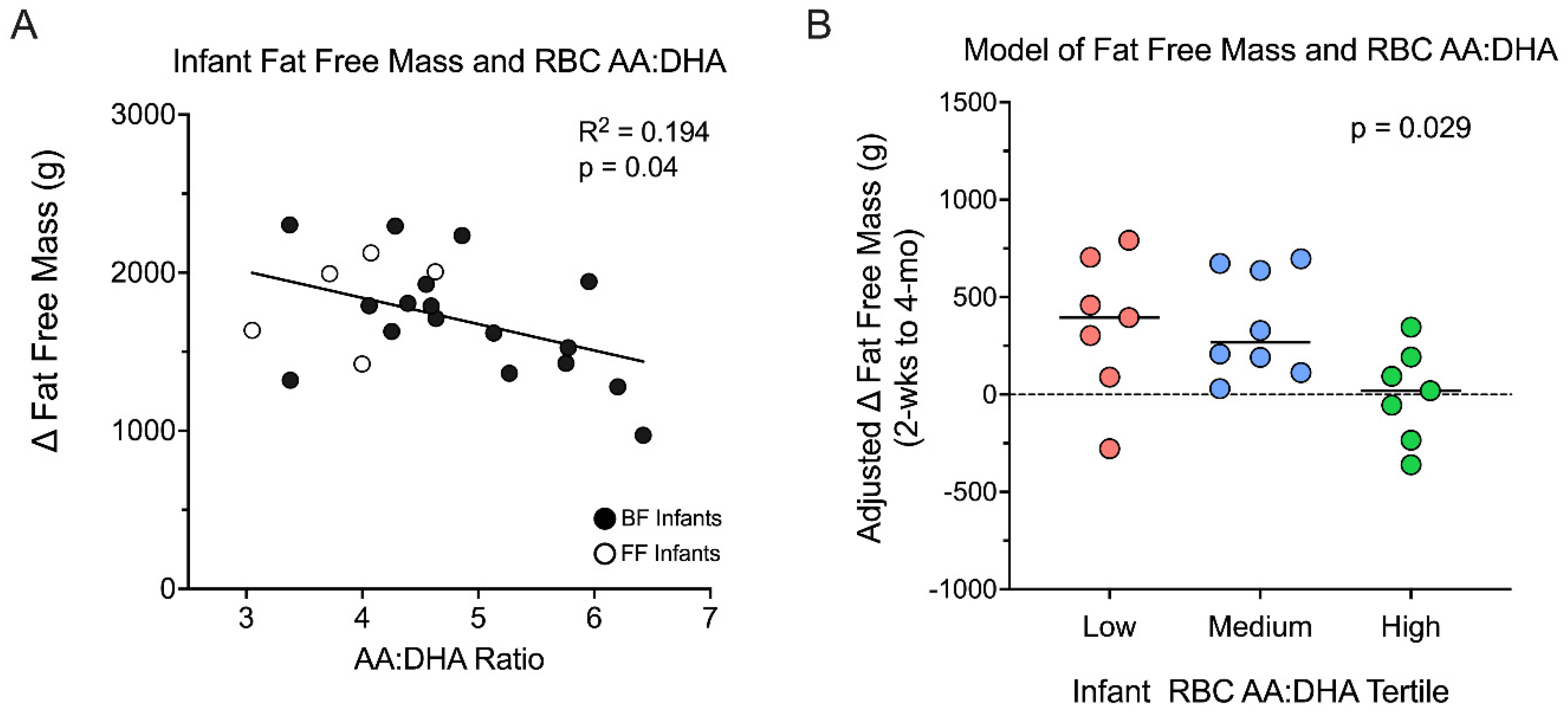
3.4. Infant RBC AA/DHA Ratio Relationships with Infant Body Composition
3.5. Infant Fat Free Mass Accumulation Is Greater in the Low AA/DHA Ratio Group
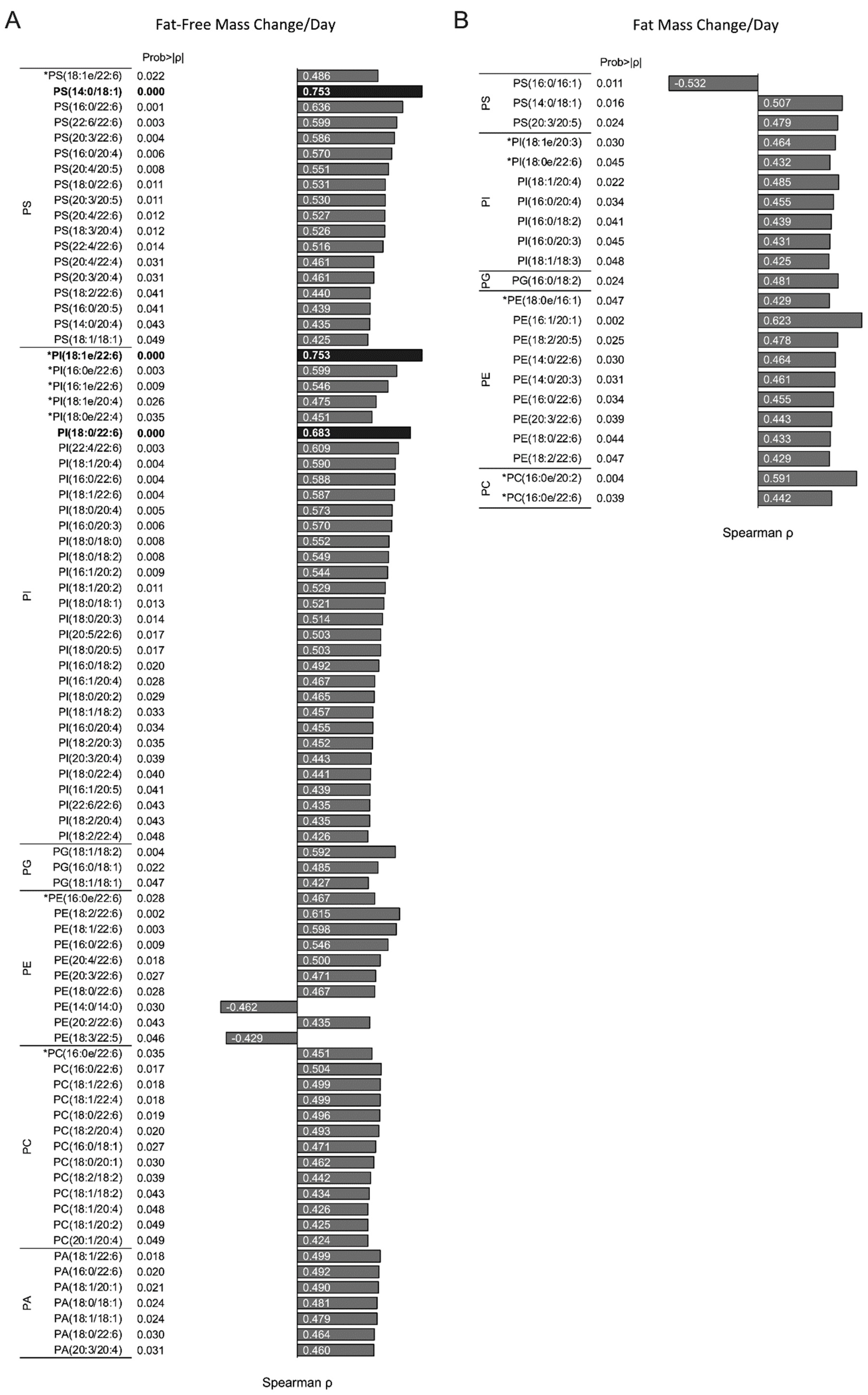
3.6. Infant RBC Phospholipid Species Containing AA and DHA Correlate with Changes Infant Fat-Free Mass Accumulation
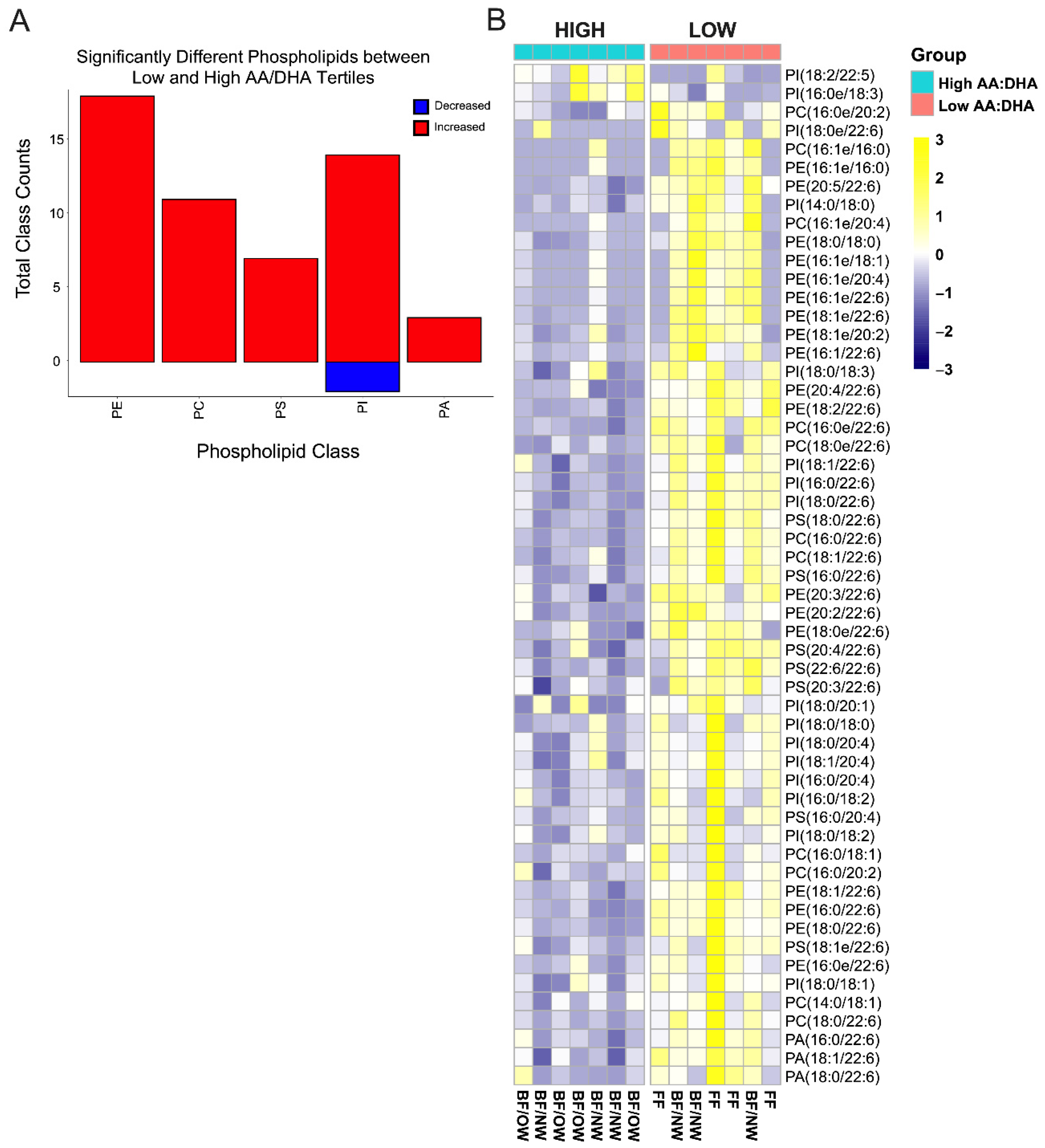
3.7. Plasmalogens Comprise Half of the Differential Infant RBC Phospholipids by Low AA/DHA Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Summermatter, S.; Marcelino, H.; Arsenijevic, D.; Buchala, A.; Aprikian, O.; Assimacopoulos-Jeannet, F.; Seydoux, J.; Montani, J.P.; Solinas, G.; Dulloo, A.G. Adipose tissue plasticity during catch-up fat driven by thrifty metabolism: Relevance for muscle-adipose glucose redistribution during catch-up growth. Diabetes 2009, 58, 2228–2237. [Google Scholar] [CrossRef]
- Ay, L.; Van Houten, V.A.; Steegers, E.A.; Hofman, A.; Witteman, J.C.; Jaddoe, V.W.; Hokken-Koelega, A.C. Fetal and postnatal growth and body composition at 6 months of age. J. Clin. Endocrinol. Metab. 2009, 94, 2023–2030. [Google Scholar] [CrossRef]
- Summermatter, S.; Mainieri, D.; Russell, A.P.; Seydoux, J.; Montani, J.P.; Buchala, A.; Solinas, G.; Dulloo, A.G. Thrifty metabolism that favors fat storage after caloric restriction: A role for skeletal muscle phosphatidylinositol-3-kinase activity and AMP-activated protein kinase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 774–785. [Google Scholar] [CrossRef]
- Dulloo, A.G. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 155–171. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Nissensohn, M.; Overby, N.C.; Fekete, K. Dietary methods and biomarkers of omega 3 fatty acids: A systematic review. Br. J. Nutr. 2012, 107 (Suppl. S2), S64–S76. [Google Scholar] [CrossRef]
