Abstract
Carbohydrates play an important role in blood glucose control in pregnant women with GDM. Carbohydrate-restricted dietary (CRD) pattern for gestational diabetes mellitus (GDM) has been widely used in clinics, but the change in insulin utilization rate beyond CRD intervention in GDM remains unclear. The aim of the present study was to explore the application of insulin in pregnancy with GDM, as well as the influence of CRD pattern on lipid metabolism and nutritional state. A retrospective study of 265 women with GDM who delivered in Peking University People’s Hospital from July 2018 to January 2020 was conducted using a questionnaire survey. Women were divided into a CRD group or a control group according to whether they had received CRD intervention during pregnancy. There was no statistically significant difference in the rate of insulin therapy between the two groups (p > 0.05), the initial gestational week of the CRD group combined with insulin treatment was significantly higher than that of the control group (p < 0.05), and the risk of insulin therapy was positively correlated with fasting plasma glucose (FPG) in early pregnancy (p < 0.05). The incidence of abnormal low-density lipoprotein cholesterol levels in the CRD group was significantly lower than that in the control group (p < 0.05). There were no significant differences in nutritional indexes between the two groups. The results indicate that CRD intervention may be effective in delaying the use of insulin and improving the blood lipids metabolism during GDM pregnancy, while nutritional status may not be significantly affected under CRD intervention, and a high FPG in early pregnancy with GDM may be a risk factor for combined insulin therapy with CRD intervention.
1. Introduction
Gestational diabetes mellitus (GDM) is one of the most common complications of pregnancy, and the number of women diagnosed with GDM is increasing worldwide [1]. GDM is a pregnancy-specific disease that harms both maternal and fetal health. Improper management of blood glucose during pregnancy can lead to premature delivery, dystocia, polyhydramnios, macrosomia, fetal growth restriction, fetal distress, fetal death in utero, neonatal respiratory distress syndrome and neonatal hypoglycemia, as well as other adverse pregnancy outcomes. In terms of long-term effects, women with a history of GDM have a significantly increased risk of long-term obesity, diabetes and cardiovascular diseases, and the offspring of GDM patients also have a significantly increased risk of developing type 2 diabetes, obesity and metabolic syndrome, posing a serious threat to the health of mothers and children [2,3].
The etiology of GDM is complicated and remains unclear. GDM might be associated with genetics; changes in hormone levels during pregnancy, which may lead to insulin resistance [4]; elevated levels of inflammatory cytokines [5,6]; and lifestyle changes, including diet and exercise.
Dietary therapy is the cornerstone of diabetes treatment. The effectiveness of medical nutritional therapy (MNT) for GDM has been widely confirmed and has been applied in clinical practice. MNT is a general term for nutritional intervention measures for nutrition-related problems under clinical conditions, including individualized nutritional assessment and the diagnosis of patients and the formulation of corresponding nutritional intervention programs [7]. The purpose of MNT intervention for GDM patients is to correct the existing abnormal glucose and lipid metabolism by adjusting the diet structure and maintaining the normal nutritional status so as to promote patients’ blood glucose to reach the standard and reduce the risk of maternal and infant complications [8]. Most studies have confirmed that about 70~85% of GDM patients can control their blood glucose through simple lifestyle changes [9]. To date, the effectiveness of a carbohydrate-restricted dietary (CRD) pattern as an MNT intervention for the glycemic control of GDM has been verified by some studies, but its impact on the drug treatment rate of GDM remains unclear.
Carbohydrate (CHO) is the macronutrient that has the greatest influence on postprandial blood glucose response. MNT for GDM includes dietary guidance on the distribution, type and quantity of CHOs [10,11,12]. It is customary in China to recommend appropriate limits on the total CHO intake of pregnant women throughout the day, while ensuring full nutrition during pregnancy, including adequate intake of protein, fat and micronutrients. A CRD pattern can be generally defined as a dietary pattern with a relatively low CHO intake [13]. According to the diagnosis and therapy guidelines of pregnancy with diabetes mellitus in China, daily CHO intake <50% E throughout pregnancy was regarded as the CRD pattern in this study [14]. Spreading CHOs throughout the day over multiple meals and snacks may help control postprandial blood glucose levels [10]. Thus, it is recommended that GDM patients eat at least five–six meals a day, that is, two–three snacks a day in addition to three main meals in the morning, noon and evening. The CRD pattern used in the present study included a restricted whole-day CHO intake and a limit of CHO intake at each meal. Evidence based on most studies demonstrates that CRD pattern plays a significant role in the management of GDM, which may improve maternal glycemia and pregnancy outcomes, but the results remain controversial mainly because of the varying degrees of CHO restriction [15,16]. A less restrictive nutritional approach may lead to similar and potentially more favorable outcomes and relieve the anxiety caused by the diagnosis and treatment of the disease [16,17].
