Predictors of Symptom-Specific Treatment Response to Dietary Interventions in Irritable Bowel Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Study Design
2.3. Dietary Interventions
2.3.1. The Traditional IBS Diet
2.3.2. The Low FODMAP Diet
2.4. Dietary Intake
2.5. Questionnaires
2.5.1. GI Symptoms
2.5.2. Non-GI Symptoms
2.6. Gut Bacterial Analysis
2.7. Data and Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Symptom Improvement during Dietary Interventions
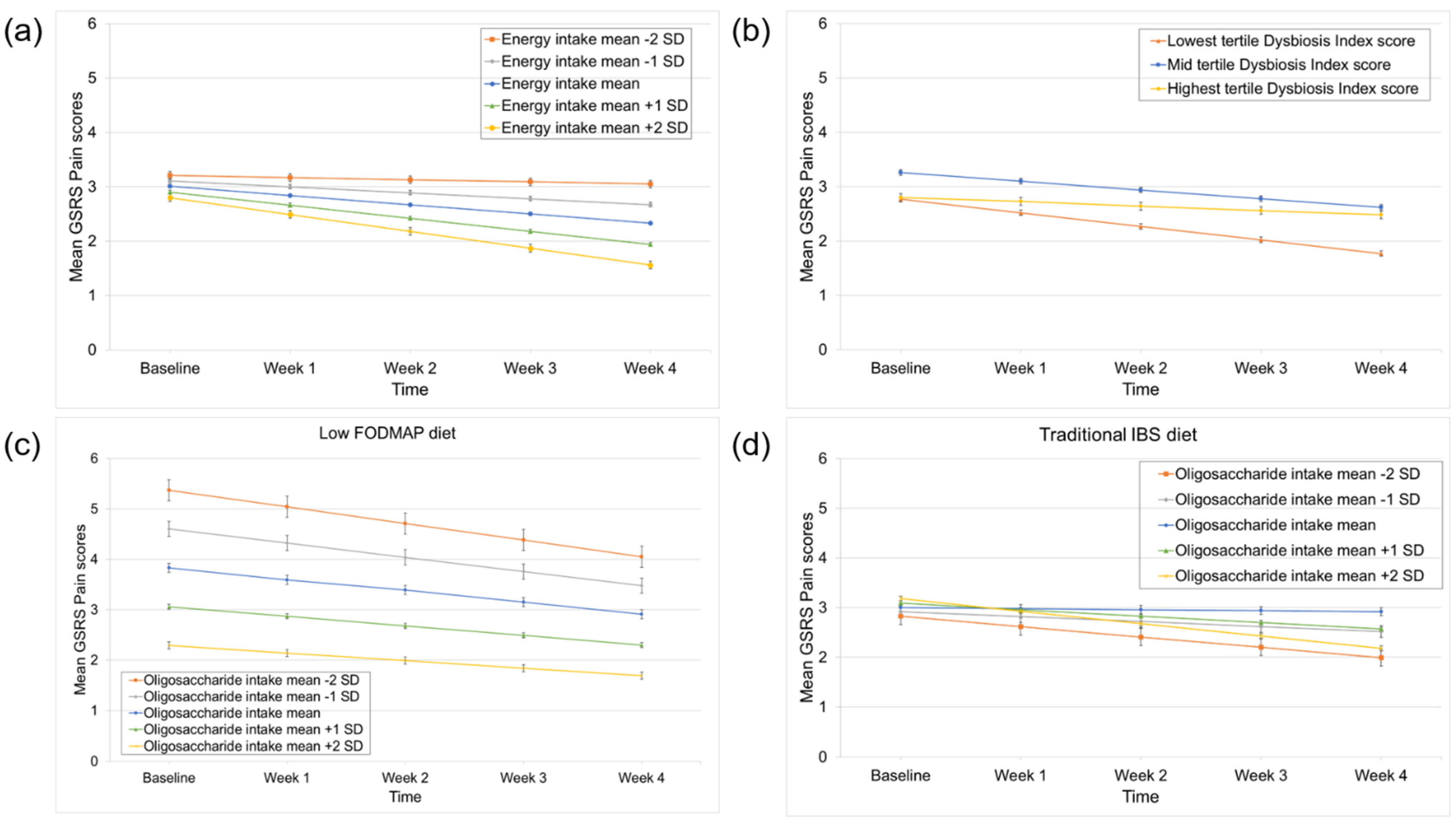
3.3. Predictors of Pain Improvement
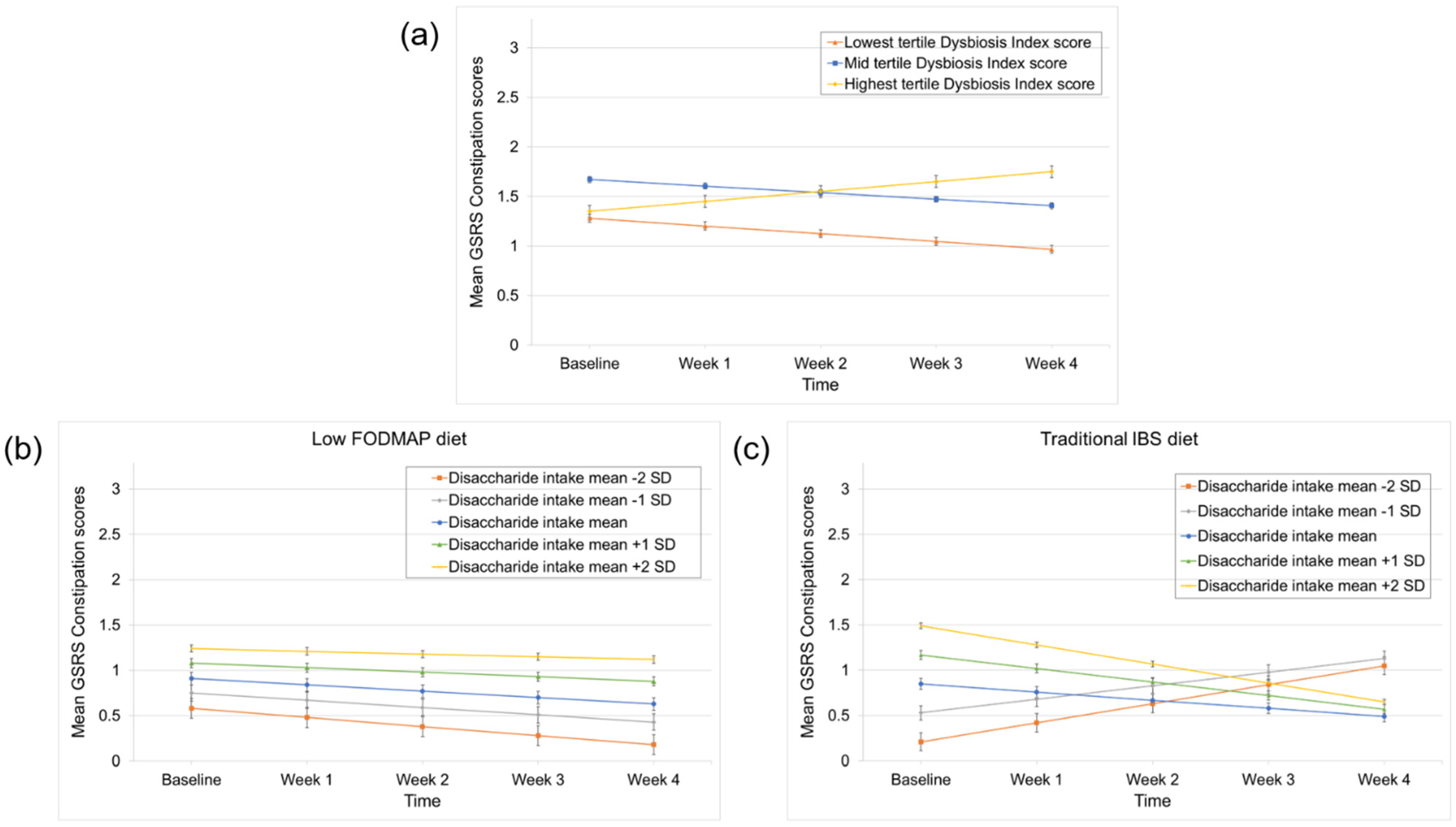
3.4. Predictors of Constipation and Diarrhea Improvement
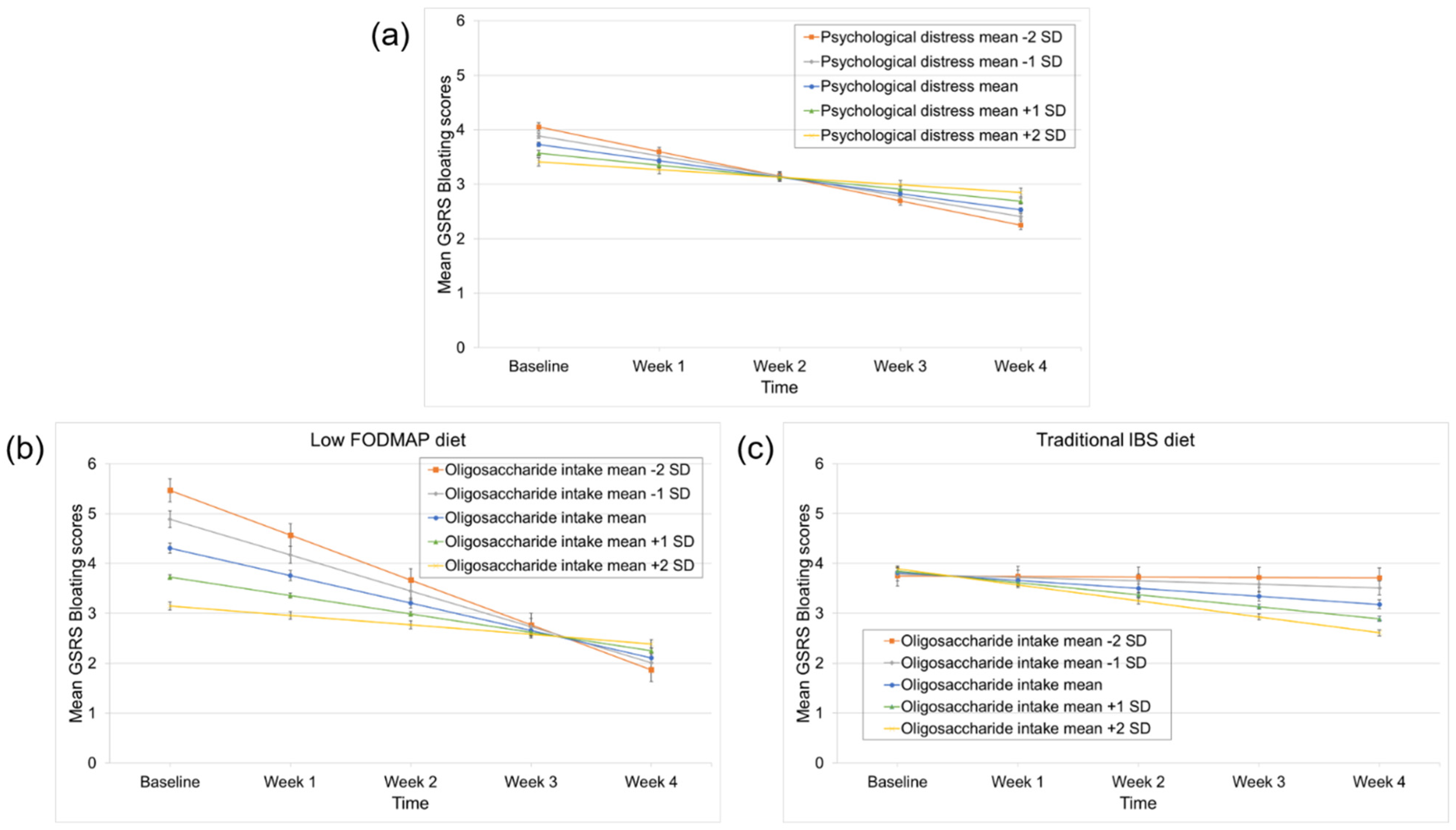
3.5. Predictors of Bloating Improvement
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e113. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 4, 1257–1492. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Lee, O.Y.; Naliboff, B.; Schmulson, M.; Mayer, E.A. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2001, 96, 3341–3347. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Törnblom, H.; Palsson, O.S.; van Tilburg, M.A.L.; Van Oudenhove, L.; Tack, J.; Whitehead, W.E. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: Consistent findings from five different patient cohorts. Gut 2018, 67, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Törnblom, H.; Van Oudenhove, L.; Sadik, R.; Abrahamsson, H.; Tack, J.; Simrén, M. Colonic transit time and IBS symptoms: What’s the link? Am. J. Gastroenterol. 2012, 107, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Manabe, N.; Wong, B.S.; Camilleri, M.; Burton, D.; McKinzie, S.; Zinsmeister, A.R. Lower functional gastrointestinal disorders: Evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol. Motil. 2010, 22, 293-e82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohman, L.; Simrén, M. Pathogenesis of IBS: Role of inflammation, immunity and neuroimmune interactions. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 163–173. [Google Scholar] [CrossRef]
- Öhman, L.; Törnblom, H.; Simrén, M. Crosstalk at the mucosal border: Importance of the gut microenvironment in IBS. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 36–49. [Google Scholar] [CrossRef]