- Cohen, R.M.; Franco, R.S.; Khera, P.K.; Smith, E.P.; Lindsell, C.J.; Ciraolo, P.J.; Palascak, M.B.; Joiner, C.H. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 2008, 112, 4284–4291. [Google Scholar] [CrossRef]
- Rudolph, M.C.; Young, B.E.; Lemas, D.J.; Palmer, C.E.; Hernandez, T.L.; Barbour, L.A.; Friedman, J.E.; Krebs, N.F.; MacLean, P.S. Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI. Int. J. Obes. (Lond.) 2017, 41, 510–517. [Google Scholar] [CrossRef]
- Young, B.E.; Levek, C.; Reynolds, R.M.; Rudolph, M.C.; MacLean, P.; Hernandez, T.L.; Friedman, J.E.; Krebs, N.F. Bioactive components in human milk are differentially associated with rates of lean and fat mass deposition in infants of mothers with normal vs. elevated BMI. Pediatr. Obes. 2018, 13, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.C.; Jackman, M.R.; Presby, D.M.; Houck, J.A.; Webb, P.G.; Johnson, G.C.; Soderborg, T.K.; de la Houssaye, B.A.; Yang, I.V.; Friedman, J.E.; et al. Low Neonatal Plasma n-6/n-3 PUFA Ratios Regulate Offspring Adipogenic Potential and Condition Adult Obesity Resistance. Diabetes 2018, 67, 651–661. [Google Scholar] [CrossRef]
- Young, B.E.; Johnson, S.L.; Krebs, N.F. Biological determinants linking infant weight gain and child obesity: Current knowledge and future directions. Adv. Nutr. 2012, 3, 675–686. [Google Scholar] [CrossRef]
- Young, B.E.; Patinkin, Z.; Palmer, C.; de la Houssaye, B.; Barbour, L.; Hernandez, T.; Friedman, J.; Krebs, N. Human Milk Insulin is Related to Maternal Plasma Insulin and BMI—But other Components of Human Milk do not Differ by BMI. Eur. J. Clin. Nutr. 2017, 71, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Rose, H.G.; Oklander, M. Improved Procedure for the Extraction of Lipids from Human Erythrocytes. J. Lipid Res. 1965, 6, 428–431. [Google Scholar] [CrossRef]
