Hydroethanolic Extract of Prunus domestica L.: Metabolite Profiling and In Vitro Modulation of Molecular Mechanisms Associated to Cardiometabolic Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fruit Extracts
2.2. Total Phenolic Contents
2.3. Antioxidant Assay
2.4. Metabolic Profiling of P. domestica Fruit Pulp Extract
2.4.1. RP-UHPLC-HRMS Analysis
2.4.2. NMR Analysis
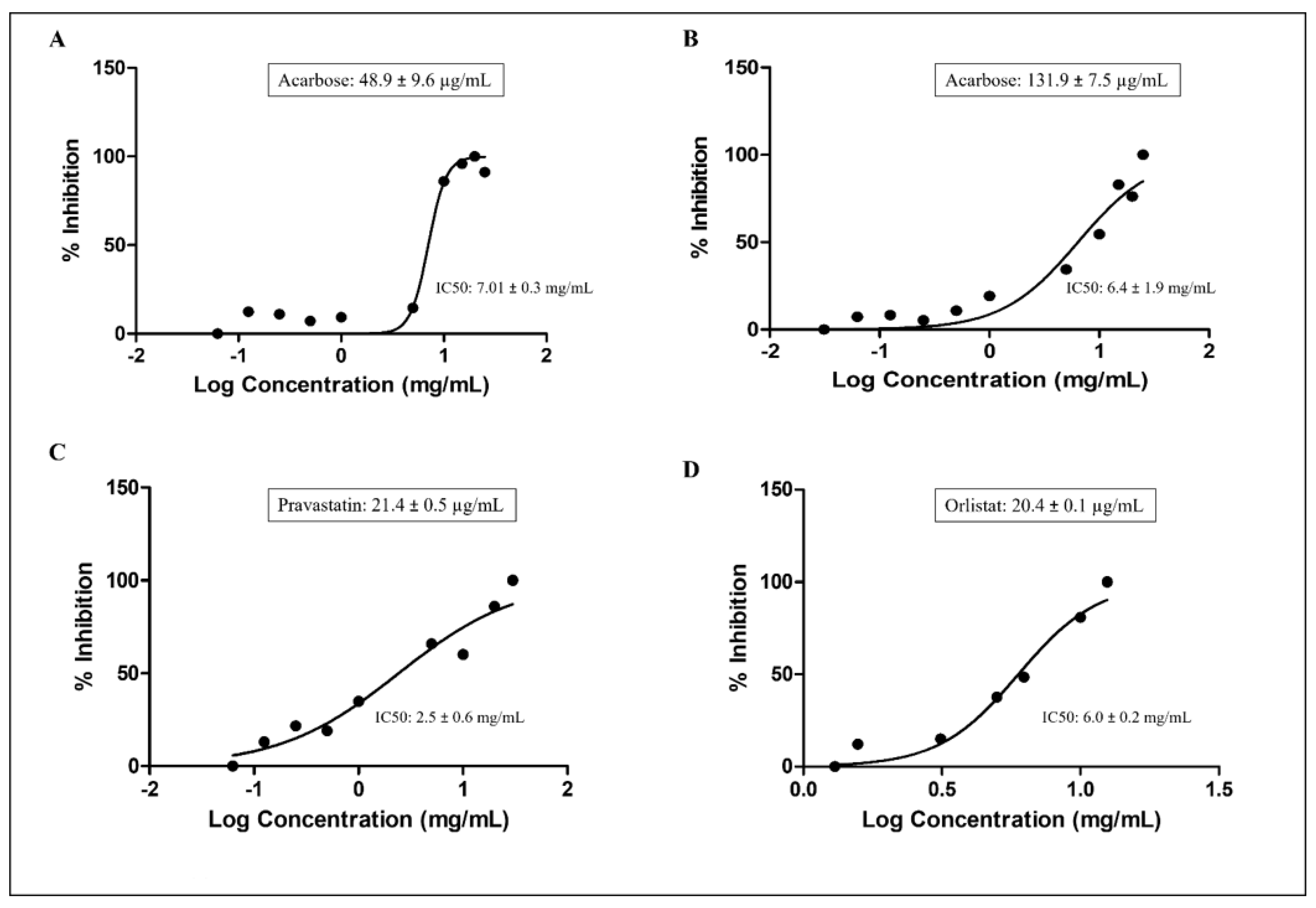
2.5. Enzyme Inhibition Assays
2.5.1. α-Amylase Inhibition Assay
2.5.2. α-Glucosidase Inhibition Assay
2.5.3. HMG-CoA Reductase Inhibition Assay
2.5.4. Pancreatic Lipase Inhibition Assay
2.6. Cell Culture
2.6.1. Nitrite, IL-1β and PGE2 Assay
2.6.2. Cell Viability
2.7. Statistical Analysis
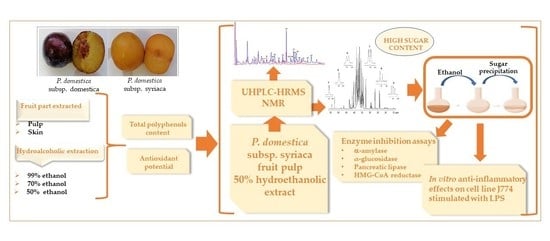
3. Results
3.1. Description of P. domestica Extracts
3.2. Total Phenolic Contents and In Vitro Antioxidant Activity
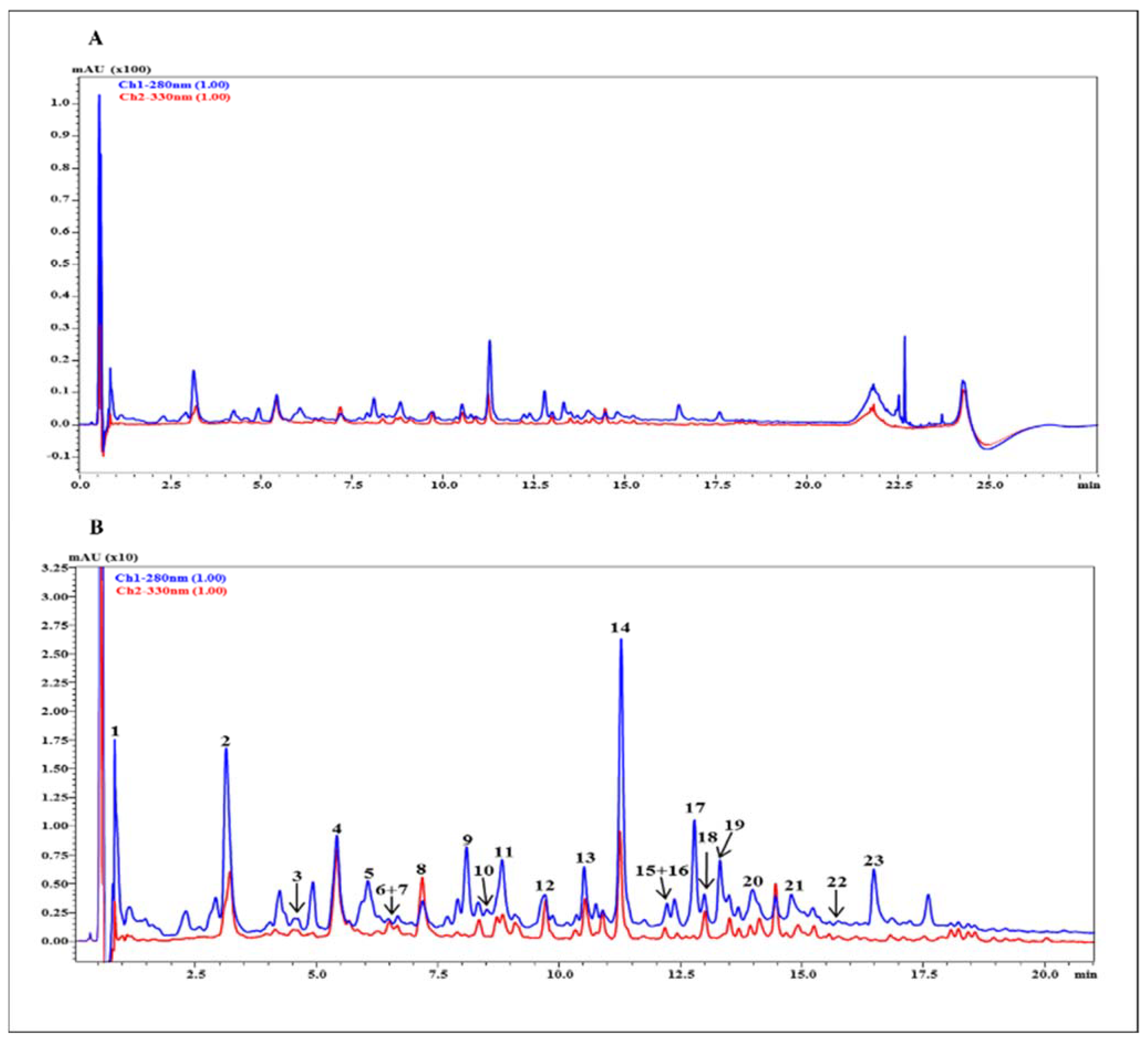
3.3. UHPLC-HRMS Profile
3.4. NMR Analysis and Quantification of Sugar and Organic Acid Contents
3.5. Preparation of P. domestica Fruit Extract without Sugar
3.6. Effect of P. domestica Subsp. Syriaca Fruit Pulp Extract on Enzyme Activities
3.7. In Vitro Anti-Inflammatory Effects of P. domestica Subsp. Syriaca Fruit Pulp Extract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Belwal, T.; Bisht, A.; Devkota, H.P.; Ullah, H.; Khan, H.; Pandey, A.; Bhatt, I.D.; Echeverría, J. Phytopharmacology and Clinical Updates of Berberis Species against Diabetes and Other Metabolic Diseases. Front. Pharmacol. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.A.; Canani, L.H.; Lisbôa, H.R.K.; Tres, G.S.; Gross, J.L. Aggregation of Features of the Metabolic Syndrome Is Associated with Increased Prevalence of Chronic Complications in Type 2 Diabetes. Diabet Med. 2004, 21, 252–255. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the Metabolic Syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Helenius-Hietala, J.; Puukka, P.; Färkkilä, M.; Jula, A. Interaction between Alcohol Consumption and Metabolic Syndrome in Predicting Severe Liver Disease in the General Population. Hepatology 2018, 67, 2141–2149. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.; Foster, M.C.; Anderson, C.A.M.; Burke, G.L.; Haq, N.; Kalyani, R.R.; Ouyang, P.; Sibley, C.T.; Tracy, R.; Woodward, M.; et al. Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 71, 1857–1865. [Google Scholar] [CrossRef]
- Scarpellini, E.; Tack, J. Obesity and Metabolic Syndrome: An Inflammatory Condition. Dig. Dis. 2012, 30, 148–153. [Google Scholar] [CrossRef]
