Dietary Inflammatory Index and the Risk of Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents
Abstract
:1. Introduction
2. Materials and Methods
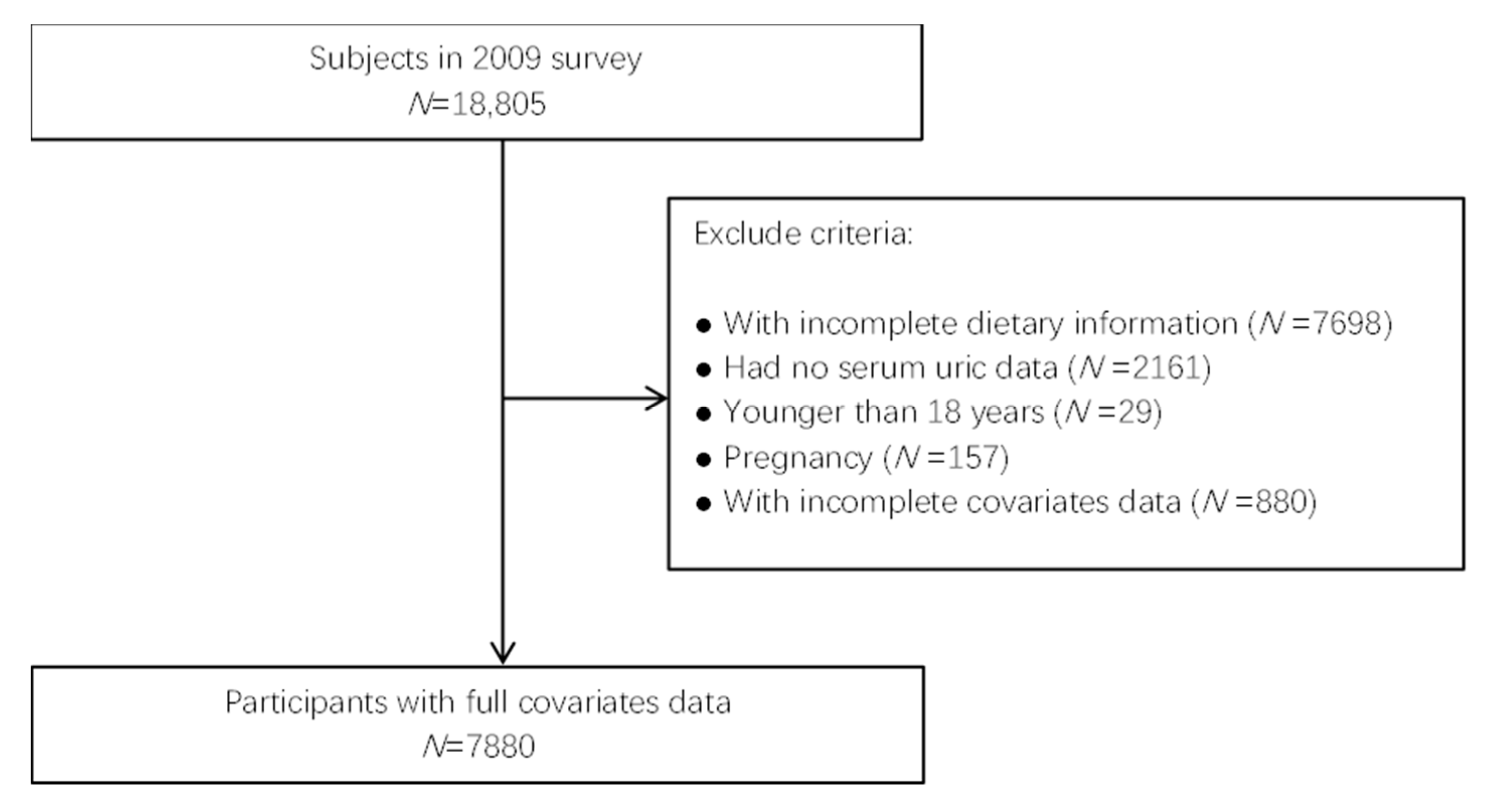
2.1. Data Collection and Samples
2.2. Definition of Hyperuricemia
2.3. Dietary Inflammatory Index
2.4. Assessment of Other Characteristics
2.5. Statistical Analysis
3. Results
3.1. Subjects Characteristics
3.2. Comparison of Dietary Intakes between Non-Hyperuricemia and Hyperuricemia Subjects
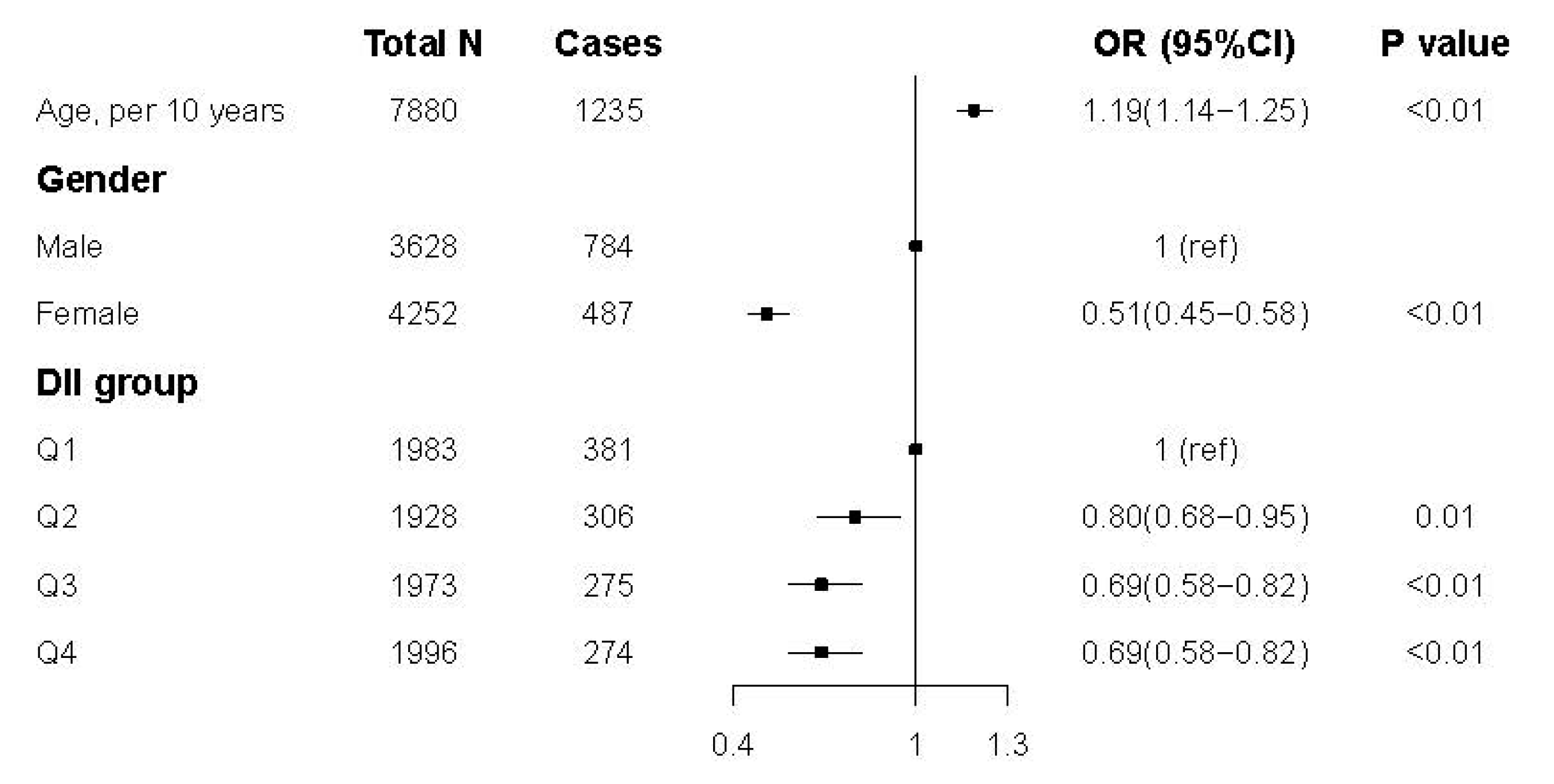
3.3. Association between DII Score and Hyperuricemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rho, Y.H.; Zhu, Y.; Choi, H.K. The epidemiology of uric acid and fructose. Semin. Nephrol. 2011, 31, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.K.; Ford, E.S.; Li, C.; Curhan, G. Prevalence of the metabolic syndrome in patients with gout: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007, 57, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, T.; Chen, J.; Li, Y.; Wang, L.; Huang, H.; Li, J. Hyperuricemia and risk of incident hypertension: A systematic review and meta-analysis of observational studies. PLoS ONE 2014, 9, e114259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, A.; Pan, C.; Wang, H.; Jin, Z.; Lee, J.H.; Wu, Q.; Jiang, Q.; Cui, L. Prevalence and Associated Risk Factors of Chronic Kidney Disease in an Elderly Population from Eastern China. Int. J. Environ. Res. Public Health 2019, 16, 4383. [Google Scholar] [CrossRef] [Green Version]
- Borghi, C.; Rosei, E.A.; Bardin, T.; Dawson, J.; Dominiczak, A.; Kielstein, J.T.; Manolis, A.J.; Perez-Ruiz, F.; Mancia, G. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens. 2015, 33, 1729–1741; discussion 1741. [Google Scholar] [CrossRef]
- Nakanishi, N.; Okamoto, M.; Yoshida, H.; Matsuo, Y.; Suzuki, K.; Tatara, K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur. J. Epidemiol. 2003, 18, 523–530. [Google Scholar] [CrossRef]
- Lu, J.; Hou, X.; Yuan, X.; Cui, L.; Liu, Z.; Li, X.; Ma, L.; Cheng, X.; Xin, Y.; Wang, C.; et al. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int. 2018, 93, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Mena-Sanchez, G.; Babio, N.; Becerra-Tomas, N.; Martinez-Gonzalez, M.A.; Diaz-Lopez, A.; Corella, D.; Zomeno, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; et al. Association between dairy product consumption and hyperuricemia in an elderly population with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 214–222. [Google Scholar] [CrossRef]