There is a close relationship between glucose and lipid metabolism in GDM. Since abnormal lipid metabolism can promote the development of GDM, controlling blood lipid levels in the normal range is beneficial to delay the occurrence of metabolic complications during pregnancy [18]. A meta-analysis of 60 studies showed that triglycerides (TGs) and non-high-density lipoprotein cholesterol were significantly higher and high-density lipoprotein cholesterol (HDLC) levels were significantly lower in women with GDM compared to women with normal pregnancy [19]. Dyslipidemia in GDM may also affect fetal growth, and a prospective study confirmed that maternal TG and free fatty acid levels are independent risk factors for higher gestational age [20]. There are several studies about the relationship between dietary patterns and blood lipids, but few focus on the pregnant population, especially individuals with glucose metabolism disorders. Yancy et al. conducted a 24-week trial to study the effect of a low-CHO diet on blood lipids and found that a low-CHO diet reduced serum TG levels and increased HDLC levels more than the low-fat diet among overweight, hyperlipidemic volunteers [21]. In this study, we intended to determine whether the CRD pattern could help in lipid management in women with GDM.
The CRD pattern might improve the glucolipid metabolism during pregnancy with GDM. Primarily, we aimed to investigate the effect of the CRD pattern on glycemic management in GDM patients by analyzing insulin use. Secondarily, we explored the effect of the CRD pattern on lipid metabolism in GDM patients and assessed the differences in nutritional indicators between patients with and without CRD intervention to observe whether the CRD pattern had an impact on the nutritional status of pregnant women with GDM.
2. Materials and Methods
2.1. Study Design
We conducted a case–control study to investigate the effect of the CRD pattern on glucose-related metabolic and nutritional states in pregnant women with GDM. A total of 265 women with GDM who delivered in Peking University People’s Hospital from July 2018 to January 2020 were included.
Eligible pregnant women aged at least 18 years were identified following diagnosis of GDM based on the 75 g oral glucose tolerance test (OGTT) diagnostic criteria published by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) in 2010 [22]. Between 24 and 28 weeks of gestation, level of blood glucose should be lower than 5.1, 10.0 and 8.5 mmol/L (92, 180 and 153 mg/dL) before and 1 h and 2 h after glucose administration, respectively. Any blood glucose level that meets or exceeds these criteria is diagnosed as GDM. Women with prior history of diabetes, dyslipidemia, serious systemic diseases, severe postpartum complications, abnormal Apgar score in neonates and less than 30 min of activity per day or more than 2 h per day were excluded. Study protocol was approved by the research ethics committee of Peking University People’s Hospital, and informed consent was taken from all participants.
General data were collected using a questionnaire survey, and GDM women were asked whether they were willing to participate in the research-related questionnaire survey during hospital delivery. The questionnaire included age, height, pre-pregnancy weight, prenatal weight, pregnancy weight gain, clinical nutrition experience during pregnancy and specific dietary intervention programs. In addition, data of maternal history, pregnancy history, medical advice during pregnancy, prenatal abdominal circumference, delivery method, birth weight and length of newborn were collected by consulting medical records.
2.2. Intervention
All participants were divided into either a CRD group or control group according to whether they had received a CRD plan from a dietician during pregnancy. According to the questionnaire, they were grouped after answering two questions—“Have you received dietary guidance related to the CRD pattern from a dietician” and “Implementation of the CRD program (50% or less)”. GDM pregnant women who “Received CRD pattern instruction” and “Executed more than 50%” were classified as the CRD group, while those who “Did not receive CRD pattern instruction” and those who received CRD pattern instruction but executed less than 50% were classified as the control group.
Women in the CRD group were required to follow a dietary plan developed by a dietician during their visit to a nutrition clinic at pregnancy. At the time of the first visit, the dietitian had taken measurements of the pregnant women with GDM, including height, weight and abdominal circumference, and conducted a dietary survey (24 h dietary review) and individualized MNT program. In general, the meal plans and the exercise plans included in the MNT programs followed the dietary guidelines for pregnant women with GDM in China.
