Genome-Wide Analysis of Disordered Eating Behavior in the Mexican Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Population
2.2. Genome-Wide Typing
2.3. Statistical Analysis
2.3.1. Population Stratification
2.3.2. Genome-Wide Associations Analysis
2.3.3. Functional Prediction
2.3.4. Methylation Quantitative Trait Loci Analysis
2.3.5. Summary Data-Based Mendelian Randomization (SMR)
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berkman, N.D.; Lohr, K.N.; Bulik, C.M. Outcomes of Eating Disorders: A Systematic Review of the Literature. Int. J. Eat. Disord. 2007, 40, 293–309. [Google Scholar] [CrossRef]
- Smink, F.R.E.; van Hoeken, D.; Hoek, H.W. Epidemiology of Eating Disorders: Incidence, Prevalence and Mortality Rates. Curr. Psychiatry Rep. 2012, 14, 406–414. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Melen, S.; Mitchison, D.; Vos, T.; Whiteford, H.; Ferrari, A.J. The Hidden Burden of Eating Disorders: An Extension of Estimates from the Global Burden of Disease Study 2019. Lancet Psychiatry 2021, 8, 320–328. [Google Scholar] [CrossRef]
- Jalali-Farahani, S.; Chin, Y.S.; Mohd Nasir, M.T.; Amiri, P. Disordered Eating and Its Association with Overweight and Health-Related Quality of Life Among Adolescents in Selected High Schools of Tehran. Child Psychiatry Hum. Dev. 2015, 46, 485–492. [Google Scholar] [CrossRef]
- Zeiler, M.; Waldherr, K.; Philipp, J.; Nitsch, M.; Dür, W.; Karwautz, A.; Wagner, G. Prevalence of Eating Disorder Risk and Associations with Health-Related Quality of Life: Results from a Large School-Based Population Screening: Prevalence of Eating Disorder Risk. Eur. Eat. Disord. Rev. 2016, 24, 9–18. [Google Scholar] [CrossRef]
- Vo, M.; Accurso, E.C.; Goldschmidt, A.B.; Le Grange, D. The Impact of DSM-5 on Eating Disorder Diagnoses. Int. J. Eat. Disord. 2017, 50, 578–581. [Google Scholar] [CrossRef]
- Pearson, C.M.; Miller, J.; Ackard, D.M.; Loth, K.A.; Wall, M.M.; Haynos, A.F.; Neumark-Sztainer, D. Stability and Change in Patterns of Eating Disorder Symptoms from Adolescence to Young Adulthood. Int. J. Eat. Disord. 2017, 50, 748–757. [Google Scholar] [CrossRef]
- Wu, X.Y.; Yin, W.Q.; Sun, H.W.; Yang, S.X.; Li, X.Y.; Liu, H.Q. The Association between Disordered Eating and Health-Related Quality of Life among Children and Adolescents: A Systematic Review of Population-Based Studies. PLoS ONE 2019, 14, e0222777. [Google Scholar] [CrossRef]
- Eichen, D.M.; Strong, D.R.; Rhee, K.E.; Rock, C.L.; Crow, S.J.; Epstein, L.H.; Wilfley, D.E.; Boutelle, K.N. Change in Eating Disorder Symptoms Following Pediatric Obesity Treatment. Int. J. Eat. Disord. 2019, 52, 299–303. [Google Scholar] [CrossRef]