- Magni, P.; Liuzzi, A.; Ruscica, M.; Dozio, E.; Ferrario, S.; Bussi, I.; Minocci, A.; Castagna, A.; Motta, M.; Savia, G. Free and Bound Plasma Leptin in Normal Weight and Obese Men and Women: Relationship with Body Composition, Resting Energy Expenditure, Insulin-sensitivity, Lipid Profile and Macronutrient Preference. Clin. Endocrinol. 2005, 62, 189–196. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory Cytokines Il-6 and TNF-α and the Development of Inflammation in Obese Subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar] [CrossRef]
- Tangvarasittichai, S.; Pongthaisong, S.; Tangvarasittichai, O. Tumor Necrosis Factor-A, Interleukin-6, C-Reactive Protein Levels and Insulin Resistance Associated with Type 2 Diabetes in Abdominal Obesity Women. Indian J. Clin. Biochem. 2016, 31, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Roy, S.; Rahaman, M. Epidemiological Predictors of Metabolic Syndrome in Urban West Bengal, India. J. Family Med. Prim. Care 2015, 4, 535. [Google Scholar] [CrossRef]
- de Filippis, A.; Ullah, H.; Baldi, A.; Dacrema, M.; Esposito, C.; Garzarella, E.U.; Santarcangelo, C.; Tantipongpiradet, A.; Daglia, M. Gastrointestinal Disorders and Metabolic Syndrome: Dysbiosis as a Key Link and Common Bioactive Dietary Components Useful for Their Treatment. Int. J. Mol. Sci. 2020, 21, 4929. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.; Ryan, D.A.; Celzo, M.F.; Stapleton, D. Metabolic Syndrome: Definition and Therapeutic Implications. Postgrad. Med. 2012, 124, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rask Larsen, J.; Dima, L.; Correll, C.U.; Manu, P. The Pharmacological Management of Metabolic Syndrome. Expert Rev. Clin. Pharmacol. 2018, 11, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Matfin, G. Developing Therapies for the Metabolic Syndrome: Challenges, Opportunities, and the Unknown. Ther. Adv. Endocrinol. Metab. 2010, 1, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Gallelli, L.; Cannataro, R.; Perri, M.; Calignano, A.; Citraro, R.; Russo, E.; Gareri, P.; Corsonello, A.; de Sarro, G. When Nutraceuticals Reinforce Drugs Side Effects: A Case Report. Curr. Drug Saf. 2016, 11, 264–266. [Google Scholar] [CrossRef]
- Li, X.T.; Liao, W.; Yu, H.J.; Liu, M.W.; Yuan, S.; Tang, B.W.; Yang, X.H.; Song, Y.; Huang, Y.; le Cheng, S.; et al. Combined Effects of Fruit and Vegetables Intake and Physical Activity on the Risk of Metabolic Syndrome among Chinese Adults. PLoS ONE 2017, 12, e0188533. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific Opinion on the Safety of Monacolins in Red Yeast Rice. EFSA J. 2018, 16, e05368. [Google Scholar]