- Bednarska, O.; Walter, S.A.; Casado-Bedmar, M.; Ström, M.; Salvo-Romero, E.; Vicario, M.; Mayer, E.A.; Keita, Å.V. Vasoactive Intestinal Polypeptide and Mast Cells Regulate Increased Passage of Colonic Bacteria in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 948–960.e3. [Google Scholar] [CrossRef]
- Tap, J.; Derrien, M.; Törnblom, H.; Brazeilles, R.; Cools-Portier, S.; Doré, J.; Störsrud, S.; Le Nevé, B.; Öhman, L.; Simrén, M. Identification of an Intestinal Microbiota Signature Associated with Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123.e8. [Google Scholar] [CrossRef] [Green Version]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Quigley, E.M.; Santos, J.; Vanner, S.; Vergnolle, N.; Zoetendal, E.G. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1305–1318.e8. [Google Scholar] [CrossRef] [Green Version]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Posserud, I.; Strid, H.; Störsrud, S.; Törnblom, H.; Svensson, U.; Tack, J.; Van Oudenhove, L.; Simrén, M. Symptom pattern following a meal challenge test in patients with irritable bowel syndrome and healthy controls. United Eur. Gastroenterol. J. 2013, 1, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Melchior, C.; Algera, J.; Colomier, E.; Törnblom, H.; Simrén, M.; Störsrud, S. Food Avoidance and Restriction in Irritable Bowel Syndrome: Relevance for Symptoms, Quality of Life and Nutrient Intake. Clin. Gastroenterol. Hepatol. 2021, in press. [CrossRef]
- Algera, J.; Colomier, E.; Simrén, M. The Dietary Management of Patients with Irritable Bowel Syndrome: A Narrative Review of the Existing and Emerging Evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 549–575. [Google Scholar] [CrossRef] [Green Version]
- Dalrymple, J.; Bullock, I. Diagnosis and management of irritable bowel syndrome in adults in primary care: Summary of NICE guidance. BMJ 2008, 336, 556–558. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Whelan, K.; Irving, P.M.; Lomer, M.C. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2011, 24, 487–495. [Google Scholar] [CrossRef] [PubMed]
