Plinia trunciflora Extract Administration Prevents HI-Induced Oxidative Stress, Inflammatory Response, Behavioral Impairments, and Tissue Damage in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
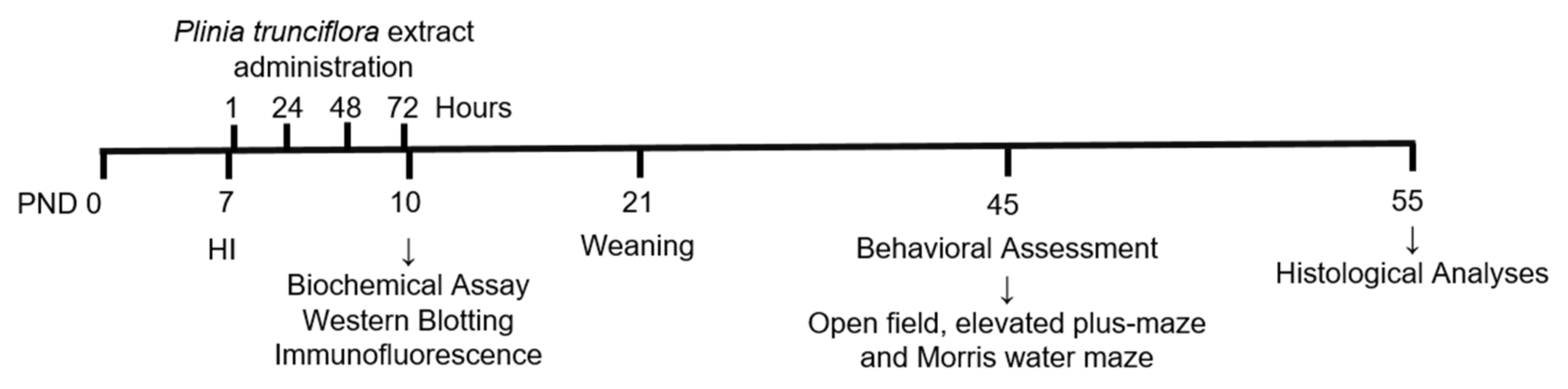
2.2. Experimental Design
2.3. Preparation and Characterization of Plant Extract
2.4. Redox Homeostasis Parameters
2.4.1. Malondialdehyde (MDA) Levels
2.4.2. Reduced Glutathione (GSH) Concentrations
2.4.3. Antioxidant Enzymes Activities
2.5. Western Blotting
2.6. Immunofluorescence Studies
2.7. Behavioral Assessment
2.7.1. Open Field Test
2.7.2. Elevated plus Maze
2.7.3. Morris Water Maze
2.8. Brain Volumetric Analysis
2.9. Statistics
3. Results
3.1. Chemical Composition
3.2. PTE Prevents HI-Induced Disruption of Redox Homeostasis and an Increase in Pro-Inflammatory Interleukin-1β
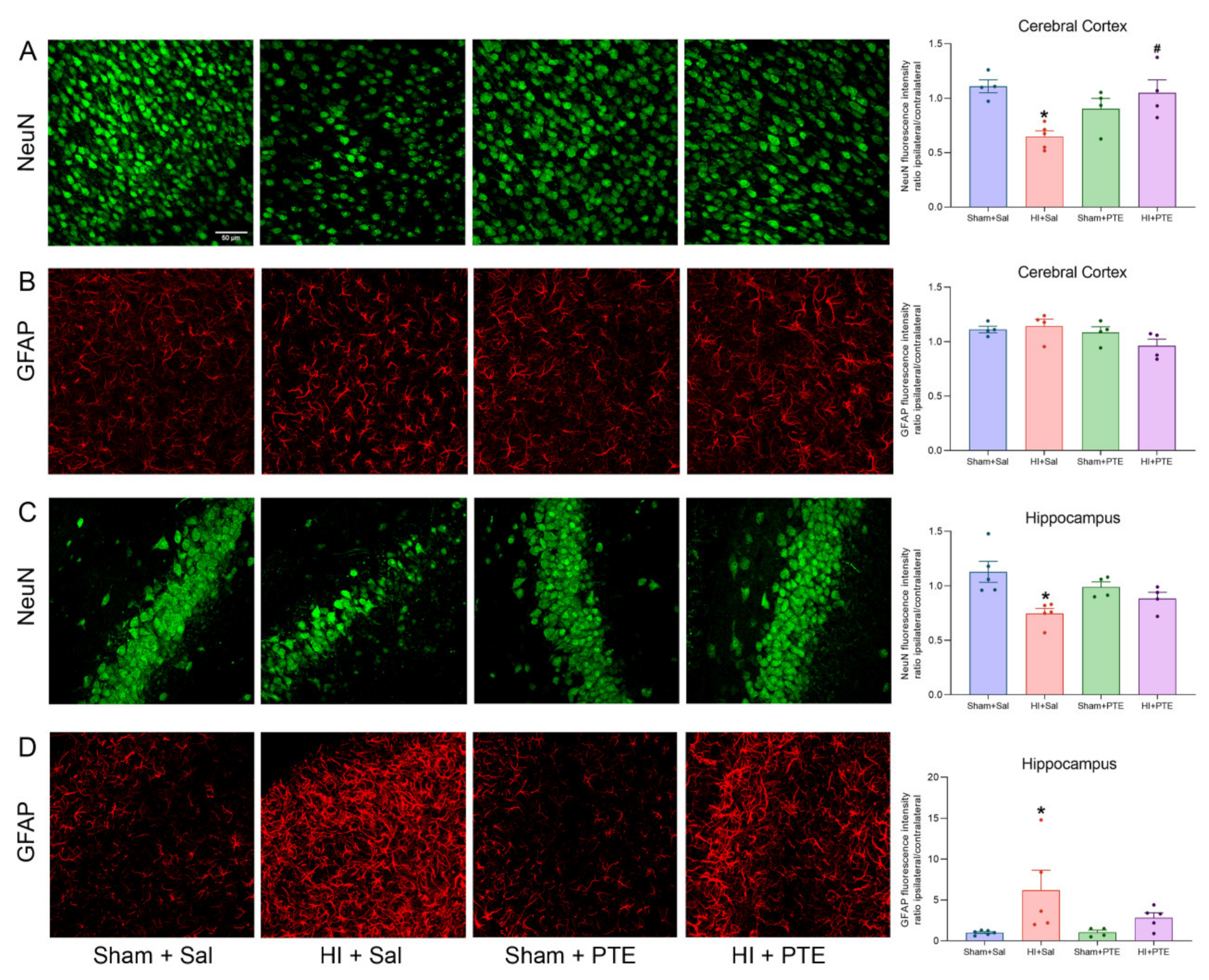
3.3. PTE Prevents HI-Induced Neuronal Loss and Greater GFAP Immunoreactivity
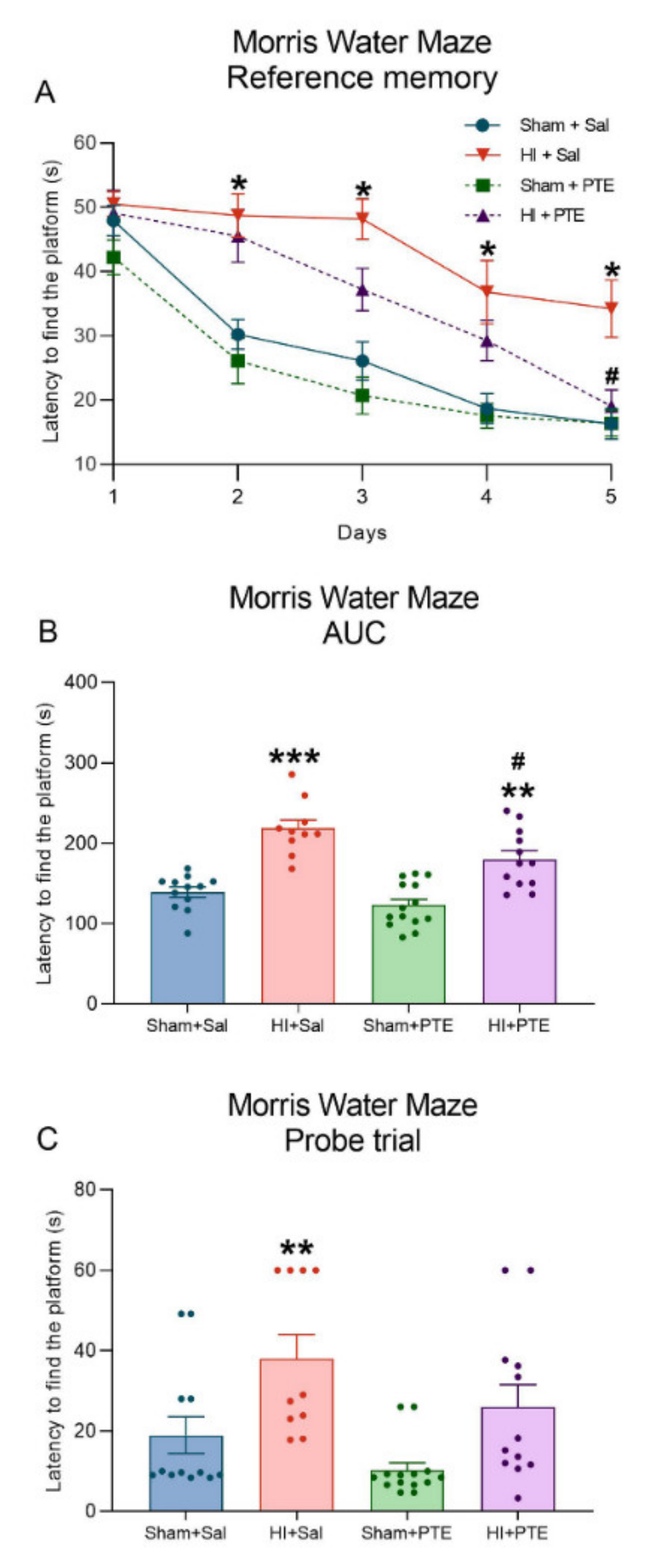
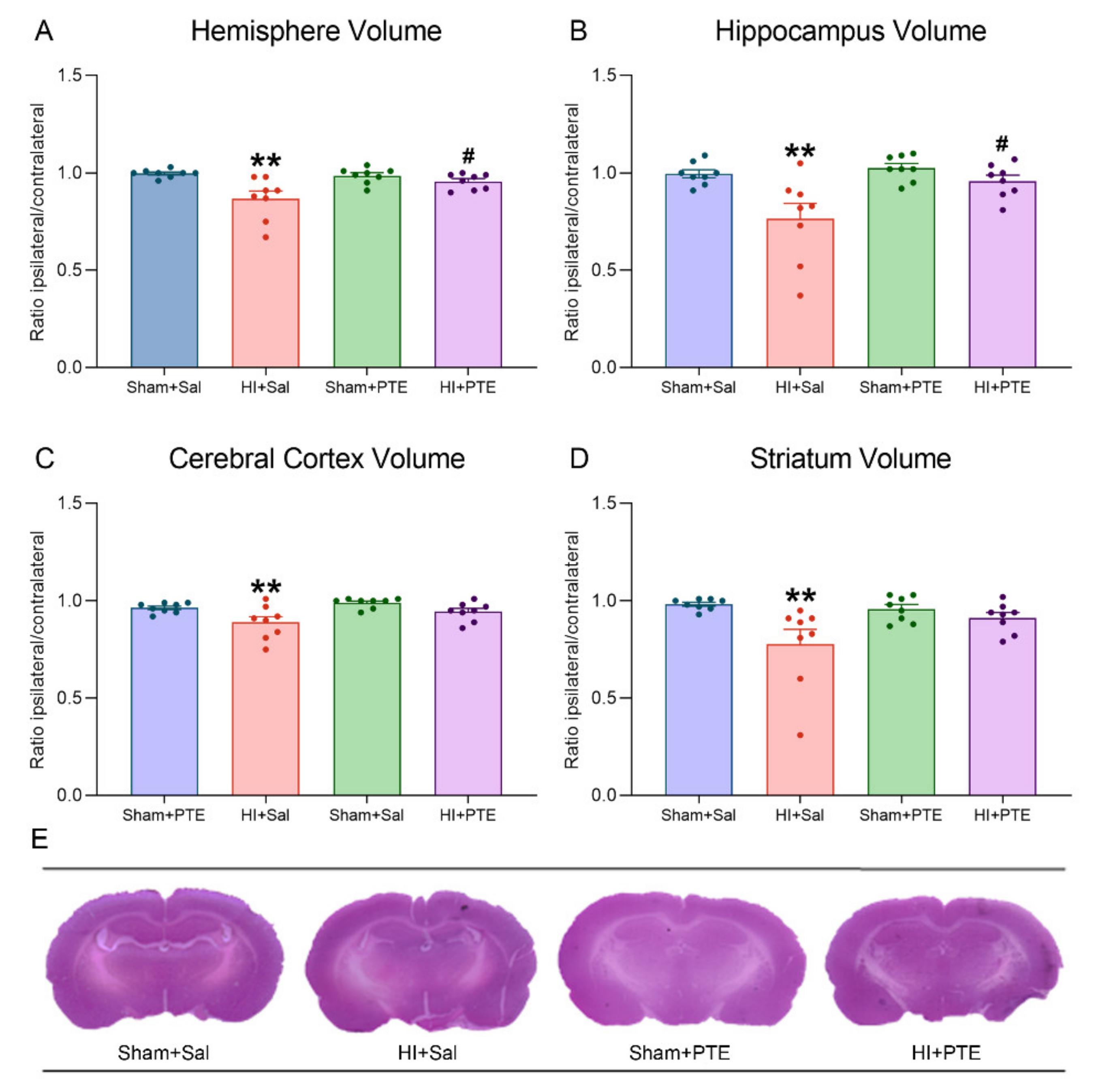
3.4. PTE Partially Prevents HI-Induced Behavioral Impairments and Brain Tissue Damage Assessed at Adult Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Ferriero, D.M. Oxidant mechanisms in neonatal hypoxia-ischemia. Dev. Neurosci. 2001, 23, 198–202. [Google Scholar] [CrossRef]
- Dilenge, M.E.; Majnemer, A.; Shevell, M.I. Long-term developmental outcome of asphyxiated term neonates. J. Child Neurol. 2001, 16, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Perlman, J.M. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1997, 100, 1004–1114. [Google Scholar] [CrossRef]
- Beilharz, E.J.; Williams, C.E.; Dragunow, M.; Sirimanne, E.S.; Gluckman, P.D. Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: Evidence for apoptosis during selective neuronal loss. Brain Res. Mol. Brain Res. 1995, 29, 1–14. [Google Scholar] [CrossRef]
- Lai, M.-C.; Yang, S.-N. Perinatal hypoxic-ischemic encephalopathy. J. Biomed. Biotechnol. 2011, 2011, 609813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankaran, S.; Pappas, A.; McDonald, S.A.; Vohr, B.R.; Hintz, S.R.; Yolton, K.; Gustafson, K.E.; Leach, T.M.; Green, C.; Bara, R.; et al. Childhood Outcomes after Hypothermia for Neonatal Encephalopathy. N. Engl. J. Med. 2012, 366, 2085–2092. [Google Scholar] [CrossRef]