According to the gestational weeks and individual needs, calorie content of the CRD meal plans were from 1800 to 2200 kcal. Calories were calculated using Henry’s [23] formula for calculating resting energy expenditure (REE), which is based on pre-pregnancy weight and height multiplied by a physical activity level factor. All patients were in their second or third trimester when meeting with a dietician, and an additional 200 kcal were added. According to the recommendation of the Institute of Medicine (IOM) [24], the estimated calorie needs were reduced by 30% if the patient was overweight (pre-pregnancy BMI ≥ 24 kg/m2) or had already reached the standard weight gain. All meal plans provided a total of five to seven meals a day. The overall macronutrient distribution of the CRD group was the same, including 45–50 E% CHO, 25–30 E% fat and 20–25 E% protein, according to a recent systematic review of CRD pattern and Chinese guidelines for GDM diagnosis and treatment [13,14]. Meanwhile, the dietary composition of the control group included 60–65 E% CHO, 20–30 E% fat and 15–20 E% protein. Daily energy intakes of the control group were equal to those of the CRD group, ranging from 1800 to 2200 kcal. After calculating each participant’s individual energy needs, light to moderate activity for 20–30 min after three meals was used to make exercise plans. The principles of MNT followed in this study and meal plans, including the distribution of energy, CHO, fat and protein content during the day, in CRD group are shown in Table 1.

Table 1.
Nutrient structure and diet compositions of CRD pattern.
Although the meal plans were conducted in home settings, the dietitian followed up every patient who received CRD intervention on a regular basis. The GDM patients visited the clinic once a week during the CRD intervention period, which lasted three to four weeks on average. Meal plans with individualized CHO distribution were handed out at the beginning of each intervention period. In each outpatient follow-up, patients were required to carry a 3-day diet diary. Before that, the participants were provided with models of common food items for estimating food weight. All participants were asked to weigh all foods and pictures of the main meals and of the plate if any leftovers were to be taken so that the dietitian could visually assess how much a patient was eating. All meals contained more than one-third of whole-grain products. The participants were asked not to ingest or drink anything besides the food components of the meal plan. In addition, women who were not consulted promptly from the time of GDM diagnosis (24–28 weeks) to the time of delivery were excluded.
2.3. Self-Monitoring of Blood Glucose (SMBG)
Each participant was instructed to carry out SMBG four times a day (before breakfast, 2 h after breakfast, lunch and dinner) with a glucometer. Patients in CRD group recorded the SMBG data in their 3-day food diary as requested by the dietician.
2.4. Laboratory Examination
FPG (fasting plasma glucose), lipid profile (TC (total cholesterol), TG, HDLC and LDLC (low-density lipoprotein cholesterol)) and nutrition-related indicators (HB (hemoglobin), ALB (albumin) and TP (total protein)) were measured during the second and third trimesters. FPG of the first trimester was measured at the first inspection. OGTT was performed at 24 to 28 weeks in all participants.
2.5. Study Outcomes
Insulin treatment rate as the primary outcome was compared between the two groups, as few studies have previously assessed the use of insulin from GDM patients, especially in China. The initial gestational age of insulin use between the two groups and the risk factors for insulin use in the CRD group were further analyzed.
Levels of serum lipid and nutrition indicators during pregnancy under CRD intervention were analyzed as secondary outcomes. This study explored the changes in lipid metabolism and nutritional status, which were associated with glucose metabolism under CRD pattern of GDM pregnancy.
2.6. Sample Size Estimate
The primary clinical endpoint was the difference in insulin utilization rate between intervention group and control group. We used SAS 9.4 to estimate the sample size based on a foreign study on insulin application in pregnant women with GDM under a high-glycemic index (GI) diet and low-GI diet, which showed that the utilization rates of insulin in the two groups were 59% and 29%, respectively [16]. A study size of 264 participants was required (α = 0.05, 1 − β = 0.8).
2.7. Statistical Analysis
SPSS 20.0 software was used for data statistical analysis. Kolmogorov–Smirnov was used for normality test. Measurement data conforming to normal distribution were expressed by ( ± s), while data that did not conform to normal distribution were represented by median (P25~P75), and a non-parametric test was used for comparison between groups. The count data were expressed by the number of cases and rate, and the comparison between groups was performed using a χ2 test, including cases of insulin use and serum lipid parameters. To analyze the initial treatment time of insulin and nutritional status, independent sample T test was used for inter-group comparison. Univariate analysis and binary logistic regression were conducted to analyze the influencing factors of insulin treatment in CRD group. p < 0. 05 was considered statistically significant.