- De Roest, R.H.; Dobbs, B.R.; Chapman, B.A.; Batman, B.; O’Brien, L.A.; Leeper, J.A.; Hebblethwaite, C.R.; Gearry, R.B. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: A prospective study. Int. J. Clin. Pract. 2013, 67, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef] [Green Version]
- Schindler, V.; Giezendanner, S.; Van Oudenhove, L.; Murray, F.R.; Buehler, J.; Bordier, V.; Hente, J.; Pohl, D. Better response to low FODMAP diet in disorders of gut-brain interaction patients with pronounced hydrogen response to a nutrient challenge test. J. Gastroenterol. Hepatol. 2021, 36, 3322–3328. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Lindsay, J.O.; Irving, P.; Probert, C.; Whelan, K. Volatile Organic Compounds in Feces Associate With Response to Dietary Intervention in Patients With Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2018, 16, 385–391.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eetemadi, A.; Tagkopoulos, I. Methane and fatty acid metabolism pathways are predictive of Low-FODMAP diet efficacy for patients with irritable bowel syndrome. Clin. Nutr. 2021, 40, 4414–4421. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, L.; Wang, X.; Fox, M.; Luo, L.; Du, L.; Chen, B.; Chen, X.; He, H.; Zhu, S.; et al. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet compared with traditional dietary advice for diarrhea-predominant irritable bowel syndrome: A parallel-group, randomized controlled trial with analysis of clinical and microbiological factors associated with patient outcomes. Am. J. Clin. Nutr. 2021, 113, 1531–1545. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut 2018, 67, 872–881. [Google Scholar] [CrossRef]
- Valeur, J.; Småstuen, M.C.; Knudsen, T.; Lied, G.A.; Røseth, A.G. Exploring Gut Microbiota Composition as an Indicator of Clinical Response to Dietary FODMAP Restriction in Patients with Irritable Bowel Syndrome. Dig. Dis. Sci. 2018, 63, 429–436. [Google Scholar] [CrossRef]