- Mavelli, I.; Rigo, A.; Federico, R.; Ciriolo, M.R.; Rotilio, G. Superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Biochem. J. 1982, 204, 535–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odorcyk, F.K.; Ribeiro, R.T.; Roginski, A.C.; Duran-Carabali, L.E.; Couto-Pereira, N.S.; Dalmaz, C.; Wajner, M.; Netto, C.A. Differential Age-Dependent Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis Induced by Neonatal Hypoxia-Ischemia in the Immature Rat Brain. Mol. Neurobiol. 2021, 58, 2297–2308. [Google Scholar] [CrossRef]
- Jin, Y.; Silverman, A.J.; Vannucci, S.J. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke 2009, 40, 3107–3112. [Google Scholar] [CrossRef] [Green Version]
- Fathali, N.; Ostrowski, R.P.; Hasegawa, Y.; Lekic, T.; Tang, J.; Zhang, J.H. Splenic Immune Cells in Experimental Neonatal Hypoxia-Ischemia. Transl. Stroke Res. 2013, 4, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Y.; Xu, Y.; Pi, G. Effect of intrauterine infection on brain development and injury. Int. J. Dev. Neurosci. 2013, 31, 543–549. [Google Scholar] [CrossRef]
- Davidson, J.O.; Wassink, G.; van den Heuij, L.G.; Bennet, L.; Gunn, A.J. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy—Where to from here? Front. Neurol. 2015, 6, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassink, G.; Davidson, J.O.; Lear, C.A.; Juul, S.E.; Northington, F.; Bennet, L.; Gunn, A.J. A working model for hypothermic neuroprotection. J. Physiol. 2018, 596, 5641–5654. [Google Scholar] [CrossRef] [PubMed]
- Pohl, F.; Lin, P.K.T. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [Green Version]
- Mohsenpour, H.; Pesce, M.; Patruno, A.; Bahrami, A.; Pour, P.M.; Farzaei, M.H. A review of plant extracts and plant-derived natural compounds in the prevention/treatment of neonatal hypoxic-ischemic brain injury. Int. J. Mol. Sci. 2021, 22, 833. [Google Scholar] [CrossRef]
- Wu, M.; Liu, F.; Guo, Q. Quercetin attenuates hypoxia-ischemia induced brain injury in neonatal rats by inhibiting TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 74, 105704. [Google Scholar] [CrossRef]
- Costa, S.L.; Silva, V.D.A.; Souza, C.S.; Santos, C.C.; Paris, I.; Muñoz, P.; Segura-Aguilar, J. Impact of Plant-Derived Flavonoids on Neurodegenerative Diseases. Neurotox. Res. 2016, 30, 41–52. [Google Scholar] [CrossRef]
- Simonyi, A.; Wang, Q.; Miller, R.L.; Yusof, M.; Shelat, P.B.; Sun, A.Y.; Sun, G.Y. Polyphenols in cerebral ischemia. Mol. Neurobiol. 2005, 31, 135–147. [Google Scholar] [CrossRef]