- Tozzi, F.; Thornton, L.M.; Klump, K.L.; Fichter, M.M.; Halmi, K.A.; Kaplan, A.S.; Strober, M.; Woodside, D.B.; Crow, S.; Mitchell, J.; et al. Symptom Fluctuation in Eating Disorders: Correlates of Diagnostic Crossover. Am. J. Psychiatry 2005, 162, 732–740. [Google Scholar] [CrossRef]
- Milos, G.; Spindler, A.; Schnyder, U.; Fairburn, C.G. Instability of Eating Disorder Diagnoses: Prospective Study. Br. J. Psychiatry 2005, 187, 573–578. [Google Scholar] [CrossRef]
- Castellini, G.; Lo Sauro, C.; Mannucci, E.; Ravaldi, C.; Rotella, C.M.; Faravelli, C.; Ricca, V. Diagnostic Crossover and Outcome Predictors in Eating Disorders According to DSM-IV and DSM-V Proposed Criteria: A 6-Year Follow-Up Study. Psychosom. Med. 2011, 73, 270–279. [Google Scholar] [CrossRef]
- Stice, E.; Marti, C.N.; Rohde, P. Prevalence, Incidence, Impairment, and Course of the Proposed DSM-5 Eating Disorder Diagnoses in an 8-Year Prospective Community Study of Young Women. J. Abnorm. Psychol. 2013, 122, 445–457. [Google Scholar] [CrossRef]
- Jacobi, C.; Hayward, C.; de Zwaan, M.; Kraemer, H.C.; Agras, W.S. Coming to Terms with Risk Factors for Eating Disorders: Application of Risk Terminology and Suggestions for a General Taxonomy. Psychol. Bull. 2004, 130, 19–65. [Google Scholar] [CrossRef]
- Hilbert, A.; Pike, K.M.; Goldschmidt, A.B.; Wilfley, D.E.; Fairburn, C.G.; Dohm, F.-A.; Walsh, B.T.; Striegel Weissman, R. Risk Factors across the Eating Disorders. Psychiatry Res. 2014, 220, 500–506. [Google Scholar] [CrossRef]
- Horwitz, T.; Lam, K.; Chen, Y.; Xia, Y.; Liu, C. A Decade in Psychiatric GWAS Research. Mol. Psychiatry 2019, 24, 378–389. [Google Scholar] [CrossRef]
- The Brainstorm Consortium; Anttila, V.; Bulik-Sullivan, B.; Finucane, H.K.; Walters, R.K.; Bras, J.; Duncan, L.; Escott-Price, V.; Falcone, G.J.; Gormley, P.; et al. Analysis of Shared Heritability in Common Disorders of the Brain. Science 2018, 360, eaap8757. [Google Scholar] [CrossRef]
- The Price Foundation Collaborative Group; Wang, K.; Zhang, H.; Bloss, C.S.; Duvvuri, V.; Kaye, W.; Schork, N.J.; Berrettini, W.; Hakonarson, H. A Genome-Wide Association Study on Common SNPs and Rare CNVs in Anorexia Nervosa. Mol. Psychiatry 2011, 16, 949–959. [Google Scholar] [CrossRef]
- Boraska, V.; Franklin, C.S.; Floyd, J.A.B.; Thornton, L.M.; Huckins, L.M.; Southam, L.; Rayner, N.W.; Tachmazidou, I.; Klump, K.L.; Treasure, J.; et al. A Genome-Wide Association Study of Anorexia Nervosa. Mol. Psychiatry 2014, 19, 1085–1094. [Google Scholar] [CrossRef]
- Duncan, L.; Yilmaz, Z.; Walters, R.; Goldstein, J.; Anttila, V.; Bulik-Sullivan, B.; Ripke, S.; Thornton, L.; Hinney, A.; Daly, M.; et al. Genome-Wide Association Study Reveals First Locus for Anorexia Nervosa and Metabolic Correlations. Am. J. Psychiatry 2017, 174, 850–858. [Google Scholar] [CrossRef]