- Vervier, K.; Moss, S.; Kumar, N.; Adoum, A.; Barne, M.; Browne, H.; Kaser, A.; Kiely, C.J.; Neville, B.A.; Powell, N.; et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut 2021, preprint. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Midenfjord, I.; Polster, A.; Sjövall, H.; Törnblom, H.; Simrén, M. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol. Motil. 2019, 31, e13619. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiklund, I.K.; Fullerton, S.; Hawkey, C.J.; Jones, R.H.; Longstreth, G.F.; Mayer, E.A.; Peacock, R.A.; Wilson, I.K.; Naesdal, J. An irritable bowel syndrome-specific symptom questionnaire: Development and validation. Scand. J. Gastroenterol. 2003, 38, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Lomer, M.C.; Gibson, P.R. Short-chain carbohydrates and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 707–717. [Google Scholar] [CrossRef]
- Liljebo, T.; Störsrud, S.; Andreasson, A. Presence of Fermentable Oligo-, Di-, Monosaccharides, and Polyols (FODMAPs) in commonly eaten foods: Extension of a database to indicate dietary FODMAP content and calculation of intake in the general population from food diary data. BMC Nutr. 2020, 6, 47. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom. Med. 2002, 64, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labus, J.S.; Bolus, R.; Chang, L.; Wiklund, I.; Naesdal, J.; Mayer, E.A.; Naliboff, B.D. The Visceral Sensitivity Index: Development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment. Pharmacol. Ther. 2004, 20, 89–97. [Google Scholar] [CrossRef]
- Casén, C.; Vebø, H.C.; Sekelja, M.; Hegge, F.T.; Karlsson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Frøyland, C.; Nestestog, R.; Engstrand, L.; et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Maruo, K.; Yamaguchi, Y.; Noma, H.; Gosho, M. Interpretable inference on the mixed effect model with the Box-Cox transformation. Stat. Med. 2017, 36, 2420–2434. [Google Scholar] [CrossRef]
- Harris, L.R.; Roberts, L. Treatments for irritable bowel syndrome: Patients’ attitudes and acceptability. BMC Complement. Altern. Med. 2008, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.L.; Yao, C.K.; Philpott, H.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised clinical trial: The efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloan, T.J.; Jalanka, J.; Major, G.A.D.; Krishnasamy, S.; Pritchard, S.; Abdelrazig, S.; Korpela, K.; Singh, G.; Mulvenna, C.; Hoad, C.L.; et al. A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects. PLoS ONE 2018, 13, e0201410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients with Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Clinical Characteristic | Dietary Intervention | |