- Odorcyk, F.K.; Sanches, E.F.; Nicola, F.C.; Moraes, J.; Pettenuzzo, L.F.; Kolling, J.; Siebert, C.; Longoni, A.; Konrath, E.L.; Wyse, A.; et al. Administration of Huperzia quadrifariata Extract, a Cholinesterase Inhibitory Alkaloid Mixture, has Neuroprotective Effects in a Rat Model of Cerebral Hypoxia-Ischemia. Neurochem. Res. 2016, 42, 1–11. [Google Scholar] [CrossRef]
- West, T.; Atzev, M.; Holtzman, D.M. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev. Neurosci. 2007, 29, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, X.; Wang, M.; Liu, Y.; Zhao, W.; Ren, X.; Li, Y.; Liu, H.; Gu, Z.; Jia, H.; Liu, J.; et al. Pretreatment of Grape Seed Proanthocyanidin Extract Exerts Neuroprotective Effect in Murine Model of Neonatal Hypoxic-ischemic Brain Injury by Its Antiapoptotic Property. Cell. Mol. Neurobiol. 2019, 39, 953–961. [Google Scholar] [CrossRef]
- Anastácio, J.R.; Netto, C.A.; Castro, C.C.; Sanches, E.F.; Ferreira, D.C.; Noschang, C.; Krolow, R.; Dalmaz, C.; Pagnussat, A. Resveratrol treatment has neuroprotective effects and prevents cognitive impairment after chronic cerebral hypoperfusion. Neurol. Res. 2014, 36, 627–633. [Google Scholar] [CrossRef]
- Anastacio, J.B.R.; Sanches, E.F.; Nicola, F.; Odorcyk, F.; Fabres, R.B.; Netto, C.A. Phytoestrogen coumestrol attenuates brain mitochondrial dysfunction and long-term cognitive deficits following neonatal hypoxia–ischemia. Int. J. Dev. Neurosci. 2019, 79, 86–95. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Lafuente, H.; Rey-Santano, M.C.; Mielgo, V.E.; Gastiasoro, E.; Rueda, M.; Pertwee, R.G.; Castillo, A.I.; Romero, J.; Martínez-Orgado, J. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr. Res. 2008, 64, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, H.; LaRocca, L.L. Brazilian Fruits & Cultivated Exotics (For Consuming in Natura); Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2006; ISBN 8586714240. [Google Scholar]