- Li, D.; Chang, X.; Connolly, J.J.; Tian, L.; Liu, Y.; Bhoj, E.J.; Robinson, N.; Abrams, D.; Li, Y.R.; Bradfield, J.P.; et al. A Genome-Wide Association Study of Anorexia Nervosa Suggests a Risk Locus Implicated in Dysregulated Leptin Signaling. Sci. Rep. 2017, 7, 3847. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-Wide Association Study Identifies Eight Risk Loci and Implicates Metabo-Psychiatric Origins for Anorexia Nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Hübel, C.; Marzi, S.J.; Breen, G.; Bulik, C.M. Epigenetics in Eating Disorders: A Systematic Review. Mol. Psychiatry 2019, 24, 901–915. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, J.; Zhang, F.; Zhu, Z.; Qi, T.; Zheng, Z.; Lloyd-Jones, L.R.; Marioni, R.E.; Martin, N.G.; Montgomery, G.W.; et al. Integrative Analysis of Omics Summary Data Reveals Putative Mechanisms Underlying Complex Traits. Nat. Commun. 2018, 9, 918. [Google Scholar] [CrossRef]
- Martínez-Magaña, J.J.; Genis-Mendoza, A.D.; Villatoro Velázquez, J.A.; Bustos-Gamiño, M.; Juárez-Rojop, I.E.; Tovilla-Zarate, C.A.; Sarmiento, E.; Saucedo, E.; Rodríguez-Mayoral, O.; Fleiz-Bautista, C.; et al. Genome-Wide Association Study of Psychiatric and Substance Use Comorbidity in Mexican Individuals. Sci. Rep. 2021, 11, 6771. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramos, D.; Martínez-Magaña, J.J.; García, A.R.; Juarez-Rojop, I.E.; Gonzalez-Castro, T.B.; Tovilla-Zarate, C.A.; Sarmiento, E.; López-Narvaez, M.L.; Nicolini, H.; Genis-Mendoza, A.D. Psychiatric Comorbidity in Mexican Adolescents with a Diagnosis of Eating Disorders Its Relationship with the Body Mass Index. Int. J. Environ. Res. Public Health 2021, 18, 3900. [Google Scholar] [CrossRef]
- Martínez-Magaña, J.J.; Genis-Mendoza, A.D.; Villatoro Velázquez, J.A.; Camarena, B.; Martín del Campo Sanchez, R.; Fleiz Bautista, C.; Bustos Gamiño, M.; Reséndiz, E.; Aguilar, A.; Medina-Mora, M.E.; et al. The Identification of Admixture Patterns Could Refine Pharmacogenetic Counseling: Analysis of a Population-Based Sample in Mexico. Front. Pharmacol. 2020, 11, 324. [Google Scholar] [CrossRef]
- Nicolini, H.; Martínez-Magaña, J.J.; Genis-Mendoza, A.D.; Villatoro Velázquez, J.A.; Camarena, B.; Fleiz Bautista, C.; Bustos-Gamiño, M.; Aguilar García, A.; Lanzagorta, N.; Medina-Mora, M.E. Cannabis Use in People With Obsessive-Compulsive Symptomatology: Results From a Mexican Epidemiological Sample. Front. Psychiatry 2021, 12, 664228. [Google Scholar] [CrossRef]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The Eating Attitudes Test: Psychometric Features and Clinical Correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef]