- Okuno, T.; Gijon, M.A.; Zarini, S.; Martin, S.A.; Barkley, R.M.; Johnson, C.A.; Ohba, M.; Yokomizo, T.; Murphy, R.C. Altered eicosanoid production and phospholipid remodeling during cell culture. J. Lipid Res. 2018, 59, 542–549. [Google Scholar] [CrossRef]
- Vinding, R.K.; Stokholm, J.; Sevelsted, A.; Sejersen, T.; Chawes, B.L.; Bonnelykke, K.; Thorsen, J.; Howe, L.D.; Krakauer, M.; Bisgaard, H. Effect of fish oil supplementation in pregnancy on bone, lean, and fat mass at six years: Randomised clinical trial. BMJ 2018, 362, k3312. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.T.; Schoenfeld, B.J.; de Oliveira, E.P. Is there sufficient evidence to supplement omega-3 fatty acids to increase muscle mass and strength in young and older adults? Clin. Nutr. 2020, 39, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Leyton, J.; Drury, P.J.; Crawford, M.A. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br. J. Nutr. 1987, 57, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef]
- Devarshi, P.P.; Grant, R.W.; Ikonte, C.J.; Hazels Mitmesser, S. Maternal Omega-3 Nutrition, Placental Transfer and Fetal Brain Development in Gestational Diabetes and Preeclampsia. Nutrients 2019, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Gharami, K.; Das, M.; Das, S. Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem. Int. 2015, 89, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Docosahexaenoic Acid. Ann. Nutr. Metab. 2016, 69 (Suppl. S1), 7–21. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern. Child Nutr. 2011, 7 (Suppl. S2), 112–123. [Google Scholar] [CrossRef]