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Myrtaceae in Lista de Espécies da Flora do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Reynertson, K.A.; Wallace, A.M.; Adachi, S.; Gil, R.R.; Yang, H.; Basile, M.J.; D’Armiento, J.; Weinstein, I.B.; Kennelly, E.J. Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora). J. Nat. Prod. 2006, 69, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Leite-Legatti, A.V.; Batista, A.G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Machado, A.R.T.; de Carvalho-Silva, L.B.; Ruiz, A.L.T.G.; et al. Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.B.; Dastmalchi, K.; Long, C.; Kennelly, E.J. Metabolite profiling of jaboticaba (Myrciaria cauliflora) and other dark-colored fruit juices. J. Agric. Food Chem. 2012, 60, 7513–7525. [Google Scholar] [CrossRef]
- Calloni, C.; Agnol, R.D.; Martínez, L.S.; de Siqueira Marcon, F.; Moura, S.; Salvador, M. Jaboticaba (Plinia trunciflora (O. Berg) Kausel) fruit reduces oxidative stress in human fibroblasts cells (MRC-5). Food Res. Int. 2015, 70, 15–22. [Google Scholar] [CrossRef]
- Sharma, L.K.; Fang, H.; Liu, J.; Vartak, R.; Deng, J.; Bai, Y. Mitochondrial respiratory complex I dysfunction promotes tumorigenesis through ROS alteration and AKT activation. Hum. Mol. Genet. 2011, 20, 4605–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taddei, M.L.; Giannoni, E.; Raugei, G.; Scacco, S.; Sardanelli, A.M.; Papa, S.; Chiarugi, P. Mitochondrial Oxidative Stress due to Complex I Dysfunction Promotes Fibroblast Activation and Melanoma Cell Invasiveness. J. Signal Transduct. 2012, 2012, 684592. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scola, G.; Kim, H.K.; Young, L.T.; Andreazza, A.C. A fresh look at complex i in microarray data: Clues to understanding disease-specific mitochondrial alterations in bipolar disorder. Biol. Psychiatry 2013, 73, e4–e5. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Mizgier, M.L.; Speisky, H.; Gotteland, M. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem. Biol. Interact. 2012, 195, 199–205. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, R.; Shen, G.X. Impact of cyanidin-3-glucoside on glycated LDL-induced NADPH oxidase activation, mitochondrial dysfunction and cell viability in cultured vascular endothelial cells. Int. J. Mol. Sci. 2012, 13, 15867–15880. [Google Scholar] [CrossRef]