- Birari, R.B.; Bhutani, K.K. Pancreatic Lipase Inhibitors from Natural Sources: Unexplored Potential. Drug Discov. 2007, 12, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Lin, S.-H.; Huang, K.-J.; Weng, C.-F.; Shiuan, D. Exploration of Natural Product Ingredients as Inhibitors of Human HMG-CoA Reductase through Structure-Based Virtual Screening. Drug Des. Devel. Ther. 2015, 9, 3313. [Google Scholar] [PubMed]
- Young Shin, J.; Young Kim, J.; Tak Kang, H.; Hwa Han, K.; Yong Shim, J. Effect of Fruits and Vegetables on Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Food Sci. Nutr. 2015, 66, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lim, M.; Kim, J. Fruit and Vegetable Consumption and the Metabolic Syndrome: A Systematic Review and Dose-Response Meta-Analysis. Br. J. Nutr. 2019, 122, 723–733. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; El-Ansary, A.E.; Mostafa, M.A.; Kamel, T.A.; Safwat, G. Evaluation of the Phytochemical, Antioxidant, Antibacterial and Anticancer Activity of Prunus Domestica Fruit. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 395–404. [Google Scholar] [CrossRef]
- Igwe, E.O.; Charlton, K.E. A Systematic Review on the Health Effects of Plums (Prunus domestica and Prunus salicina). Phytother. Res. 2016, 30, 701–731. [Google Scholar] [CrossRef]
- Furchner-Evanson, A.; Petrisko, Y.; Howarth, L.; Nemoseck, T.; Kern, M. Type of Snack Influences Satiety Responses in Adult Women. Appetite 2010, 54, 564–569. [Google Scholar] [CrossRef]
- Nilsson, A.; Ostman, E.; Holst, J.; Björck, I. Including Indigestible Carbohydrates in the Evening Meal of Healthy Subjects Improves Glucose Tolerance, Lowers Inflammatory Markers, and Increases Satiety after a Subsequent Standardized Breakfast. J. Nutr. 2008, 138, 732–739. [Google Scholar] [CrossRef]
- di Matteo, G.; Spano, M.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L.; Ingallina, C.; Sobolev, A.P. NMR Characterization of Ten Apple Cultivars from the Piedmont Region. Foods 2021, 10, 289. [Google Scholar] [CrossRef]
- Ingallina, C.; Spano, M.; Sobolev, A.P.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L. Characterization of Local Products for Their Industrial Use: The Case of Italian Potato Cultivars Analyzed by Untargeted and Targeted Methodologies. Foods 2020, 9, 1216. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kok, J.M.L.; Jee, J.M.; Chew, L.Y.; Wong, C.L. The Potential of the Brown Seaweed Sargassum Polycystum against Acne Vulgaris. J. Appl. Phycol. 2016, 28, 3127–3133. [Google Scholar] [CrossRef]
- Mass Bank of North America. Available online: https://mona.fiehnlab.ucdavis.edu/ (accessed on 18 July 2021).