- Donahue, S.M.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef]
- Massiera, F.; Saint-Marc, P.; Seydoux, J.; Murata, T.; Kobayashi, T.; Narumiya, S.; Guesnet, P.; Amri, E.Z.; Negrel, R.; Ailhaud, G. Arachidonic acid and prostacyclin signaling promote adipose tissue development: A human health concern? J. Lipid Res. 2003, 44, 271–279. [Google Scholar] [CrossRef] [PubMed]
- O’Tierney-Ginn, P.F.; Davina, D.; Gillingham, M.; Barker, D.J.P.; Morris, C.; Thornburg, K.L. Neonatal fatty acid profiles are correlated with infant growth measures at 6 months. J. Dev. Orig. Health Dis. 2017, 8, 474–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pedersen, L.; Lauritzen, L.; Brasholt, M.; Buhl, T.; Bisgaard, H. Polyunsaturated fatty acid content of mother’s milk is associated with childhood body composition. Pediatr. Res. 2012, 72, 631–636. [Google Scholar] [CrossRef]
- Farahnak, Z.; Yuan, Y.; Vanstone, C.A.; Weiler, H.A. Maternal and neonatal red blood cell n-3 polyunsaturated fatty acids inversely associate with infant whole-body fat mass assessed by dual-energy X-ray absorptiometry. Appl. Physiol. Nutr. Metab. 2020, 45, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Metherel, A.H.; Bazinet, R.P. Updates to the n-3 polyunsaturated fatty acid biosynthesis pathway: DHA synthesis rates, tetracosahexaenoic acid and (minimal) retroconversion. Prog. Lipid Res. 2019, 76, 101008. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Park, W.J.; Kothapalli, K.S.; Brenna, J.T. The fatty acid desaturase 2 (FADS2) gene product catalyzes Delta4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 2015, 29, 3911–3919. [Google Scholar] [CrossRef]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Tulodziecka, K.; Diaz-Rohrer, B.B.; Farley, M.M.; Chan, R.B.; Di Paolo, G.; Levental, K.R.; Waxham, M.N.; Levental, I. Remodeling of the postsynaptic plasma membrane during neural development. Mol. Biol. Cell 2016, 27, 3480–3489. [Google Scholar] [CrossRef]
- Wolfs, D.; Lynes, M.D.; Tseng, Y.H.; Pierce, S.; Bussberg, V.; Darkwah, A.; Tolstikov, V.; Narain, N.R.; Rudolph, M.C.; Kiebish, M.A.; et al. Brown Fat-Activating Lipokine 12,13-diHOME in Human Milk Is Associated With Infant Adiposity. J. Clin. Endocrinol. Metab. 2021, 106, e943–e956. [Google Scholar] [CrossRef]
| Characteristic | BF/NW (n = 8) | BF/OW (n = 9) | FF (n = 5) | p-Value |
|---|---|---|---|---|
| Maternal age (yrs) | 29.9 ± 2.7 | 31.4 ± 4.4 | 27.4 ± 4.1 | 0.1976 |
| Pre-Pregnancy BMI (kg/m2) | 20.9 ± 2.2 | 30.5 ± 3.9 | 33.2 ± 7.1 | <0.0001 |
| Gestational age (wks) | 39.6 ± 0.8 | 39.4 ± 0.9 | 39.6 ± 0.9 | 0.938 |
| Race | Asian: 12.5% White 87.5% | Asian 1: 22.2% African American: 11.1% White: 66.7% | Hawaiian/Pacific Islander: 20.0% White: 80.0% | 0.342 |
| Ethnicity | Hispanic: 12.5% Non-Hispanic: 87.5% | Hispanic: 25.0% Non-Hispanic: 75.0% | Hispanic: 60.0% Non-Hispanic: 40.0% | 0.185 |
| Infant sex (% male) | 62.5% | 77.8% | 20% | 0.098 |
| Birth weight (g) | 3130 ± 357 | 3473 ± 544 | 3781 ± 366 | 0.056 |
| Breastfeeding Exposure (months) | 3.9 ± 0.42 | 4.0 ± 0.06 | 0.8 ± 0.41 | <0.0001 |
| Milk | Infant RBCs | p-Value | R2 |
|---|---|---|---|
| 16:0 | 16:0 | 0.715 | |
| 18:2 n6 | 18:2 n6 | 0.397 | |
| 18:3 n3 | 18:3 n3 | 0.999 | |
| 20:4 n6 | 20:4 n6 | 0.128 | |
| 20:5 n3 | 20:5 n3 | 0.997 | |
| 22:6 n3 | 22:6 n3 | 0.0038 | 0.44 |