- García-García, E.; Vázquez-Velázquez, V.; López-Alvarenga, J.C.; Arcila-Martínez, D. [Internal validity and diagnostic utility of the Eating Disorder Inventory in Mexican women]. Salud Publica Mex. 2003, 45, 206–210. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Yanovski, S.; Wadden, T.; Wing, R.; Marcus, M.D.; Stunkard, A.; Devlin, M.; Mitchell, J.; Hasin, D.; Horne, R.L. Binge Eating Disorder: Its Further Validation in a Multisite Study. Int. J. Eat. Disord. 1993, 13, 137–153. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Williams, J.B.; Gibbon, M.; First, M.B. The Structured Clinical Interview for DSM-III-R (SCID). I: History, Rationale, and Description. Arch. Gen. Psychiatry 1992, 49, 624–629. [Google Scholar] [CrossRef]
- Endicott, J.; Spitzer, R.L. A Diagnostic Interview: The Schedule for Affective Disorders and Schizophrenia. Arch. Gen. Psychiatry 1978, 35, 837–844. [Google Scholar] [CrossRef]
- Hartz, S.M.; Pato, C.N.; Medeiros, H.; Cavazos-Rehg, P.; Sobell, J.L.; Knowles, J.A.; Bierut, L.J.; Pato, M.T. Comorbidity of Severe Psychotic Disorders With Measures of Substance Use. JAMA Psychiatry 2014, 71, 248. [Google Scholar] [CrossRef]
- Docherty, A.R.; Bigdeli, T.B.; Edwards, A.C.; Bacanu, S.; Lee, D.; Neale, M.C.; Wormley, B.K.; Walsh, D.; O’Neill, F.A.; Riley, B.P.; et al. Genome-Wide Gene Pathway Analysis of Psychotic Illness Symptom Dimensions Based on a New Schizophrenia-Specific Model of the OPCRIT. Schizophr. Res. 2015, 164, 181–186. [Google Scholar] [CrossRef]
- Pato, M.T.; Sobell, J.L.; Medeiros, H.; Abbott, C.; Sklar, B.M.; Buckley, P.F.; Bromet, E.J.; Escamilla, M.A.; Fanous, A.H.; Lehrer, D.S.; et al. The Genomic Psychiatry Cohort: Partners in Discovery. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162, 306–312. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. GigaScience 2015, 4, s13742-015. [Google Scholar] [CrossRef] [PubMed]
- Conomos, M.P.; Miller, M.B.; Thornton, T.A. Robust Inference of Population Structure for Ancestry Prediction and Correction of Stratification in the Presence of Relatedness. Genet. Epidemiol. 2015, 39, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Harry, D. The Human Genome Diversity Project and Its Implications for Indigenous Peoples. Genewatch Bull. Comm. Responsib. Genet. 1996, 10, 8–9. [Google Scholar]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Wang, P.; Tang, B.; Teng, X.; Li, C.; Liu, X.; Zou, D.; Song, S.; Zhang, Z. GWAS Atlas: A Curated Resource of Genome-Wide Variant-Trait Associations in Plants and Animals. Nucleic Acids Res. 2020, 48, D927–D932. [Google Scholar] [CrossRef]