|---|---|---|
| Low FODMAP Diet | Traditional IBS Diet | |
| Gender (%) (n = 67) | ||
| Female | 85.8 | 83.6 |
| Male | 15.2 | 16.4 |
| Age (years) (n = 67) | 45 ± 15 | 40 ± 17 |
| BMI (kg/m2) (n = 65) | 24.39 ± 3.93 | 24.07 ± 3.75 |
| IBS subtypes (%) (n = 67) | ||
| IBS-C | 27.3 | 31.3 |
| IBS-D | 30.3 | 28.4 |
| IBS-nonCnonD | 42.4 | 40.3 |
| IBS-SSS (score) (n = 67) | 317 ± 70 | 306 ± 66 |
| GSRS-IBS total (score) (n = 67) | 3.91 ±0.95 | 3.64 ± 0.75 |
| GSRS-IBS pain | 4.05 ± 1.00 | 4.13 ± 0.93 |
| GSRS-IBS bloating | 4.98 ± 1.08 | 5.13 ± 1.38 |
| GSRS-IBS constipation | 3.03 ± 1.57 | 3.29 ± 1.65 |
| GSRS-IBS diarrhea | 3.41 ± 1.01 | 3.29 ± 1.12 |
| PHQ-12 (score) (n = 66) | 8.16 ± 3.89 | 7.47 ± 3.40 |
| VSI (score) (n = 66) | 49.13 ± 12.83 | 47.59 ±16.85 |
| HADS total (score) (n = 67) | 13.55 ± 7.39 | 10.74 ± 6.74 |
| FODMAP intake (gram/day) (n = 67) | 18.49 ± 9.90 | 18.49 ± 7.92 |
| Oligosaccharide intake (gram/day) | 2.72 ± 1.21 | 2.87 ± 1.42 |
| Disaccharide intake (gram/day) | 8.80 ± 8.33 | 8.47 ± 5.69 |
| Monosaccharide intake (gram/day) | 5.91 ± 5.42 | 6.03 ± 5.49 |
| Polyols intake (gram/day) | 1.07 ±1.56 | 1.12 ± 1.44 |
| Energy intake (Kcal/day) (n = 67) | 2097 ± 430 | 2100 ± 451 |
| Dysbiosis index score (% 1/2/3/4/5) (n = 60) | 10.3/20.7/48.3/10.3/10.3 | 6.7/16.7/53.3/13.3/10.0 |
| Pain | Constipation | Diarrhea | Bloating | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variables: PHQ-12 and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 3.02 | 0.11 | <0.0001 | 1.48 | 0.11 | <0.0001 | 1.32 | 0.05 | <0.0001 | 3.72 | 0.15 | <0.0001 |
| Time | −0.18 | 0.03 | <0.0001 | −0.03 | 0.02 | 0.18 | −0.06 | 0.01 | <0.0001 | −0.30 | 0.04 | <0.0001 |
| PHQ-12 | 0.37 | 0.11 | 0.001 | 0.01 | 0.11 | 0.91 | 0.13 | 0.05 | 0.01 | 0.24 | 0.15 | 0.11 |
| Time × PHQ-12 | −0.01 | 0.03 | 0.67 | 0.01 | 0.02 | 0.60 | −0.008 | 0.01 | 0.54 | 0.01 | 0.04 | 0.70 |