| Total n6/n3 | Total n6/n3 | 0.95 | |
| AA to DHA | AA to DHA | 0.0005 | 0.56 |
| AA to DHA + EPA | AA to DHA + EPA | 0.0007 | 0.55 |
| Outcome | p-Value | Relationship (Tukey) |
|---|---|---|
| ∆ FFM/day | 0.029 | high < mid, p = 0.032 high < low, p = 0.075 |
| ∆ Fat Mass/day | 0.144 | |
| ∆ % Fat/day | 0.479 | |
| ∆ WLZ/day | 0.190 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, B.E.; Kyere-Davies, G.; Farriester, J.W.; Varshney, R.; MacLean, P.S.; Krebs, N.F.; Rudolph, M.C. Infant Red Blood Cell Arachidonic to Docosahexaenoic Acid Ratio Inversely Associates with Fat-Free Mass Independent of Breastfeeding Exclusivity. Nutrients 2022, 14, 4238. https://doi.org/10.3390/nu14204238
Young BE, Kyere-Davies G, Farriester JW, Varshney R, MacLean PS, Krebs NF, Rudolph MC. Infant Red Blood Cell Arachidonic to Docosahexaenoic Acid Ratio Inversely Associates with Fat-Free Mass Independent of Breastfeeding Exclusivity. Nutrients. 2022; 14(20):4238. https://doi.org/10.3390/nu14204238
Chicago/Turabian StyleYoung, Bridget E., Gertrude Kyere-Davies, Jacob W. Farriester, Rohan Varshney, Paul S. MacLean, Nancy F. Krebs, and Michael C. Rudolph. 2022. "Infant Red Blood Cell Arachidonic to Docosahexaenoic Acid Ratio Inversely Associates with Fat-Free Mass Independent of Breastfeeding Exclusivity" Nutrients 14, no. 20: 4238. https://doi.org/10.3390/nu14204238
APA StyleYoung, B. E., Kyere-Davies, G., Farriester, J. W., Varshney, R., MacLean, P. S., Krebs, N. F., & Rudolph, M. C. (2022). Infant Red Blood Cell Arachidonic to Docosahexaenoic Acid Ratio Inversely Associates with Fat-Free Mass Independent of Breastfeeding Exclusivity. Nutrients, 14(20), 4238. https://doi.org/10.3390/nu14204238