- Liu, R.; Han, C.; Wu, D.; Xia, X.; Gu, J.; Guan, H.; Shan, Z.; Teng, W. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2015, 2015, 762820. [Google Scholar] [CrossRef] [Green Version]
- Ota-Kontani, A.; Hirata, H.; Ogura, M.; Tsuchiya, Y.; Harada-Shiba, M. Comprehensive analysis of mechanism underlying hypouricemic effect of glucosyl hesperidin. Biochem. Biophys. Res. Commun. 2020, 521, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Rumpa, N.N.; Paul, S.; Hossen, M.S.; Tanvir, E.M.; Hossan, T.; Saha, M.; Alam, N.; Karim, N.; Khalil, M.I.; et al. Antioxidant Potential, Subacute Toxicity, and Beneficiary Effects of Methanolic Extract of Pomelo (Citrus grandis L. Osbeck) in Long Evan Rats. J. Toxicol. 2019, 2019, 2529569. [Google Scholar] [CrossRef]
- Jimenez-Sousa, M.A.; Lopez, E.; Fernandez-Rodriguez, A.; Tamayo, E.; Fernandez-Navarro, P.; Segura-Roda, L.; Heredia, M.; Gomez-Herreras, J.I.; Bustamante, J.; Garcia-Gomez, J.M.; et al. Genetic polymorphisms located in genes related to immune and inflammatory processes are associated with end-stage renal disease: A preliminary study. BMC Med. Genet. 2012, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, X.; Lee, M.H.; Li, H. Vitamin C alleviates hyperuricemia nephropathy by reducing inflammation and fibrosis. J. Food Sci. 2021, 86, 3265–3276. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, D.; Xu, C.; Wu, Y.; Duan, H.; Li, S.; Tan, Q. Heritability and Genome-Wide Association Analyses of Serum Uric Acid in Middle and Old-Aged Chinese Twins. Front. Endocrinol. (Lausanne) 2018, 9, 75. [Google Scholar] [CrossRef]
- Chang, W.C. Dietary intake and the risk of hyperuricemia, gout and chronic kidney disease in elderly Taiwanese men. Aging Male 2011, 14, 195–202. [Google Scholar] [CrossRef]
- Aihemaitijiang, S.; Zhang, Y.; Zhang, L.; Yang, J.; Ye, C.; Halimulati, M.; Zhang, W.; Zhang, Z. The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients 2020, 12, 3835. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nothlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Neale, E.P.; Batterham, M.J.; Tapsell, L.C. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: A meta-analysis. Nutr. Res. 2016, 36, 391–401. [Google Scholar] [CrossRef]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; Nunes Dos Santos, C.; Pinto, P.; Re, R.; Remond, D.; et al. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 2017, 57, 2497–2525. [Google Scholar] [CrossRef]