- Sacchet, C.; Mocelin, R.; Sachett, A.; Bevilaqua, F.; Chitolina, R.; Kuhn, F.; Boligon, A.A.; Athayde, M.L.; Roman Junior, W.A.; Rosemberg, D.B.; et al. Antidepressant-Like and Antioxidant Effects of Plinia trunciflora in Mice. Evid. Based Complementary Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciência e Tecnol. Aliment. 2005, 25, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Beara, I.N.; Lesjak, M.M.; Jovin, E.D.; Balog, K.J.; Anačkov, G.T.; Orčić, D.Z.; Mimica-Dukić, N.M. Plantain (Plantago L.) species as novel sources of flavonoid antioxidants. J. Agric. Food Chem. 2009, 57, 9268–9273. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Yagi, K. Simple Procedure for Specific Assay of Lipid Hydroperoxides in Serum or Plasma. Methods Mol. Biol. 1998, 108, 107–110. [Google Scholar] [CrossRef]
- Ribeiro, R.T.; Zanatta, Â.; Amaral, A.U.; Leipnitz, G.; de Oliveira, F.H.; Seminotti, B.; Wajner, M. Experimental Evidence that In Vivo Intracerebral Administration of L-2-Hydroxyglutaric Acid to Neonatal Rats Provokes Disruption of Redox Status and Histopathological Abnormalities in the Brain. Neurotox. Res. 2018, 33, 681–692. [Google Scholar] [CrossRef]
- Browne, R.W.; Armstrong, D. Reduced Glutathione and Glutathione Disulfide. Methods Mol. Biol. 1998, 108, 347–352. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Wendel, A. [44] Glutathione peroxidase. Methods Enzymol. 1981, 77, 325–333. [Google Scholar] [CrossRef]
- Ribeiro, R.T.; Seminotti, B.; Zanatta, Â.; de Oliveira, F.H.; Amaral, A.U.; Leipnitz, G.; Wajner, M. Neuronal Death, Glial Reactivity, Microglia Activation, Oxidative Stress and Bioenergetics Impairment Caused by Intracerebroventricular Administration of D-2-hydroxyglutaric Acid to Neonatal Rats. Neuroscience 2021, 471, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Durán-Carabali, L.; Arcego, D.; Sanches, E.; Odorcyk, F.; Marques, M.; Tosta, A.; Reichert, L.; Carvalho, A.S.; Dalmaz, C.; Netto, C. Preventive and therapeutic effects of environmental enrichment in Wistar rats submitted to neonatal hypoxia-ischemia. Behav. Brain Res. 2019, 359, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.F.; Arteni, N.S.; Nicola, F.; Boisserand, L.; Willborn, S.; Netto, C. Early hypoxia-ischemia causes hemisphere and sex-dependent cognitive impairment and histological damage. Neuroscience 2013, 237, 208–215. [Google Scholar] [CrossRef]