| Independent variables: HADS and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 3.01 | 0.12 | <0.0001 | 1.48 | 0.11 | <0.0001 | 1.33 | 0.05 | <0.0001 | 3.73 | 0.15 | <0.0001 |
| Time | −0.17 | 0.03 | <0.0001 | −0.03 | 0.02 | 0.15 | −0.06 | 0.01 | <0.0001 | −0.30 | 0.04 | <0.0001 |
| HADS | 0.03 | 0.12 | 0.80 | −0.10 | 0.11 | 0.37 | 0.03 | 0.05 | 0.55 | −0.16 | 0.15 | 0.28 |
| Time × HADS | 0.01 | 0.03 | 0.70 | 0.01 | 0.02 | 0.75 | 0.004 | 0.01 | 0.72 | 0.08 | 0.04 | 0.03 |
| Independent variables: VSI and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 3.02 | 0.11 | <0.0001 | 1.48 | 0.11 | <0.0001 | 1.32 | 0.05 | <0.0001 | 3.72 | 0.15 | <0.0001 |
| Time | −0.18 | 0.03 | <0.0001 | −0.03 | 0.02 | 0.18 | −0.06 | 0.01 | <0.0001 | −0.30 | 0.04 | <0.0001 |
| VSI | 0.29 | 0.11 | 0.01 | 0.17 | 0.11 | 0.11 | 0.14 | 0.05 | 0.01 | 0.11 | 0.15 | 0.47 |
| Time × VSI | 0.01 | 0.03 | 0.68 | −0.001 | 0.02 | 0.97 | −0.01 | 0.01 | 0.60 | 0.06 | 0.04 | 0.11 |
| Independent variables: FODMAP intake and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 3.01 | 0.12 | <0.0001 | 1.48 | 0.10 | <0.0001 | 1.33 | 0.05 | <0.0001 | 3.73 | 0.15 | <0.0001 |
| Time | −0.17 | 0.03 | <0.0001 | −0.03 | 0.02 | 0.15 | −0.06 | 0.01 | <0.0001 | −0.30 | 0.04 | <0.0001 |
| FODMAP intake | 0.02 | 0.12 | 0.83 | 0.18 | 0.10 | 0.09 | 0.05 | 0.05 | 0.33 | −0.12 | 0.15 | 0.41 |
| Time × FODMAP intake | 0.01 | 0.03 | 0.80 | 0.01 | 0.02 | 0.71 | −0.01 | 0.01 | 0.69 | 0.001 | 0.04 | 0.97 |
| Independent variables: Energy intake and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 3.01 | 0.12 | <0.0001 | 1.48 | 0.11 | <0.0001 | 1.33 | 0.05 | <0.0001 | 3.73 | 0.14 | <0.0001 |
| Time | −0.17 | 0.03 | <0.0001 | −0.03 | 0.02 | 0.15 | −0.06 | 0.01 | <0.0001 | −0.30 | 0.04 | <0.0001 |
| Energy intake | −0.10 | 0.12 | 0.39 | 0.01 | 0.11 | 0.94 | −0.06 | 0.05 | 0.24 | −0.34 | 0.14 | 0.03 |
| Time × Energy intake | −0.07 | 0.03 | 0.03 | −0.01 | 0.02 | 0.72 | −0.02 | 0.01 | 0.20 | 0.01 | 0.04 | 0.81 |
| Independent variables: Dysbiosis Index (DI) score (in tertiles) and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 2.80 | 0.25 | <0.0001 | 1.35 | 0.23 | <0.0001 | 1.42 | 0.11 | <0.0001 | 3.72 | 0.32 | <0.0001 |