3. Results
3.1. Participants
At the beginning of the study, 353 patients with GDM were invited to fill in the questionnaire to enter the trial; however, 65 participants refused to cooperate, and 23 questionnaires were excluded due to unavailable data. Finally, 265 participants (control group (n = 113) and CRD group (n = 152)) were included in the analysis (Figure 1).
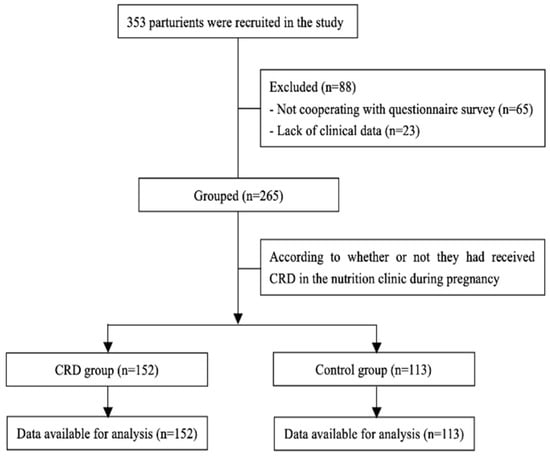
Figure 1.
Flow of participants in the study.
The baseline characteristics of the participants in the two groups are shown in Table 2. Mean height, median of age, pre-pregnancy weight, BMI, gestational weight gain, antenatal abdominal perimeter, delivery mode and infant birth length were not statistically different between the CRD and control groups. The median of infant birthweight and the proportion of parturient women in the CRD group were significantly higher than those in the control group.

Table 2.
Baseline characteristics.
3.2. Differences in Insulin Treatment Rate and Risk Factors of Insulin Use
There was no statistically significant difference in insulin application rate between the two groups. When compared with parturients in the control group, the initial gestational weeks of insulin treatment of parturients in the CRD group were significantly later, as shown in Table 3.

Table 3.
Comparison of insulin treatment rate and the initial gestational weeks.
Univariate analysis was conducted for the CRD group. Compared with the CRD intervention alone, the women treated with insulin were older, had higher FPG in the early and middle trimesters and had higher glucose levels at 60 min of OGTT, as shown in Table 4.

Table 4.
Univariate comparison of risk factors for insulin use in CRD group.
Logistic regression analysis of risk factors for insulin use in the CRD group was performed, showing that GDM mothers with higher FPG in the first trimester had a higher risk of insulin use during pregnancy (OR = 8.203, 95%CI 2.115 to 31.812; p = 0.002).
3.3. Effect of CRD Pattern on Lipid Metabolism
Lipid levels between the CRD group and the control group were compared by T test and no statistically significant difference was found in both the second and third trimesters (Figure 2).
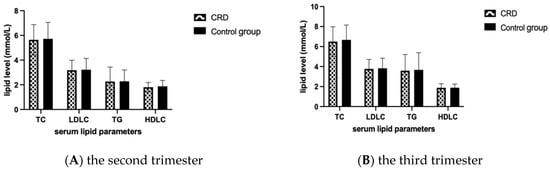
Figure 2.
Comparison of lipid-related indexes between the two groups. Mean and SD of TC, TG, HDLC and LDLC levels in the second and third trimester in each group are presented in panel (A) and (B), respectively. TC, total cholesterol; TG, triglyceride; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol.
According to the χ2 test, there was a statistically significant difference between the CRD group and the control group in the proportion of abnormal LDLC in the second trimester (Table 5).

Table 5.
Comparison of dyslipidemia rate between the two groups.
3.4. Nutrition Status
We conducted a statistical analysis on the nutritional status of pregnant women with GDM in the middle and third trimesters, and we found that there was no statistical difference in TP, ALB or HB between the CRD group and the control group (p > 0.05), as shown in Table 6.

Table 6.
Comparison of nutritional status between the two groups.
4. Discussion
Nutrition is critical to the treatment and prevention of GDM for the health of both mother and offspring. In addition, nutritional intervention of GDM may serve as a starting point for healthy dietary pattern transformation during pregnancy, which is conducive to the persistence of a healthy dietary pattern after delivery and has a protective effect on long-term metabolic diseases, such as type 2 diabetes [10]. Close attention to the amount or type of dietary CHO can have important benefits on the pathophysiology of GDM. As a result, most dietary guidelines currently recommend either to restrict CHO intake or to eat low-GI CHOs instead of those that are more quickly digested [25].