- Sirius. Available online: https://bio.informatik.uni-jena.de/software/sirius/ (accessed on 18 July 2021).
- Cicolari, S.; Dacrema, M.; Tsetegho Sokeng, A.J.; Xiao, J.; Atchan Nwakiban, A.P.; di Giovanni, C.; Santarcangelo, C.; Magni, P.; Daglia, M. Hydromethanolic Extracts from Adansonia digitata L. Edible Parts Positively Modulate Pathophysiological Mechanisms Related to the Metabolic Syndrome. Molecules 2020, 25, 2858. [Google Scholar] [CrossRef] [PubMed]
- Nwakiban, A.P.A.; Sokeng, A.J.; Dell’Agli, M.; Bossi, L.; Beretta, G.; Gelmini, F.; Tchamgoue, A.D.; Agbor, G.A.; Kuiaté, J.R.; Daglia, M.; et al. Hydroethanolic Plant Extracts from Cameroon Positively Modulate Enzymes Relevant to Carbohydrate/Lipid Digestion and Cardio-Metabolic Diseases. Food Funct. 2019, 10, 6533–6542. [Google Scholar] [CrossRef]
- Galuppo, M.; Rossi, A.; Giacoppo, S.; Pace, S.; Bramanti, P.; Sautebin, L.; Mazzon, E. Use of Mometasone Furoate in Prolonged Treatment of Experimental Spinal Cord Injury in Mice: A Comparative Study of Three Different Glucocorticoids. Pharmacol. Res. 2015, 99, 316–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Capitani, D.; Sobolev, A.P.; Delfini, M.; Vista, S.; Antiochia, R.; Proietti, N.; Bubici, S.; Ferrante, G.; Carradori, S.; Salvador, F.R.D.; et al. NMR Methodologies in the Analysis of Blueberries. Electrophoresis 2014, 35, 1615–1626. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Viel, S. Liquid State 1H High Field NMR in Food Analysis. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 66, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Capitani, D.; Mannina, L.; Proietti, N.; Sobolev, A.P.; Tomassini, A.; Miccheli, A.; di Cocco, M.E.; Capuani, G.; de Salvador, R.; Delfini, M. Monitoring of Metabolic Profiling and Water Status of Hayward Kiwifruits by Nuclear Magnetic Resonance. Talanta 2010, 82, 1826–1838. [Google Scholar] [CrossRef]
- WHO Hypertension. Available online: https://www.who.int/ne%0Aws-room/fact-sheets/detail/hypertension (accessed on 23 June 2021).
- WHO Diabetes. Available online: https://www.who.int/news-roo%0Am/fact-sheets/detail/diabetes (accessed on 23 June 2021).
- WHO Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 23 June 2021).