| Time | −0.08 | 0.07 | 0.24 | 0.01 | 0.05 | 0.05 | −0.07 | 0.03 | 0.01 | −0.25 | 0.08 | 0.003 |
| DI score (lowest vs. highest tertile) | −0.03 | 0.32 | 0.92 | −0.08 | 0.30 | 0.79 | −0.25 | 0.15 | 0.09 | −0.34 | 0.41 | 0.41 |
| DI score (mid vs. highest tertile) | 0.45 | 0.30 | 0.13 | 0.31 | 0.28 | 0.26 | −0.02 | 0.13 | 0.87 | 0.25 | 0.39 | 0.51 |
| DI score (lowest vs. mid tertile) | −0.49 | 0.26 | 0.06 | −0.39 | 0.24 | 0.11 | −0.22 | 0.12 | 0.06 | −0.59 | 0.34 | 0.08 |
| Time × DI score (lowest vs. highest tertile) | −0.17 | 0.09 | 0.05 | −0.18 | 0.06 | 0.01 | 0.03 | 0.04 | 0.36 | −0.06 | 0.10 | 0.59 |
| Time × DI score (mid vs. highest tertile) | −0.08 | 0.08 | 0.31 | −0.17 | 0.06 | 0.01 | 0.01 | 0.03 | 0.81 | −0.05 | 0.10 | 0.62 |
| Time × DI score (lowest vs. mid tertile) | −0.09 | 0.07 | 0.20 | −0.01 | 0.05 | 0.81 | 0.03 | 0.03 | 0.40 | −0.008 | 0.09 | 0.92 |
| Independent variables: Oligosaccharide intake and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 3.34 | 0.22 | <0.0001 | 1.50 | 0.20 | <0.0001 | 1.29 | 0.10 | <0.0001 | 4.00 | 0.28 | <0.0001 |
| Time | −0.12 | 0.06 | 0.06 | −0.04 | 0.05 | 0.33 | −0.04 | 0.02 | 0.11 | −0.33 | 0.07 | <0.0001 |
| Oligosaccharide intake | −0.27 | 0.15 | 0.07 | −0.02 | 0.14 | 0.91 | 0.04 | 0.07 | 0.60 | −0.22 | 0.19 | 0.25 |
| Time × Oligosaccharide intake | −0.05 | 0.04 | 0.25 | −0.01 | 0.03 | 0.80 | −0.01 | 0.02 | 0.42 | 0.03 | 0.05 | 0.53 |
| Independent variables: Polyols intake and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 3.07 | 0.14 | <0.0001 | 1.50 | 0.13 | <0.0001 | 1.39 | 0.06 | <0.0001 | 3.67 | 0.19 | <0.0001 |
| Time | −0.17 | 0.04 | <0.0001 | −0.04 | 0.03 | 0.15 | −0.06 | 0.02 | 0.001 | −0.26 | 0.05 | <0.0001 |
| Polyols intake | 0.05 | 0.08 | 0.47 | 0.02 | 0.07 | 0.79 | 0.05 | 0.04 | 0.16 | −0.06 | 0.11 | 0.56 |
| Time × Polyols intake | 0.0001 | 0.02 | >0.99 | −0.01 | 0.02 | 0.61 | 0.001 | 0.01 | 0.93 | 0.03 | 0.02 | 0.21 |
| Independent variables: Disaccharide intake and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 2.62 | 0.22 | <0.0001 | 0.89 | 0.19 | <0.0001 | 1.27 | 0.10 | <0.0001 | 3.70 | 0.30 | <0.0001 |