Studies have confirmed that the quantity and type of dietary CHO may have an influence on maternal glucose, and women with GDM were recently advised to limit total intake or choose low-GI CHO by nutritional recommendations [26]. Related studies have set energy restrictions through changing the type or reducing the quantity of CHOs with or without increasing physical activity to slow or reduce weight gain during GDM pregnancy [27]. Although several trials [28] that assessed dietary regimens focusing on CHOs in GDM pregnant women did not show consistency in perinatal outcomes, the meta-analysis by Wan et al. [29], who focused on all Chinese-language studies, showed that CHO-modified dietary intervention strategies were associated with improved glycemic control, as well as maternal and infant outcomes in ethnic Chinese women with GDM.
The relevant guidelines and a number of studies have confirmed that MNT is the initial treatment for GDM, and drug therapy should be performed after MNT has a poor intervention effect [11,12]. Currently, most studies focus on the levels of glucose-related indicators during pregnancy and perinatal outcomes, and they explore the effectiveness of CRD pattern in the blood glucose management of GDM [11]. The use of insulin during pregnancy as an indicator of the effectiveness of CRD intervention was observed in the present study. We explored whether GDM women who had received CRD intervention during pregnancy had a lower insulin treatment rate. Although no significant difference was found in the rate of insulin treatment between the two groups, the onset of insulin treatment in the CRD group was significantly later than that in the control group. A review of the dietary interventions for GDM showed that a low-GI diet characterized by the intake of high-quality complex CHO can reduce insulin utilization and the risk of macrosomia [30]. The use of insulin may be affected by a variety of factors in clinical practice, and the compliance with a CRD regimen in GDM patients also affects the effect of nutritional intervention. The results of this study confirmed the treatment effectiveness for GDM from the perspective that CRD intervention delayed the timing of insulin therapy.
In this study, the risk factors of insulin therapy in the CRD group were preliminarily explored, and it was found that the higher the FPG in the first trimester, the higher the risk of insulin use during GDM pregnancy. Previous studies have shown that women with GDM who fail to achieve good glycemic control through lifestyle changes alone may have a range of characteristics, including baseline characteristics, such as BMI, age and diagnostic parameters of diabetes [31]. Similarly, we found that gestational age, early pregnancy FPG and the level of OGTT 60 min were significantly higher in the CRD combined with insulin group than those in the non-insulin group by univariate analysis. Some studies have speculated that women with GDM who require MNT+ insulin therapy may require more rigorous risk screening and early diagnosis as a special type of GDM [22]. Barnes RA et al. found that gestational age > 30 years, early diagnosis of GDM (<24 weeks of gestation) and fasting venous glucose level (≥5.3 mmol/L) were independent predictors of insulin therapy for GDM [32], which is consistent with the present study. Therefore, early risk control and early identification of GDM risk factors are crucial to improve the effectiveness of GDM intervention.
The glycolipid metabolism of GDM has a certain interaction. In order to maintain the normal development of the fetus and their own energy metabolism, the pregnant women’s ability to absorb fat is significantly enhanced, forming physiological hyperlipidemia [18]. Studies have found that lipid metabolism disorders begin in the early stages of the disease and play an important role in the development of metabolic diseases during pregnancy [33]. We found that CRD intervention can significantly reduce the incidence of an abnormal LDLC level in the middle of GDM pregnancy. This study preliminarily confirmed that CRD pattern plays a certain role in the improvement of the LDLC level of GDM in the second trimester, which is consistent with a series of studies that found that specific forms of MNT intervention can improve lipid metabolism, control weight gain during pregnancy and improve pregnancy outcomes [34].
Adequate nutrition during pregnancy is important for the health of both mother and child. Recent studies showed that, under the conditions of CHO restriction, fuel sources shift from glucose and fatty acids to fatty acids and ketones, and CRD pattern leads to appetite reduction and weight loss [35]. In order to observe whether CRD pattern causes malnutrition in pregnant women with GDM in the study, common nutrition-related indicators of pregnant women in the CRD group and the control group were compared, and no significant statistical differences were found between any indicators. It was preliminarily confirmed that a CRD pattern with 45%–50% E CHO has no risk of malnutrition.