- Arteni, N.S.; Pereira, L.O.; Rodrigues, A.L.; Lavinsky, D.; Achaval, M.E.; Netto, C.A. Lateralized and sex-dependent behavioral and morphological effects of unilateral neonatal cerebral hypoxia-ischemia in the rat. Behav. Brain Res. 2010, 210, 92–98. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2013, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Kalt, W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J. Agric. Food Chem. 2010, 58, 3950–3956. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.M.; Soares, M.S.P.; Gutierres, J.M.; Gerzson, M.F.B.; Carvalho, F.B.; Azambuja, J.H.; Schetinger, M.R.C.; Stefanello, F.M.; Spanevello, R.M. Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J. Nutr. Biochem. 2018, 56, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.L.; Simon, J.E.; Lila, M.A.; Rochet, J.-C. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson′s disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, J.; Yu, S.-W.; Baek, S.-H.; Nair, K.M.; Bae, O.-N.; Bhatt, A.; Kassab, M.; Nair, M.G.; Majid, A. Neuroprotective effect of cyanidin-3-O-glucoside anthocyanin in mice with focal cerebral ischemia. Neurosci. Lett. 2011, 500, 157–161. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: An outlined review. Eur. J. Pharmacol. 2019, 858, 172500. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacomo, C.D.; Acquaviva, R.; Santangelo, R.; Sorrenti, V.; Vanella, L.; Volti, G.L.; D’Orazio, N.; Vanella, A.; Galvano, F. Effect of Treatment with Cyanidin-3-O-β-D-Glucoside on Rat Ischemic/Reperfusion Brain Damage. Evid. Based Complementary Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Odorcyk, F.K.; Duran-Carabali, L.E.; Rocha, D.S.; Sanches, E.F.; Martini, A.P.; Venturin, G.T.; Greggio, S.; da Costa, J.C.; Kucharski, L.C.; Zimmer, E.R.; et al. Differential glucose and beta-hydroxybutyrate metabolism confers an intrinsic neuroprotection to the immature brain in a rat model of neonatal hypoxia ischemia. Exp. Neurol. 2020, 330, 113317. [Google Scholar] [CrossRef] [PubMed]
- Gee, C.E.; Benquet, P.; Raineteau, O.; Rietschin, L.; Kirbach, S.W.; Gerber, U. NMDA receptors and the differential ischemic vulnerability of hippocampal neurons. Eur. J. Neurosci. 2006, 23, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Towfighi, J.; Mauger, D.; Vannucci, R.C.; Vannucci, S.J. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: A light microscopic study. Brain Res. Dev. Brain Res. 1997, 100, 149–160. [Google Scholar] [CrossRef]