- Domingo-Fernández, D.; Hoyt, C.T.; Bobis-Álvarez, C.; Marín-Llaó, J.; Hofmann-Apitius, M. ComPath: An Ecosystem for Exploring, Analyzing, and Curating Mappings across Pathway Databases. NPJ SYST. BIOL. APPL. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) Project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium Human Genomics. The Genotype-Tissue Expression (GTEx) Pilot Analysis: Multitissue Gene Regulation in Humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.L.; Martínez-Magaña, J.J.; Ruiz-Ramos, D.; García, A.R.; Gonzalez, L.; Tovilla-Zarate, C.A.; Sarmiento, E.; Juárez-Rojop, I.E.; Nicolini, H.; Gonzalez-Castro, T.B.; et al. Individuals Diagnosed with Binge-Eating Disorder Have DNA Hypomethylated Sites in Genes of the Metabolic System: A Pilot Study. Nutrients 2021, 13, 1413. [Google Scholar] [CrossRef]
- Manichaikul, A.; Mychaleckyj, J.C.; Rich, S.S.; Daly, K.; Sale, M.; Chen, W.-M. Robust Relationship Inference in Genome-Wide Association Studies. Bioinform. Oxf. Engl. 2010, 26, 2867–2873. [Google Scholar] [CrossRef]
- Dong, S.; Boyle, A.P. Predicting Functional Variants in Enhancer and Promoter Elements Using RegulomeDB. Hum. Mutat. 2019, 40, 1292–1298. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Hu, H.; Bakshi, A.; Robinson, M.R.; Powell, J.E.; Montgomery, G.W.; Goddard, M.E.; Wray, N.R.; Visscher, P.M.; et al. Integration of Summary Data from GWAS and EQTL Studies Predicts Complex Trait Gene Targets. Nat. Genet. 2016, 48, 481–487. [Google Scholar] [CrossRef]
- Szyf, M.; Bick, J. DNA Methylation: A Mechanism for Embedding Early Life Experiences in the Genome. Child Dev. 2013, 84, 49–57. [Google Scholar] [CrossRef]
- Gibbs, J.R.; van der Brug, M.P.; Hernandez, D.G.; Traynor, B.J.; Nalls, M.A.; Lai, S.-L.; Arepalli, S.; Dillman, A.; Rafferty, I.P.; Troncoso, J.; et al. Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain. PLoS Genet. 2010, 6, e1000952. [Google Scholar] [CrossRef]
- Hannon, E.; Spiers, H.; Viana, J.; Pidsley, R.; Burrage, J.; Murphy, T.M.; Troakes, C.; Turecki, G.; O’Donovan, M.C.; Schalkwyk, L.C.; et al. Methylation Quantitative Trait Loci in the Developing Brain and Their Enrichment in Schizophrenia-Associated Genomic Regions. Nat. Neurosci. 2016, 19, 48–54. [Google Scholar] [CrossRef]
- Pavlides, J.M.W.; Zhu, Z.; Gratten, J.; McRae, A.F.; Wray, N.R.; Yang, J. Predicting Gene Targets from Integrative Analyses of Summary Data from GWAS and EQTL Studies for 28 Human Complex Traits. Genome Med. 2016, 8, 84. [Google Scholar] [CrossRef]