5. Conclusions
In summary, the CRD pattern can effectively delay the use of insulin during pregnancy with GDM, which confirms the effectiveness of CRD pattern in blood glucose management from this perspective. High FPG in early pregnancy may be the most important risk factor for GDM in pregnancy treated with a CRD pattern combined with insulin. CRD pattern may have a certain regulation effect on lipid metabolism during GDM pregnancy, and no obvious adverse effect was found on the nutritional status of pregnant women. Further study about the effect of CRD pattern on other metabolisms of GDM pregnant women is urgently needed.
Author Contributions
Q.G. and M.C. designed and directed the conduction of experiments; M.C. and X.L. performed the experiments; X.L., L.L., S.Z., C.Y. and L.W. were involved in the collection and collation of data; M.C. analyzed the data and wrote the original manuscript; Q.G. and P.L. revised and edited the article content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Peking University People’s Hospital Research and Development Fund (RDX2019-10).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Peking University People’s Hospital (2019PHB263-02, 10 August 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
We thank all the participants in this study. Thanks to the research platform provided by People’s Hospital of Peking University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Srichumchit, S.; Luewan, S.; Tongsong, T. Outcomes of pregnancy with gestational diabetes mellitus. Int. J. Gynecol. Obstet. 2015, 131, 251–254. [Google Scholar] [CrossRef]
- American Diabetes Association. 14. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. S1), S144–S151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, G.L. The Role of Inflammatory Cytokines in Diabetes and Its Complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef]
- Salmi, A.A.; Zaki, M.N.; Zakaria; Aliza, G.N.; Rasool, H.A. Arterial stiffness, inflammatory and pro-atherogenic markers in gestational diabetes mellitus. Vasa 2012, 41, 96–104. [Google Scholar] [CrossRef] [PubMed]
- López-Tinoco, C.; Roca, M.; Fernández-Deudero, A.; García-Valero, A.; Bugatto, F.; Aguilar-Diosdado, M.; Bartha, J. Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus. Cytokine 2012, 58, 14–19. [Google Scholar] [CrossRef]
- Jia, W.; Weng, J.; Zhu, D.; Ji, L.; Lu, J.; Zhou, Z.; Zou, D.; Guo, L.; Ji, Q.; Chen, L.; et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes/Metabolism Res. Rev. 2019, 35, e3158. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Yang, H. Gestational diabetes mellitus, programing and epigenetics. J. Matern. Neonatal Med. 2013, 27, 1266–1269. [Google Scholar] [CrossRef]
- Standards of medical care in diabetes—2015: Summary of revisions. Diabetes Care 2015, 38, S4. [CrossRef] [Green Version]
- Moreno-Castilla, C.; Mauricio, D.; Hernandez, M. Role of Medical Nutrition Therapy in the Management of Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2016, 16, 22. [Google Scholar] [CrossRef]
- Dolatkhah, N.; Hajifaraji, M.; Shakouri, S.K. Nutrition Therapy in Managing Pregnant Women with Gestational Diabetes Mellitus: A Literature Review. J. Fam. Reprod. Heal. 2018, 12, 57–72. [Google Scholar]
- ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [CrossRef]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obstetrics Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association; Group of Pregnancy with Diabetes Mellitus, Chinese Society of Perinatal Medicine, Chinese Medical Association; Obstetrics Subgroup Chinese Society of Obstetrics and Gynecology Chinese Medical Association; Group of Pregnancy with Diabetes Mellitus Chinese Society of Perinatal Medicine Chinese Medical Association. Diagnosis and therapy guideline of pregnancy with diabetes mellitus. Zhonghua Fu Chan Ke Za Zhi 2014, 49, 561–569. [Google Scholar]
- Sweeting, A.; Mijatovic, J.; Brinkworth, G.; Markovic, T.; Ross, G.; Brand-Miller, J.; Hernandez, T. The Carbohydrate Threshold in Pregnancy and Gestational Diabetes: How Low Can We Go? Nutrients 2021, 13, 2599. [Google Scholar] [CrossRef]