- Lan, X.B.; Wang, Q.; Yang, J.M.; Ma, L.; Zhang, W.J.; Zheng, P.; Sun, T.; Niu, J.G.; Liu, N.; Yu, J.Q. Neuroprotective effect of Vanillin on hypoxic-ischemic brain damage in neonatal rats. Biomed. Pharmacother. 2019, 118, 109196. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; McCullough, L.D. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol. Sin. 2013, 34, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, M.; Zitta, K.; Groenendaal, F.; van Bel, F.; Peeters-Scholte, C. Neuroprotective strategies following perinatal hypoxia-ischemia: Taking aim at NOS. Free Radic. Biol. Med. 2019, 142, 123–131. [Google Scholar] [CrossRef]
- Li, S.-J.; Liu, W.; Wang, J.-L.; Zhang, Y.; Zhao, D.-J.; Wang, T.-J.; Li, Y.-Y. The role of TNF-α, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 905–909. [Google Scholar] [PubMed]
- Meireles, M.; Marques, C.; Norberto, S.; Santos, P.; Fernandes, I.; Mateus, N.; Faria, A.; Calhau, C. Anthocyanin effects on microglia M1/M2 phenotype: Consequence on neuronal fractalkine expression. Behav. Brain Res. 2016, 305, 223–228. [Google Scholar] [CrossRef]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Chung, J.I.; Kim, M.O. Anthocyanins Improve Hippocampus-Dependent Memory Function and Prevent Neurodegeneration via JNK/Akt/GSK3β Signaling in LPS-Treated Adult Mice. Mol. Neurobiol. 2019, 56, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Mohsen, M.A.E.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P.E. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar] [CrossRef]
- Filloux, F.M.; Adair, J.; Narang, N. The temporal evolution of striatal dopamine receptor binding and mRNA expression following hypoxia-ischemia in the neonatal rat. Dev. Brain Res. 1996, 94, 81–91. [Google Scholar] [CrossRef]
- Nunes Azevedo, P.; Zanirati, G.; Teribele Venturin, G.; Garcia Schu, G.; Elena Durán–Carabali, L.; Kawa Odorcyk, F.; Vinicius Soares, A.; de Oliveira Laguna, G.; Alexandre Netto, C.; Rigon Zimmer, E.; et al. Long-term changes in metabolic brain network drive memory impairments in rats following neonatal hypoxia-ischemia. Neurobiol. Learn. Mem. 2020, 171, 107207. [Google Scholar] [CrossRef]
- Sanches, E.F.; Arteni, N.; Nicola, F.; Aristimunha, D.; Netto, C.A. Sexual dimorphism and brain lateralization impact behavioral and histological outcomes following hypoxia–ischemia in P3 and P7 rats. Neuroscience 2015, 290, 581–593. [Google Scholar] [CrossRef] [PubMed]



| Sham + Sal | HI + Sal | Sham + PTE | HI + PTE | |
|---|---|---|---|---|