- Johansson-Persson, A.; Ulmius, M.; Cloetens, L.; Karhu, T.; Herzig, K.H.; Onning, G. A high intake of dietary fiber influences C-reactive protein and fibrinogen, but not glucose and lipid metabolism, in mildly hypercholesterolemic subjects. Eur. J. Nutr. 2014, 53, 39–48. [Google Scholar] [CrossRef]
- Khayyatzadeh, S.S.; Moohebati, M.; Mazidi, M.; Avan, A.; Tayefi, M.; Parizadeh, S.M.; Ebrahimi, M.; Heidari-Bakavoli, A.; Azarpazhooh, M.R.; Esmaily, H.; et al. Nutrient patterns and their relationship to metabolic syndrome in Iranian adults. Eur. J. Clin. Investig. 2016, 46, 840–852. [Google Scholar] [CrossRef]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hebert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 2021, 23, e13349. [Google Scholar] [CrossRef]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hebert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kwon, M.; Lee, H.Y.; Shivappa, N.; Hebert, J.R.; Sohn, C.; Na, W.; Kim, M.K. Higher Pro-Inflammatory Dietary Score is Associated with Higher Hyperuricemia Risk: Results from the Case-Controlled Korean Genome and Epidemiology Study_Cardiovascular Disease Association Study. Nutrients 2019, 11, 1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Das, G.; Heredia, J.B.; de Lourdes Pereira, M.; Coy-Barrera, E.; Rodrigues Oliveira, S.M.; Gutierrez-Grijalva, E.P.; Cabanillas-Bojorquez, L.A.; Shin, H.S.; Patra, J.K. Korean traditional foods as antiviral and respiratory disease prevention and treatments: A detailed review. Trends Food Sci. Technol. 2021, 116, 415–433. [Google Scholar] [CrossRef]
- Yokose, C.; McCormick, N.; Rai, S.K.; Lu, N.; Curhan, G.; Schwarzfuchs, D.; Shai, I.; Choi, H.K. Effects of Low-Fat, Mediterranean, or Low-Carbohydrate Weight Loss Diets on Serum Urate and Cardiometabolic Risk Factors: A Secondary Analysis of the Dietary Intervention Randomized Controlled Trial (DIRECT). Diabetes Care 2020, 43, 2812–2820. [Google Scholar] [CrossRef]