- Mandlekar, S.; Hong, J.L.; Tony Kong, A.N. Modulation of Metabolic Enzymes by Dietary Phytochemicals: A Review of Mechanisms Underlying Beneficial versus Unfavorable Effects. Curr. Drug Metab. 2006, 7, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; de Filippis, A.; Khan, H.; Xiao, J.; Daglia, M. An Overview of the Health Benefits of Prunus Species with Special Reference to Metabolic Syndrome Risk Factors. Food Chem. Toxicol. 2020, 144, 111574. [Google Scholar] [CrossRef] [PubMed]
- Moracci, L.; Traldi, P.; Agostini, M. Mass Spectrometry for a Holistic View of Natural Extracts of Phytotherapeutic Interest. Mass Spectrom. Rev. 2020, 39, 553–573. [Google Scholar] [CrossRef]
- Kumar Singla, R.; Singh, R.; Kumar Dubey, A. Important Aspects of Post-Prandial Antidiabetic Drug, Acarbose. Curr. Top. Med. Chem. 2016, 16, 2625–2633. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of Phytochemicals, Antioxidant Capacity, and Antidiabetic Activity of Novel Smoothies from Selected Prunus Fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Zhou, B.; Li, H.; Zeng, J.; Gao, W. Anti-Diabetic Activity in Type 2 Diabetic Mice and α-Glucosidase Inhibitory, Antioxidant and Anti-Inflammatory Potential of Chemically Profiled Pear Peel and Pulp Extracts (Pyrus spp.). J. Funct. Foods 2015, 13, 276–288. [Google Scholar] [CrossRef]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry Components Inhibit α-Glucosidase in Vitro: Synergies between Acarbose and Polyphenols from Black Currant and Rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Nor-Liyana, J.; Siroshini, K.T.; Nurul-Syahirah, M.B.; Chang, W.L.; Nurul-Husna, S.; Daryl, J.A.; Khairul-Kamilah, A.K.; Hasnah, B. Phytochemical Analysis of Elateriospermum Tapos and Its Inhibitory Effects on Alpha-Amylase, Alpha-Glucosidase and Pancreatic Lipase. J. Trop. For. Sci. 2019, 31, 240–248. [Google Scholar]
- Popović, B.M.; Blagojević, B.; Kucharska, A.Z.; Agić, D.; Magazin, N.; Milović, M.; Serra, A.T. Exploring Fruits from Genus Prunus as a Source of Potential Pharmaceutical Agents–In Vitro and in Silico Study. Food Chem. 2021, 358, 129812. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; de Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the Special Contribution of the Europea. Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef]
- Susilowati, R.; Jannah, J.; Maghfuroh, Z.; Kusuma, M.T. Antihyperlipidemic Effects of Apple Peel Extract in High-Fat Diet-Induced Hyperlipidemic Rats. J. Adv. Pharm. Technol. Res. 2020, 11, 128. [Google Scholar] [CrossRef]
- Baskaran, G.; Salvamani, S.; Ahmad, S.A.; Shaharuddin, N.A.; Pattiram, P.D.; Shukor, M.Y. HMG-CoA Reductase Inhibitory Activity and Phytocomponent Investigation of Basella Alba Leaf Extract as a Treatment for Hypercholesterolemia. Drug Des. Devel. Ther. 2015, 9, 509. [Google Scholar] [CrossRef]
- Hartanti, L.; Yonas, S.M.K.; Mustamu, J.J.; Wijaya, S.; Setiawan, H.K.; Soegianto, L. Influence of Extraction Methods of Bay Leaves (Syzygium Polyanthum) on Antioxidant and HMG-CoA Reductase Inhibitory Activity. Heliyon 2019, 5, e01485. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, D.; Khan, M.S.; Khan, A.; Khan, M.; Ahmad, S.; Srivastava, A.K.; Bagga, P. In Vitro Screening for β-Hydroxy-β-Methylglutaryl-Coa Reductase Inhibitory and Antioxidant Activity of Sequentially Extracted Fractions of Ficus Palmata Forsk. Biomed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Salvamani, S.; Gunasekaran, B.; Shukor, M.Y.; Shaharuddin, N.A.; Sabullah, M.K.; Ahmad, S.A. Anti-HMG-CoA Reductase, Antioxidant, and Anti-Inflammatory Activities of Amaranthus Viridis Leaf Extract as a Potential Treatment for Hypercholesterolemia. Evid. Based Complement. Alternat. Med. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Pilitsi, E.; Farr, O.M.; Polyzos, S.A.; Perakakis, N.; Nolen-Doerr, E.; Papathanasiou, A.E.; Mantzoros, C.S. Pharmacotherapy of Obesity: Available Medications and Drugs under Investigation. Metabolism 2019, 92, 170–192. [Google Scholar] [CrossRef]
- FDA Orlistat (Marketed as Alli and Xenical) Information. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/orlistat-marketed-alli-and-xenical-information (accessed on 2 August 2021).
- FDA FDA Drug Safety Communication: Completed Safety Review of Xenical/Alli (Orlistat) and Severe Liver Injury. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-completed-safety-review-xenicalalli-orlistat-and-severe-liver-injury (accessed on 2 August 2021).
- European Medicines Agency European Medicines Agency Starts Review of Orlistat-Containing Medicines. Available online: https://www.ema.europa.eu/en/news/european-medicines-agency-starts-review-orlistat-containing-medicines (accessed on 2 August 2021).