- Piazza, R.; Magistroni, V.; Redaelli, S.; Mauri, M.; Massimino, L.; Sessa, A.; Peronaci, M.; Lalowski, M.; Soliymani, R.; Mezzatesta, C.; et al. SETBP1 Induces Transcription of a Network of Development Genes by Acting as an Epigenetic Hub. Nat. Commun. 2018, 9, 2192. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhou, Q.; Wei, Q.; Li, J.; Feng, C.; Mao, X. SEMG1 May Be the Candidate Gene for Idiopathic Asthenozoospermia. Andrologia 2014, 46, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Shuvalov, O.; Kizenko, A.; Petukhov, A.; Fedorova, O.; Daks, A.; Bottrill, A.; Snezhkina, A.V.; Kudryavtseva, A.V.; Barlev, N. SEMG1/2 Augment Energy Metabolism of Tumor Cells. Cell Death Dis. 2020, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
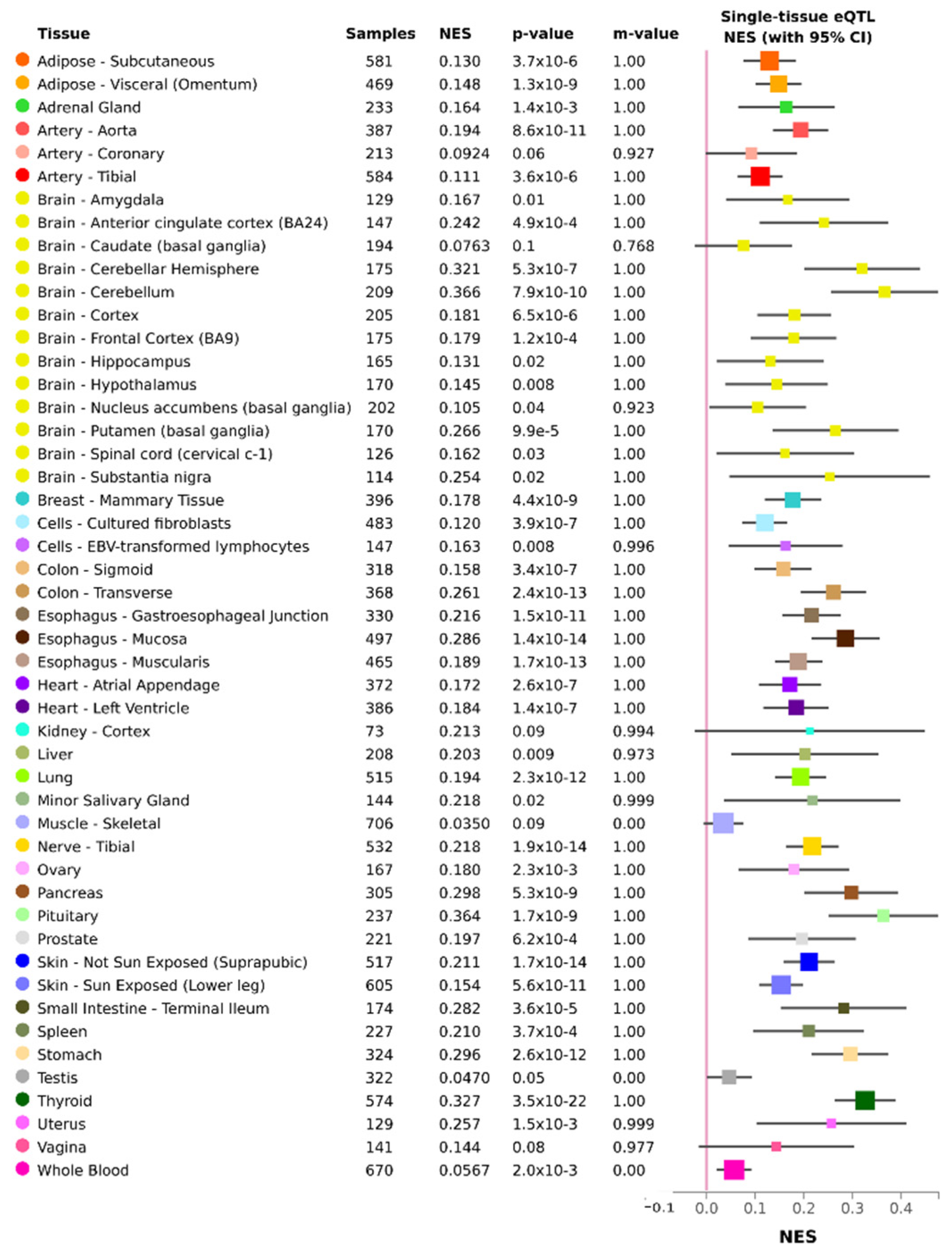
| Characteristic | MeDaCrosR (n = 168) | INPRFM (n = 166) | MxGDAR (n = 1469) |
|---|---|---|---|
| Age, mean (s.d) | 13.96 (1.94) | 19.32 (4.82) | 35.86 (15.77) |
| Gender | |||
| Male, n(%) | 42 (0.25) | 16 (9.64) | 388 (26.41) |
| Female, n(%) | 126 (0.75) | 150 (90.36) | 1081 (73.59) |
| SNP | Band | Position | A1/A2 | MAF Cases | MAF Controls | OR | L95 | U95 | p-Value | Gene | Effect |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs17030129 | 1p36.31 | 1:7059150 | A/G | 0.3787 | 0.3145 | 1.685 | 1.325 | 2.141 | 2.03 × 10−5 | CAMTA1 | Intron |
| rs11120813 | 1:7062993 | A/G | 0.4414 | 0.3720 | 1.646 | 1.306 | 2.075 | 2.45 × 10−5 | |||
| rs6690584 | 1:7078434 | G/T | 0.4401 | 0.3645 | 1.718 | 1.359 | 2.170 | 5.87 × 10−5 | |||
| rs7521204 | 1p36.13 | 1:19138295 | T/C | 0.5329 | 0.4193 | 1.672 | 1.330 | 2.100 | 1.03 × 10−5 | Intergenic | - |
| rs12024738 | 1q31.1 | 1:190694813 | A/G | 0.5285 | 0.4238 | 1.577 | 1.267 | 1.964 | 4.65 × 10−5 | LINC01720 | Intron |
| rs4626924 | 1q42.3 | 1:234909298 | C/T | 0.2260 | 0.2862 | 0.5861 | 0.4536 | 0.7573 | 4.38 × 10−5 | LOC107985364 | |
| rs867286 | 2p21 | 2:45982030 | A/G | 0.4505 | 0.3821 | 1.655 | 1.321 | 2.074 | 1.18 × 10−5 | PRKCE | Intron |
| rs11677196 | 2p12 | 2:75830221 | A/G | 0.2949 | 0.3754 | 0.5947 | 0.4688 | 0.7546 | 1.83 × 10−5 | Intergenic | - |
| rs3205060 | 2q31.1 | 2:175425346 | G/A | 0.4249 | 0.3410 | 1.657 | 1.318 | 2.084 | 1.57 × 10−5 | WIPF1 | 3′-UTR |
| rs7569439 | 2q35 | 2:220590633 | C/T | 0.3091 | 0.3712 | 0.57 | 0.4472 | 0.7266 | 5.67 × 10−6 | Intergenic | - |
| rs35542515 | 4:161798045 | A/C | 0.2733 | 0.2063 | 1.93 | 1.472 | 2.529 | 1.91 × 10−6 | |||
| rs2748991 | 6p12.2 | 6:52596516 | C/T | 0.4234 | 0.3099 | 1.662 | 1.303 | 2.118 | 4.16 × 10−5 | ||
| rs3801220 | 7p14.1 | 7:42247876 | G/A | 0.5494 | 0.4506 | 1.729 | 1.379 | 2.167 | 2.11 × 10−6 | GLI3 | Intron |
| rs3801232 | 7:42253313 | T/C | 0.5284 | 0.4282 | 1.778 | 1.412 | 2.238 | 9.70 × 10−7 | |||
| rs4724100 | 7:42264679 | C/T | 0.5254 | 0.4316 | 1.726 | 1.371 | 2.174 | 3.50 × 10−6 | |||
| rs4507768 | 8q13.3 | 8:70642018 | A/G | 0.1272 | 0.1703 | 0.5027 | 0.3635 | 0.6952 | 3.23 × 10−5 | SLCO5A1 | Intron |