- Han, Q.X.; Zhang, D.; Zhao, Y.L.; Liu, L.; Li, J.; Zhang, F.; Luan, F.X.; Liu, D.W.; Liu, Z.S.; Cai, G.Y.; et al. Risk Factors For Hyperuricemia In Chinese Centenarians And Near-Centenarians. Clin. Interv. Aging 2019, 14, 2239–2247. [Google Scholar] [CrossRef] [Green Version]
- Barja, G.; Lopez-Torres, M.; Perez-Campo, R.; Rojas, C.; Cadenas, S.; Prat, J.; Pamplona, R. Dietary vitamin C decreases endogenous protein oxidative damage, malondialdehyde, and lipid peroxidation and maintains fatty acid unsaturation in the guinea pig liver. Free Radic. Biol. Med. 1994, 17, 105–115. [Google Scholar] [CrossRef]
- Yanardag, R.; Ozsoy-Sacan, O.; Ozdil, S.; Bolkent, S. Combined effects of vitamin C, vitamin E, and sodium selenate supplementation on absolute ethanol-induced injury in various organs of rats. Int. J. Toxicol. 2007, 26, 513–523. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Yu, X.; Guo, L.; Zhang, X.; Xia, D. The flavonoid-rich fraction from rhizomes of Smilax glabra Roxb. ameliorates renal oxidative stress and inflammation in uric acid nephropathy rats through promoting uric acid excretion. Biomed. Pharmacother 2019, 111, 162–168. [Google Scholar] [CrossRef]
- Haidari, F.; Keshavarz, S.A.; Mohammad Shahi, M.; Mahboob, S.A.; Rashidi, M.R. Effects of Parsley (Petroselinum crispum) and its Flavonol Constituents, Kaempferol and Quercetin, on Serum Uric Acid Levels, Biomarkers of Oxidative Stress and Liver Xanthine Oxidoreductase Aactivity inOxonate-Induced Hyperuricemic Rats. Iran J. Pharm Res. 2011, 10, 811–819. [Google Scholar] [PubMed]
- Lipkowitz, M.S. Regulation of uric acid excretion by the kidney. Curr. Rheumatol. Rep. 2012, 14, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mendez Landa, C.E. Renal Effects of Hyperuricemia. Contrib. Nephrol. 2018, 192, 8–16. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function—Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, S.L.; Singh, J.A. How do dietary interventions affect serum urate and gout? Nat. Rev. Rheumatol. 2021, 17, 191–192. [Google Scholar] [CrossRef]
- Mehmood, A.; Zhao, L.; Wang, C.; Nadeem, M.; Raza, A.; Ali, N.; Shah, A.A. Management of hyperuricemia through dietary polyphenols as a natural medicament: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1433–1455. [Google Scholar] [CrossRef]