| Time | −0.13 | 0.06 | 0.04 | 0.01 | 0.05 | 0.82 | −0.05 | 0.03 | 0.07 | −0.30 | 0.07 | 0.0003 |
| Disaccharide intake | 0.16 | 0.08 | 0.05 | 0.24 | 0.07 | 0.001 | 0.03 | 0.04 | 0.48 | 0.01 | 0.10 | 0.92 |
| Time × Disaccharide intake | −0.02 | 0.02 | 0.45 | −0.02 | 0.02 | 0.26 | −0.004 | 0.001 | 0.68 | −0.01 | 0.03 | 0.80 |
| Independent variables: Monosaccharide intake and time | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 3.05 | 0.19 | <0.0001 | 1.59 | 0.18 | <0.0001 | 1.32 | 0.09 | <0.0001 | 3.81 | 0.25 | <0.0001 |
| Time | −0.18 | 0.05 | 0.001 | −0.07 | 0.04 | 0.08 | −0.04 | 0.02 | 0.03 | −0.25 | 0.06 | 0.0001 |
| Monosaccharide intake | −0.04 | 0.11 | 0.75 | −0.07 | 0.10 | 0.49 | 0.003 | 0.05 | 0.96 | −0.06 | 0.14 | 0.67 |
| Time × Monosaccharide intake | 0.004 | 0.03 | 0.89 | 0.03 | 0.02 | 0.22 | −0.01 | 0.01 | 0.64 | −0.03 | 0.04 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomier, E.; Van Oudenhove, L.; Tack, J.; Böhn, L.; Bennet, S.; Nybacka, S.; Störsrud, S.; Öhman, L.; Törnblom, H.; Simrén, M. Predictors of Symptom-Specific Treatment Response to Dietary Interventions in Irritable Bowel Syndrome. Nutrients 2022, 14, 397. https://doi.org/10.3390/nu14020397
Colomier E, Van Oudenhove L, Tack J, Böhn L, Bennet S, Nybacka S, Störsrud S, Öhman L, Törnblom H, Simrén M. Predictors of Symptom-Specific Treatment Response to Dietary Interventions in Irritable Bowel Syndrome. Nutrients. 2022; 14(2):397. https://doi.org/10.3390/nu14020397
Chicago/Turabian StyleColomier, Esther, Lukas Van Oudenhove, Jan Tack, Lena Böhn, Sean Bennet, Sanna Nybacka, Stine Störsrud, Lena Öhman, Hans Törnblom, and Magnus Simrén. 2022. "Predictors of Symptom-Specific Treatment Response to Dietary Interventions in Irritable Bowel Syndrome" Nutrients 14, no. 2: 397. https://doi.org/10.3390/nu14020397
APA StyleColomier, E., Van Oudenhove, L., Tack, J., Böhn, L., Bennet, S., Nybacka, S., Störsrud, S., Öhman, L., Törnblom, H., & Simrén, M. (2022). Predictors of Symptom-Specific Treatment Response to Dietary Interventions in Irritable Bowel Syndrome. Nutrients, 14(2), 397. https://doi.org/10.3390/nu14020397