| rs10114881 | 9q21.13 | 9:76676071 | T/C | 0.5254 | 0.4298 | 1.628 | 1.293 | 2.049 | 3.34 × 10−5 | Intergenic | - |
| rs12241514 | 10p12.31 | 10:21602923 | A/G | 0.1257 | 0.2088 | 0.4525 | 0.3293 | 0.6219 | 1.02 × 10−6 | ||
| rs1865020 | 10q22.3 | 10:78688976 | C/T | 0.4566 | 0.3764 | 1.634 | 1.301 | 2.052 | 2.45 × 10−5 | KCNMA1 | Intron |
| rs7918074 | 10q26.3 | 10:134277154 | A/G | 0.2380 | 0.1547 | 1.922 | 1.448 | 2.551 | 6.20 × 10−6 | LOC105378569 | |
| rs10870311 | 10:134290526 | A/C | 0.3228 | 0.2279 | 1.751 | 1.347 | 2.275 | 2.77 × 10−5 | Intergenic | - | |
| rs10772471 | 12p13.2 | 12:11600364 | A/G | 0.3802 | 0.2754 | 1.66 | 1.301 | 2.117 | 4.58 × 10−5 | LOC440084 | Intron |
| rs7297606 | 12q24.3 | 12:119568596 | A/G | 0.1886 | 0.1605 | 1.918 | 1.415 | 2.599 | 2.66 × 10−5 | SRRM4 | Missense (p.Ser243Asn) |
| rs4075945 | 12:119569784 | T/C | 0.1886 | 0.1609 | 1.915 | 1.413 | 2.599 | 2.78 × 10−5 | Intron | ||
| rs12809631 | 12:131045190 | A/C | 0.1467 | 0.1954 | 0.5341 | 0.3992 | 0.7146 | 2.41 × 10−5 | RIMBP2 | ||
| rs2144067 | 14q32.31 | 14:101952406 | T/C | 0.2156 | 0.2330 | 0.5547 | 0.4198 | 0.7330 | 3.42 × 10−5 | Intergenic | - |
| rs1007904 | 14:101955905 | A/G | 0.2380 | 0.2589 | 0.5720 | 0.4370 | 0.7488 | 4.80 × 10−5 | |||
| rs7163468 | 15q12 | 15:26587077 | T/C | 0.2036 | 0.1243 | 1.915 | 1.399 | 2.621 | 4.96 × 10−5 | ||
| rs3922665 | 15:26590830 | G/A | 0.2425 | 0.1552 | 1.885 | 1.413 | 2.514 | 1.60 × 10−5 | |||
| rs8041059 | 15q21.3 | 15:58743709 | T/C | 0.2710 | 0.1999 | 1.732 | 1.329 | 2.256 | 4.72 × 10−5 | LIPC | Intron |
| rs11073665 | 15q25.3 | 15:87295120 | G/A | 0.4027 | 0.3281 | 1.626 | 1.295 | 2.041 | 2.78 × 10−5 | AGBL1 | |
| rs17135764 | 16p13.3 | 16:2111779 | T/C | 0.2440 | 0.3144 | 0.5455 | 0.4249 | 0.7003 | 1.99 × 10−6 | TSC2 | Intron |
| rs11862729 | 16p13.12 | 16:14146098 | G/A | 0.2395 | 0.1789 | 1.809 | 1.364 | 2.4000 | 3.87 × 10−5 | Intergenic | - |
| rs12454763 | 18q12.3 | 18:42434615 | A/G | 0.4102 | 0.3341 | 1.673 | 1.328 | 2.108 | 1.26 × 10−5 | SETBP1 | Intron |
| rs991014 | 18:42439886 | A/G | 0.4096 | 0.3349 | 1.705 | 1.350 | 2.154 | 7.49 × 10−6 | |||
| rs1042122 | 19q13.3 | 19:49989424 | C/T | 0.2769 | 0.3567 | 0.5677 | 0.4447 | 0.7246 | 5.46 × 10−6 | FLT3LG | Missense (p.Phe177Leu) |
| rs10419198 | 19:50038017 | T/C | 0.3084 | 0.3833 | 0.6054 | 0.4798 | 0.7638 | 4.85 × 10−5 | RCN3 | Intron | |
| rs6074170 | 20p12.2 | 20:10671078 | A/G | 0.4162 | 0.3501 | 1.5940 | 1.2730 | 1.9970 | 4.85 × 10−5 | Intergenic | - |
| rs4813048 | 20:11169603 | T/C | 0.2575 | 0.1821 | 1.8440 | 1.3870 | 2.4520 | 2.55 × 10−5 | |||
| rs6043684 | 20p12.1 | 20:16023836 | A/C | 0.4096 | 0.3066 | 1.6330 | 1.2980 | 2.0560 | 2.88 × 10−5 | MACROD2 | Intron |