- Becerra-Tomas, N.; Mena-Sanchez, G.; Diaz-Lopez, A.; Martinez-Gonzalez, M.A.; Babio, N.; Corella, D.; Freixer, G.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; et al. Cross-sectional association between non-soy legume consumption, serum uric acid and hyperuricemia: The PREDIMED-Plus study. Eur. J. Nutr. 2020, 59, 2195–2206. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hébert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 3628) | Non-Hyperuricemia (n = 2880) | Hyperuricemia (n = 748) | p Value | Total (n = 4252) | Non-Hyperuricemia (n = 3765) | Hyperuricemia | p Value | |
| Age (years) | 50.8(15.0) | 51.0 (15.0) | 50.3 (15.1) | 0.29 | 50.6(14.8) | 49.6 (14.6) | 58.2 (14.0) | |
| DII 1 group 1 | <0.01 | <0.01 | ||||||
| Q1 (−0.49 to 2.30) | 1081 | 828 (77%) | 253 (23%) | 902 | 774 (86%) | 128 (14%) | 0.01 | |
| Q2 (−1.29 to −0.40) | 926 | 718 (78%) | 208 (22%) | 1002 | 904 (90%) | 98 (10%) | ||
| Q3 (−2.09 to −1.30) | 886 | 724 (82%) | 162 (18%) | 1087 | 974 (90%) | 113 (10%) | ||
| Q4 (−4.00 to −2.10) | 735 | 609 (83%) | 126 (17%) | 1261 | 1113 (88%) | 148 (12%) | ||
| Marital status | 0.89 | <0.01 | ||||||
| Single | 279 | 224 (80%) | 55 (20%) | 147 | 136 (93%) | 11 (7%) | ||
| Married | 3129 | 2482 (79%) | 647 (21%) | 3551 | 3175 (89%) | 376 (11%) | ||
| Other | 220 | 173 (79%) | 47 (21%) | 554 | 454 (82%) | 100 (18%) | ||
| Region | <0.01 | <0.01 | ||||||
| Urban | 1516 | 1138 (75%) | 378 (25%) | 1697 | 1453 (86%) | 244 (14%) | ||
| Rural | 2112 | 1741 (82%) | 371 (18%) | 2555 | 2312 (90%) | 243 (10%) | ||
| Education | <0.01 | <0.01 | ||||||
| None | 528 | 429 (81%) | 99 (19%) | 1329 | 1133 (85%) | 196 (15%) | ||
| Primary school | 703 | 568 (81%) | 135 (19%) | 865 | 772 (89%) | 93 (11%) | ||
| Lower middle school | 1375 | 1117 (81%) | 258 (19%) | 1215 | 1099 (90%) | 116 (10%) | ||
| Upper middle school and above | 1022 | 765 (75%) | 257 (25%) | 843 | 761 (90%) | 82 (10%) | ||
| Current smoke status | 0.17 | 0.63 | ||||||
| No | 1381 | 1079 (78%) | 302 (22%) | 4073 | 3609 (89%) | 464 (11%) | ||
| Yes | 2247 | 1800 (80%) | 447 (20%) | 179 | 156 (87%) | 23 (13%) | ||
| BMI 2 (kg/m2) | <0.01 | <0.01 | ||||||
| ≤18.5 | 1935 | 198 (10%) | 18 (1%) | 2284 | 245 (11%) | 16 (1%) | ||
| 18.5–24 | 216 | 1636 (757%) | 299 (138%) | 261 | 2093 (802%) | 191 (73%) | ||
| 24–28 | 1157 | 833 (72%) | 324 (28%) | 1254 | 1084 (86%) | 170 (14%) | ||