- Buchholz, T.; Melzig, M.F. Polyphenolic Compounds as Pancreatic Lipase Inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Stewart, D. The Inhibitory Effects of Berry Polyphenols on Digestive Enzymes. Biofactors 2005, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gholamhoseinian, A.; Shahouzehi, B.; Sharifi-Far, F. Inhibitory Effect of Some Plant Extracts on Pancreatic Lipase. Int. J. Pharmacol. 2010, 6, 18–24. [Google Scholar] [CrossRef]
- Jaradat, N.; Zaid, A.N.; Hussein, F.; Zaqzouq, M.; Aljammal, H.; Ayesh, O. Anti-Lipase Potential of the Organic and Aqueous Extracts of Ten Traditional Edible and Medicinal Plants in Palestine; a Comparison Study with Orlistat. Medicines 2017, 4, 89. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Laskowski, P. Inhibitory Potential against Digestive Enzymes Linked to Obesity and Type 2 Diabetes and Content of Bioactive Compounds in 20 Cultivars of the Peach Fruit Grown in Poland. Plant Foods Hum. Nutr. 2018, 73, 314–320. [Google Scholar] [CrossRef]
- Welty, F.K.; Alfaddagh, A.; Elajami, T.K. Targeting Inflammation in Metabolic Syndrome. Transl. Res. 2016, 167, 257–280. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Michalska-Ciechanowska, A.; Martinez-Rodriguez, A.J. Modulation of Antibacterial, Antioxidant, and Anti-Inflammatory Properties by Drying of Prunus domestica L. Plum Juice Extracts. Microorganisms 2020, 8, 119. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kumar, A.; Zhang, J.Y.; Johnson, S.A.; Chai, S.C.; Arjmandi, B.H. Evidence for Anti-Inflammatory and Antioxidative Properties of Dried Plum Polyphenols in Macrophage RAW 264.7 Cells. Food Funct. 2015, 6, 1719–1725. [Google Scholar] [CrossRef]
- Sunil, M.A.; Sunitha, V.S.; Santhakumaran, P.; Mohan, M.C.; Jose, M.S.; Radhakrishnan, E.K.; Mathew, J. Protective Effect of (+)–Catechin against Lipopolysaccharide-Induced Inflammatory Response in RAW 264.7 Cells through Downregulation of NF-ΚB and P38 MAPK. Inflammopharmacology 2021, 29, 1139–1155. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.Y.; Son, D.J.; Lee, H.; Yoo, H.S.; Song, S.; Oh, K.W.; Han, D.C.; Kwon, B.M.; Hong, J.T. Inhibitory Effect of 2′-Hydroxycinnamaldehyde on Nitric Oxide Production through Inhibition of NF-ΚB Activation in RAW 264.7 Cells. Biochem. Pharmacol. 2005, 69, 791–799. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, Y.; Su, X.; Liu, K.; Zhang, Y.; Pang, W.; Wang, J. Inonotus Sanghuang Polyphenols Attenuate Inflammatory Response via Modulating the Crosstalk between Macrophages and Adipocytes. Front. Immunol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Overman, A.; Bumrungpert, A.; Kennedy, A.; Martinez, K.; Chuang, C.C.; West, T.; Dawson, B.; Jia, W.; McIntosh, M. Polyphenol-Rich Grape Powder Extract (GPE) Attenuates Inflammation in Human Macrophages and in Human Adipocytes Exposed to Macrophage-Conditioned Media. Int. J. Obes. 2010, 34, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczyński, R. Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
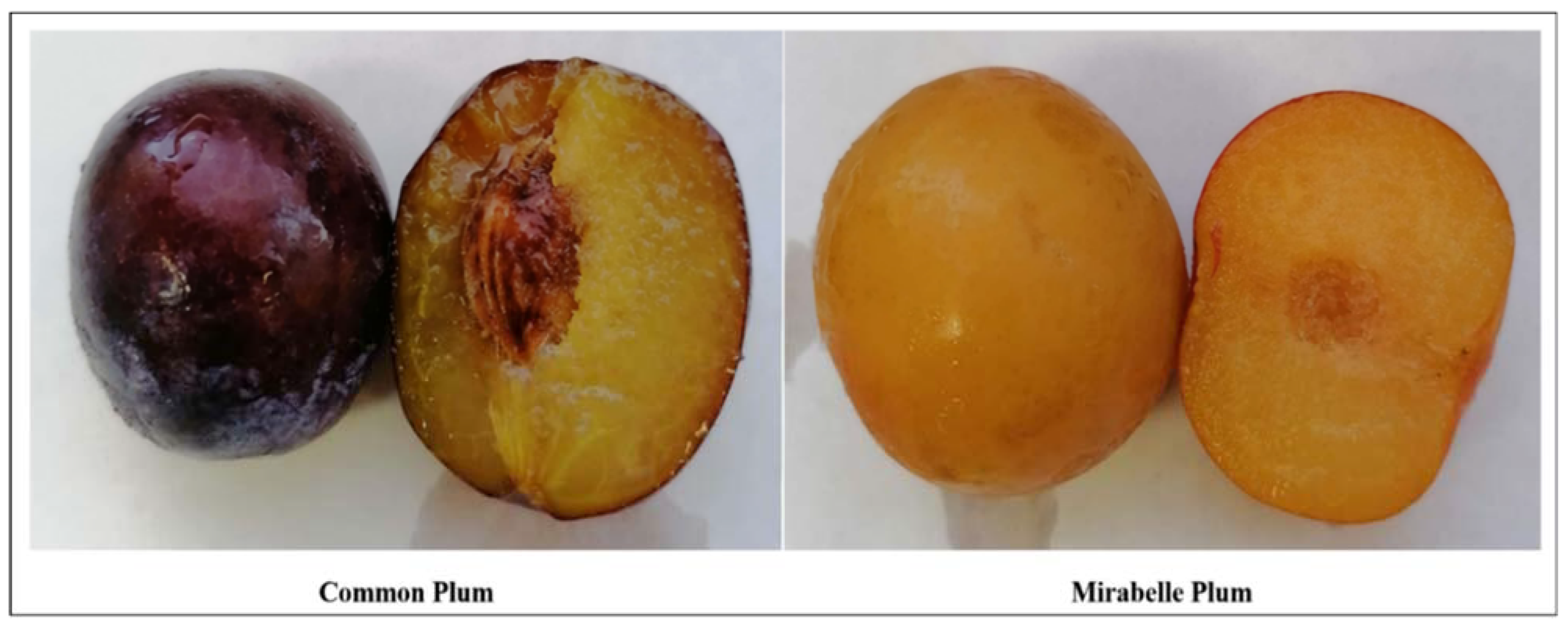


| Prunus Variety | Common Name | Skin Color | Fruit Part Extracted | Ethanol (%) | Dry Extract (g/g) 1 | Extraction Yield (%) |
|---|---|---|---|---|---|---|
| P. domestica subsp. domestica | Common plum | Purple | Skin | 99 | 0.42 | 42.0 |