| rs6104082 | 20q13.12 | 20:43897362 | C/T | 0.3423 | 0.2827 | 1.6750 | 1.3060 | 2.1470 | 4.70 × 10−5 | LOC105372630 | |
| rs2824006 | 21q21.1 | 21:18099779 | C/T | 0.4386 | 0.3584 | 1.5930 | 1.2760 | 1.9900 | 4.00 × 10−5 | Intergenic | - |
| rs2824065 | 21:18187408 | C/T | 0.3997 | 0.2968 | 1.6910 | 1.3380 | 2.1370 | 1.09 × 105 | |||
| rs71330155 | 21:22059184 | A/C | 0.1587 | 0.2234 | 0.5276 | 0.3922 | 0.7098 | 2.38 × 10−5 |
| SNP | CpG | Gene | Location | Beta | SE | p-Value |
|---|---|---|---|---|---|---|
| rs12024738 | cg12412036 | LOC440704 | TSS200 | −0.0869 | 0.0114 | 2.4216 × 10−14 |
| rs12245880 | cg09420738 | 0.1041 | 0.0155 | 1.7351 × 10−11 | ||
| rs12454763 | cg12522870 | SETBP1 | Body | −0.0775 | 0.0109 | 1.6124 × 10−12 |
| rs991014 | −0.0775 | 0.0109 | 1.6124 × 10−12 | |||
| rs10419198 | cg06378142 | PRR12 | Body | −0.3768 | 0.0502 | 6.2212 × 10−14 |
| rs2233903 | cg15921833 | SEMG1 | TSS1500 | 0.2124 | 0.0153 | 5.1650 × 10−44 |
| rs6104082 | 0.1895 | 0.0195 | 2.3470 × 10−22 |
| SNP | CpG | Beta SMR | SE SMR | p-Value SMR |
|---|---|---|---|---|
| rs991014 | cg12522870 | −1.9889 | 0.5852 | 6.7589 × 10−4 |
| rs6104082 | cg15921833 | 0.7180 | 0.2085 | 5.7263 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Magaña, J.J.; Hernandez, S.; Garcia, A.R.; Cardoso-Barajas, V.; Sarmiento, E.; Camarena, B.; Caballero, A.; Gonzalez, L.; Villatoro-Velazquez, J.A.; Medina-Mora, M.E.; et al. Genome-Wide Analysis of Disordered Eating Behavior in the Mexican Population. Nutrients 2022, 14, 394. https://doi.org/10.3390/nu14020394
Martínez-Magaña JJ, Hernandez S, Garcia AR, Cardoso-Barajas V, Sarmiento E, Camarena B, Caballero A, Gonzalez L, Villatoro-Velazquez JA, Medina-Mora ME, et al. Genome-Wide Analysis of Disordered Eating Behavior in the Mexican Population. Nutrients. 2022; 14(2):394. https://doi.org/10.3390/nu14020394
Chicago/Turabian StyleMartínez-Magaña, José Jaime, Sandra Hernandez, Ana Rosa Garcia, Valeria Cardoso-Barajas, Emmanuel Sarmiento, Beatriz Camarena, Alejandro Caballero, Laura Gonzalez, Jorge Ameth Villatoro-Velazquez, Maria Elena Medina-Mora, and et al. 2022. "Genome-Wide Analysis of Disordered Eating Behavior in the Mexican Population" Nutrients 14, no. 2: 394. https://doi.org/10.3390/nu14020394
APA StyleMartínez-Magaña, J. J., Hernandez, S., Garcia, A. R., Cardoso-Barajas, V., Sarmiento, E., Camarena, B., Caballero, A., Gonzalez, L., Villatoro-Velazquez, J. A., Medina-Mora, M. E., Bustos-Gamiño, M., Fleiz-Bautista, C., Tovilla-Zarate, C. A., Juárez-Rojop, I. E., Nicolini, H., & Genis-Mendoza, A. D. (2022). Genome-Wide Analysis of Disordered Eating Behavior in the Mexican Population. Nutrients, 14(2), 394. https://doi.org/10.3390/nu14020394