| Open Field test | ||||
| Time spent in central zone | 12.76 ± 1.99 | 15.59 ± 2.76 | 16.81 ± 2.49 | 11.46 ± 1.90 |
| Time spent in peripheral zone | 287.2 ± 1.99 | 284.4 ± 2.76 | 283.2 ± 2.49 | 288.3 ± 2.06 |
| Crossing | 114.3 ± 6.74 | 175.2 ± 12.67 *** | 145.4 ± 4.12 | 153.6 ± 7.07 * |
| Plus Maze | ||||
| Time spent in open arms | 47.33 ± 7.00 | 93.4 ± 13.06 * | 53.5 ± 7.19 | 64.17 ± 5.12 |
| Time spent in closed arms | 189.0 ± 9.74 | 155.1 ± 19.1 | 192.7 ± 9.77 | 154.8 ± 11.71 |
| Ratio time in open/closed arms | 0.25 ± 0.04 | 0.75 ± 0.19 * | 0.31 ± 0.05 | 0.45 ± 0.05 |
| Rearings | 6.41 ± 0.58 | 10.6 ± 1.25 * | 8.42 ± 1.05 | 11.83 ± 1.16 * |
| Head dipping | 2.66 ± 0.46 | 7.6 ± 2.17 * | 3.85 ± 0.62 | 5.41 ± 1.39 |
| * Injury effect (HI vs. control) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.V.S.; Ribeiro, R.T.; Durán-Carabali, L.E.; Martini, A.P.R.; Hoeper, E.; Sanches, E.F.; Konrath, E.L.; Dalmaz, C.; Wajner, M.; Netto, C.A. Plinia trunciflora Extract Administration Prevents HI-Induced Oxidative Stress, Inflammatory Response, Behavioral Impairments, and Tissue Damage in Rats. Nutrients 2022, 14, 395. https://doi.org/10.3390/nu14020395
Carvalho AVS, Ribeiro RT, Durán-Carabali LE, Martini APR, Hoeper E, Sanches EF, Konrath EL, Dalmaz C, Wajner M, Netto CA. Plinia trunciflora Extract Administration Prevents HI-Induced Oxidative Stress, Inflammatory Response, Behavioral Impairments, and Tissue Damage in Rats. Nutrients. 2022; 14(2):395. https://doi.org/10.3390/nu14020395
Chicago/Turabian StyleCarvalho, Andrey Vinicios S., Rafael T. Ribeiro, Luz Elena Durán-Carabali, Ana Paula R. Martini, Eduarda Hoeper, Eduardo F. Sanches, Eduardo Luis Konrath, Carla Dalmaz, Moacir Wajner, and Carlos Alexandre Netto. 2022. "Plinia trunciflora Extract Administration Prevents HI-Induced Oxidative Stress, Inflammatory Response, Behavioral Impairments, and Tissue Damage in Rats" Nutrients 14, no. 2: 395. https://doi.org/10.3390/nu14020395
APA StyleCarvalho, A. V. S., Ribeiro, R. T., Durán-Carabali, L. E., Martini, A. P. R., Hoeper, E., Sanches, E. F., Konrath, E. L., Dalmaz, C., Wajner, M., & Netto, C. A. (2022). Plinia trunciflora Extract Administration Prevents HI-Induced Oxidative Stress, Inflammatory Response, Behavioral Impairments, and Tissue Damage in Rats. Nutrients, 14(2), 395. https://doi.org/10.3390/nu14020395