| 70 | 0.39 | 39.0 | ||||
| 50 | 0.38 | 38.0 | ||||
| Pulp | 99 | 0.60 | 59.5 | |||
| 70 | 0.63 | 63.0 | ||||
| 50 | 0.67 | 67.0 | ||||
| P. domestica subsp. syriaca | Mirabelle plum | Yellow | Skin | 99 | 0.37 | 37.0 |
| 70 | 0.41 | 41.0 | ||||
| 50 | 0.43 | 43.0 | ||||
| Pulp | 99 | 0.45 | 45.0 | |||
| 70 | 0.46 | 46.0 | ||||
| 50 | 0.49 | 49.0 |
| Prunus Variety | Fruit Part Extracted (Ethanol %) | TPC (GAE/g on Dry Weight Basis) | Trolox Equivalent Concentration (µM/g on Dry Weight Basis) |
|---|---|---|---|
| P. domestica subsp. domestica | skin (99%) | 9.1 ± 1.0 | 1282.4 ± 84.1 a |
| skin (70%) | 12.8 ± 0.9 a | 1826.2 ± 216.4 | |
| skin (50%) | 11.0 ± 0.6 b | 1944.1 ± 138.1 b | |
| pulp (99%) | 7.2 ± 1.0 | 630.5 ± 44.1 a | |
| pulp (70%) | 11.3 ± 0.2 a | 1611.9 ± 289.5 | |
| pulp (50%) | 9.7 ± 0.2 b | 1290.7 ± 155.5 b | |
| P. domestica subsp. syriaca | skin (99%) | 7.0 ± 0.2 | 708.0 ± 25.1 |
| skin (70%) | 11.2 ± 1.4 | 1597.4 ± 88.2 c | |
| skin (50%) | 7.9 ± 0.8 c | 1602.1 ± 368.1 | |
| pulp (99%) | 6.5 ± 0.4 | 578.5 ± 53.5 | |
| pulp (70%) | 10.0 ± 0.9 | 727.7 ± 43.9 c | |
| pulp (50%) | 12.9 ± 1.7 c | 1119.4 ± 93.1 |
| Peak | rt | Compound | [M-H]- | MS/MS | Molecular Formula | Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | 0.60 | Citric acid | 191.0227 | 111.0103; 173.0103 | C6H8O7 | 1.57 |
| 2 | 3.12 | Chlorogenic acid | 353.0874 | 173.0489; 191.0576 | C16H18O9 | −1.13 |
| 3 | 4.68 | Coumaroylquinic acid Isomer | 337.0945 | 163.0417; 119.0558 | C16H18O8 | 4.75 |
| 4 | 5.45 | Catechin | 289.0729 | 245.0816 | C15H14O6 | 3.81 |
| 5 | 6.08 | (+) Epicatechin dimer B type | 577.1328 | 407.0787; 289.0728 | C30H26O12 | −4.16 |
| 6 | 6.50 | Feruloylquinic acid | 367.1053 | 193.0531; 134.0390 | C17H20O9 | 4.90 |
| 7 | 6.70 | Coumaroylquinic acid isomer | 337.0928 | 163.0447; 191.0594 | C16H18O8 | 1.19 |
| 8 | 7.20 | Coumaroylquinic acid isomer | 337.0952 | 173.0458; 163.0418 | C16H18O8 | 2.30 |
| 9 | 8.12 | (+) Epicatechin | 289.0735 | 245.0816 | C15H14O6 | 5.88 |
| 10 | 8.48 | (+) Epicatechin trimer B type | 865.1979 | 407.0790; 287.0569; 577.1344 | C45H38O18 | 3.40 |
| 11 | 8.86 | (+) Epicatechin dimer B type isomer | 577.1344 | 407.0790; 289.0732 | C30H26O12 | −1.39 |
| 12 | 9.70 | Quinic acid derivative | 393.1777 | 149.0465; 191.0561 | C17H30O10 | 2.80 |
| 13 | 10.50 | Feruloyl-coumaroylquinic acid derivative | 559.1665 | 337.0947; 193.0514 | C24H32O15 | −0.54 |
| 14 | 11.29 | Feruloyl-coumaroylquinic acid derivative | 559.1670 | 337.0949; 193.0510 | C24H32O15 | −0.50 |
| 15 | 12.19 | Feruloyl-coumaroylquinic acid derivative | 559.1677 | 337.0946; 193.0514 | C24H32O15 | 1.61 |
| 16 | 12.32 | (+) Epicatechin dimer B type isomer | 577.1358 | 407.0831; 289.0742 | C30H26O12 | 1.04 |
| 17 | 12.74 | (+) Epicatechin B type trimer isomer | 865.2015 | 407.0778; 287.0569; 577.1344; 543.0905 | C45H38O18 | 3.47 |
| 18 | 13.20 | Quercetin-rutinoside | 609.1477 | 301.0351; 271.0254; 255.0320 | C27H30O16 | 3.47 |
| 19 | 13.48 | (+) Epicatechin A type trimer | 863.1823 | 575.1180; 423.0711; 285.0393 | C45H36O18 | −0.20 |
| 20 | 14.04 | (+) Epicatechin A type trimer isomer | 863.1828 | 575.1180; 423.0711; 285.0393 | C45H36O18 | −0.12 |
| 21 | 14.82 | (+) Epicatechin A type dimer | 575.1197 | 423.0746; 285.0395 | C30H24O12 | 1.22 |