| ≥28 | 320 | 212 (66%) | 108 (34%) | 453 | 343 (76%) | 110 (24%) | ||
| Nutrients | Non-Hyperuricemia | Hyperuricemia | p |
|---|---|---|---|
| Energy (kcal) | 1919.38 ± 570.52 | 1879.58 ± 590.00 | 0.03 |
| Carbohydrate (g/1000 kcal) | 159.51 ± 54.98 | 153.51 ± 54.48 | 0.11 |
| Protein (g/1000 kcal) | 38.93 ± 1.49 | 42.44 ± 1.57 | <0.01 |
| Fat (g/1000 kcal) | 21.47 ± 1.30 | 24.41 ± 1.37 | <0.01 |
| Cholesterol (mg/1000 kcal) | 300.77 ± 28.8 | 302.04 ± 28.5 | 0.39 |
| MUFAs 1 (g/1000 kcal) | 3.72 ± 0.32 | 4.10 ± 0.33 | <0.01 |
| PUFAs 2 (g/1000 kcal) | 4.09 ± 0.34 | 4.23 ± 0.33 | 0.02 |
| Saturated fatty acid (g/1000 kcal) | 10.73 ± 2.88 | 11.44 ± 2.84 | <0.01 |
| Fiber (g/1000 kcal) | 10.86 ± 0.99 | 16.64 ± 1.45 | 0.16 |
| Vitamin A (RAE/1000 kcal) | 453.41 ± 77.17 | 484.82 ± 80.47 | 0.10 |
| Vitamin B1 (mg/1000 kcal) | 0.52 ± 0.21 | 0.52 ± 0.20 | 0.10 |
| Vitamin B2 (mg/1000 kcal) | 0.47 ± 0.21 | 0.48 ± 0.21 | <0.01 |
| Vitamin C (mg/1000 kcal) | 48.05 ± 3.05 | 47.97 ± 2.68 | 0.28 |
| Alcohol (g/1000 kcal) | 0.92 ± 0.70 | 1.52 ± 0.56 | <0.01 |
| DII 1 Group | Male | Female |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Q1 | 1 (ref) | 1(ref) |
| Q2 | 1.00 (0.81, 1.24) | 0.63 (0.47, 0.84) |
| Q3 | 0.77 (0.62, 0.97) | 0.64 (0.48, 0.84) |
| Q4 | 0.75 (0.58, 0.95) | 0.66 (0.50, 0.86) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Huang, X.; Wang, R.; Halimulati, M.; Aihemaitijiang, S.; Zhang, Z. Dietary Inflammatory Index and the Risk of Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients 2021, 13, 4504. https://doi.org/10.3390/nu13124504
Ye C, Huang X, Wang R, Halimulati M, Aihemaitijiang S, Zhang Z. Dietary Inflammatory Index and the Risk of Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients. 2021; 13(12):4504. https://doi.org/10.3390/nu13124504
Chicago/Turabian StyleYe, Chen, Xiaojie Huang, Ruoyu Wang, Mairepaiti Halimulati, Sumiya Aihemaitijiang, and Zhaofeng Zhang. 2021. "Dietary Inflammatory Index and the Risk of Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents" Nutrients 13, no. 12: 4504. https://doi.org/10.3390/nu13124504
APA StyleYe, C., Huang, X., Wang, R., Halimulati, M., Aihemaitijiang, S., & Zhang, Z. (2021). Dietary Inflammatory Index and the Risk of Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients, 13(12), 4504. https://doi.org/10.3390/nu13124504