- Farabi, S.S.; Hernandez, T.L. Low-Carbohydrate Diets for Gestational Diabetes. Nutrients 2019, 11, 1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, T.L.; Van Pelt, R.E.; Anderson, M.A.; Reece, M.S.; Reynolds, R.M.; de la Houssaye, B.A.; Heerwagen, M.; Donahoo, W.T.; Daniels, L.J.; Chartier-Logan, C.; et al. Women with Gestational Diabetes Mellitus Randomized to a Higher–Complex Carbohydrate/Low-Fat Diet Manifest Lower Adipose Tissue Insulin Resistance, Inflammation, Glucose, and Free Fatty Acids: A Pilot Study. Diabetes Care 2015, 39, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Ma, Y.; Zhang, L.; Li, N.; Zhang, J. The application of metabolomics analysis in the research of gestational diabetes mellitus and preeclampsia. J. Obstet. Gynaecol. Res. 2020, 46, 1310–1318. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Spracklen, C.N.; Smith, C.J.; Robinson, J.G.; Saftlas, A.F. Maternal lipid levels during pregnancy and gestational diabetes: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 643–651. [Google Scholar] [CrossRef]
- Son, G.H.; Kwon, J.Y.; Kim, Y.H.; Park, Y.W. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2010, 89, 700–704. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Olsen, M.K.; Guyton, J.R.; Bakst, R.P.; Westman, E.C. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: A randomized, controlled trial. Ann. Intern. Med. 2004, 140, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Lowe, L.P.; Dyer, A.R.; Oats, J.J.N.; Buchanan, T.A. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy: Response to Weinert. Diabetes Care 2010, 33, e98. [Google Scholar] [CrossRef] [Green Version]
- Henry, C.J. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines; Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar]
- Salto, R.; Manzano, M.; Girón, M.D.; Cano, A.; Castro, A.; Vílchez, J.D.; Cabrera, E.; López-Pedrosa, J.M. A Slow-Digesting Carbohydrate Diet during Rat Pregnancy Protects Offspring from Non-Alcoholic Fatty Liver Disease Risk through the Modulation of the Carbohydrate-Response Element and Sterol Regulatory Element Binding Proteins. Nutrients 2019, 11, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustad, V.A.; Huynh, D.T.; López-Pedrosa, J.M.; Campoy, C.; Rueda, R. The Role of Dietary Carbohydrates in Gestational Diabetes. Nutrients 2020, 12, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louie, J.C.Y.; Markovic, T.P.; Ross, G.P.; Foote, D.; Brand-Miller, J.C. Timing of Peak Blood Glucose after Breakfast Meals of Different Glycemic Index in Women with Gestational Diabetes. Nutrients 2012, 5, 1–9. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Van Pelt, R.E.; Anderson, M.A.; Daniels, L.J.; West, N.A.; Donahoo, W.T.; Friedman, J.E.; Barbour, L.A. A Higher-Complex Carbohydrate Diet in Gestational Diabetes Mellitus Achieves Glucose Targets and Lowers Postprandial Lipids: A Randomized Crossover Study. Diabetes Care 2014, 37, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.S.; Nankervis, A.; Teede, H.; Aroni, R. Dietary intervention strategies for ethnic Chinese women with gestational diabetes mellitus: A systematic review and meta-analysis. Nutr. Diet. 2019, 76, 211–232. [Google Scholar] [CrossRef]
- Mahajan, A.; Donovan, L.E.; Vallee, R.; Yamamoto, J.M. Evidenced-Based Nutrition for Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2019, 19, 94. [Google Scholar] [CrossRef]
- Krispin, E.; Katz, A.A.; Shmuel, E.; Toledano, Y.; Hadar, E. Characterization of women with gestational diabetes who failed to achieve glycemic control by lifestyle modifications. Arch. Gynecol. Obstet. 2021, 303, 677–683. [Google Scholar] [CrossRef]
- Barnes, R.A.; Wong, T.; Ross, G.P.; Jalaludin, B.B.; Wong, V.W.; Smart, C.E.; Collins, C.E.; MacDonald-Wicks, L.; Flack, J.R. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia 2016, 59, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, X.; Chen, T.; Xu, W.; Feng, F.; Zhao, S.; Wang, Z.; Hu, Y.; Xie, B. Maternal lipids, BMI and IL-17/IL-35 imbalance in concurrent gestational diabetes mellitus and preeclampsia. Exp. Ther. Med. 2018, 16, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.K.; Cheng, D.C.; Yang, Y.M.; Wang, X.H.; Chen, Y.; Zhang, L.; Xiu, L.; Xu, X.M. The Role of High-Content Complex Dietary Fiber in Medical Nutrition Therapy for Gestational Diabetes Mellitus. Front Pharmacol. 2021, 12, 684898. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).