| 22 | 15.75 | Quercetin-rhamnoside | 447.0924 | 301.0371; 255. | C21H20O11 | −0.9 |
| 23 | 16.52 | (+) Epicatechin A type dimer isomer | 575.1187 | 423.0716; 285.0398 | C30H24O12 | −1.39 |
| Peak | Compound | Retention Time | Area % |
|---|---|---|---|
| 1 | Citric acid | 0.6 | 7.92 |
| 2 | Chlorogenic acid | 3.12 | 15.43 |
| 3 | Coumaroylquinic acid Isomer | 4.68 | 0.74 |
| 4 | Catechin | 5.45 | 8.61 |
| 5 | (+) Epicatechin dimer B type | 6.08 | 5.74 |
| 6 | Feruloylquinic acid | 6.5 | 0.21 |
| 7 | Coumaroylquinic acid isomer | 6.7 | 0.39 |
| 8 | Coumaroylquinic acid isomer | 7.2 | 2.42 |
| 9 | (+) Epicatechin | 8.12 | 4.54 |
| 10 | (+) Epicatechin trimer B type | 8.48 | 0.24 |
| 11 | (+) Epicatechin dimer B type isomer | 8.86 | 5.47 |
| 12 | Quinic acid derivative | 9.7 | 1.98 |
| 13 | Feruloyl-coumaroylquinic acid derivative | 10.5 | 3.01 |
| 14 | Feruloyl-coumaroylquinic acid derivative | 11.29 | 19.55 |
| 15 | Feruloyl-coumaroylquinic acid derivative | 12.19 | 1.49 |
| 16 | Feruloyl-coumaroylquinic acid derivative | 12.32 | 1.47 |
| 17 | (+) Epicatechin dimer B type isomer | 12.74 | 6.27 |
| 18 | (+) Epicatechin B type trimer isomer | 13.2 | 0.79 |
| 19 | Quercetin-rutinoside | 13.48 | 3.20 |
| 20 | (+) Epicatechin A type trimer | 14.04 | 4.05 |
| 21 | (+) Epicatechin A type trimer isomer | 14.82 | 1.81 |
| 22 | (+) Epicatechin A type dimer | 15.75 | 0.16 |
| 23 | Quercetin-rhamnoside | 16.52 | 4.50 |
| Compound | Chemical Shift (ppm) of Selected Resonances Used for Quantification | μg/mg Dry Weight |
|---|---|---|
| Quinic acid | 1.88 (CH2-1) | 7.50 |
| Citric acid | 2.54 (α,γ-CH) | 0.84 |
| Malic acid | 4.30 (α-CH) | 38.49 |
| Xylose | 5.20 (CH-1) | 0.56 |
| Glucose | 5.25 (CH-1) | 106.59 |
| Sucrose | 5.42 (CH-1) | 31.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Sommella, E.; Santarcangelo, C.; D’Avino, D.; Rossi, A.; Dacrema, M.; Minno, A.D.; Di Matteo, G.; Mannina, L.; Campiglia, P.; et al. Hydroethanolic Extract of Prunus domestica L.: Metabolite Profiling and In Vitro Modulation of Molecular Mechanisms Associated to Cardiometabolic Diseases. Nutrients 2022, 14, 340. https://doi.org/10.3390/nu14020340
Ullah H, Sommella E, Santarcangelo C, D’Avino D, Rossi A, Dacrema M, Minno AD, Di Matteo G, Mannina L, Campiglia P, et al. Hydroethanolic Extract of Prunus domestica L.: Metabolite Profiling and In Vitro Modulation of Molecular Mechanisms Associated to Cardiometabolic Diseases. Nutrients. 2022; 14(2):340. https://doi.org/10.3390/nu14020340
Chicago/Turabian StyleUllah, Hammad, Eduardo Sommella, Cristina Santarcangelo, Danilo D’Avino, Antonietta Rossi, Marco Dacrema, Alessandro Di Minno, Giacomo Di Matteo, Luisa Mannina, Pietro Campiglia, and et al. 2022. "Hydroethanolic Extract of Prunus domestica L.: Metabolite Profiling and In Vitro Modulation of Molecular Mechanisms Associated to Cardiometabolic Diseases" Nutrients 14, no. 2: 340. https://doi.org/10.3390/nu14020340
APA StyleUllah, H., Sommella, E., Santarcangelo, C., D’Avino, D., Rossi, A., Dacrema, M., Minno, A. D., Di Matteo, G., Mannina, L., Campiglia, P., Magni, P., & Daglia, M. (2022). Hydroethanolic Extract of Prunus domestica L.: Metabolite Profiling and In Vitro Modulation of Molecular Mechanisms Associated to Cardiometabolic Diseases. Nutrients, 14(2), 340. https://doi.org/10.3390/nu14020340