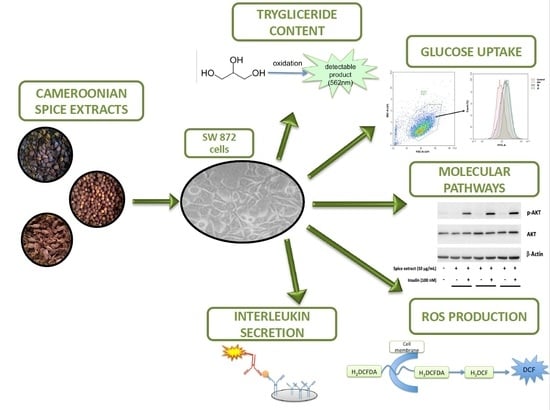
Cameroonian Spice Extracts Modulate Molecular Mechanisms Relevant to Cardiometabolic Diseases in SW 872 Human Liposarcoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Plant Extracts
2.3. Cell Cultures and Differentation
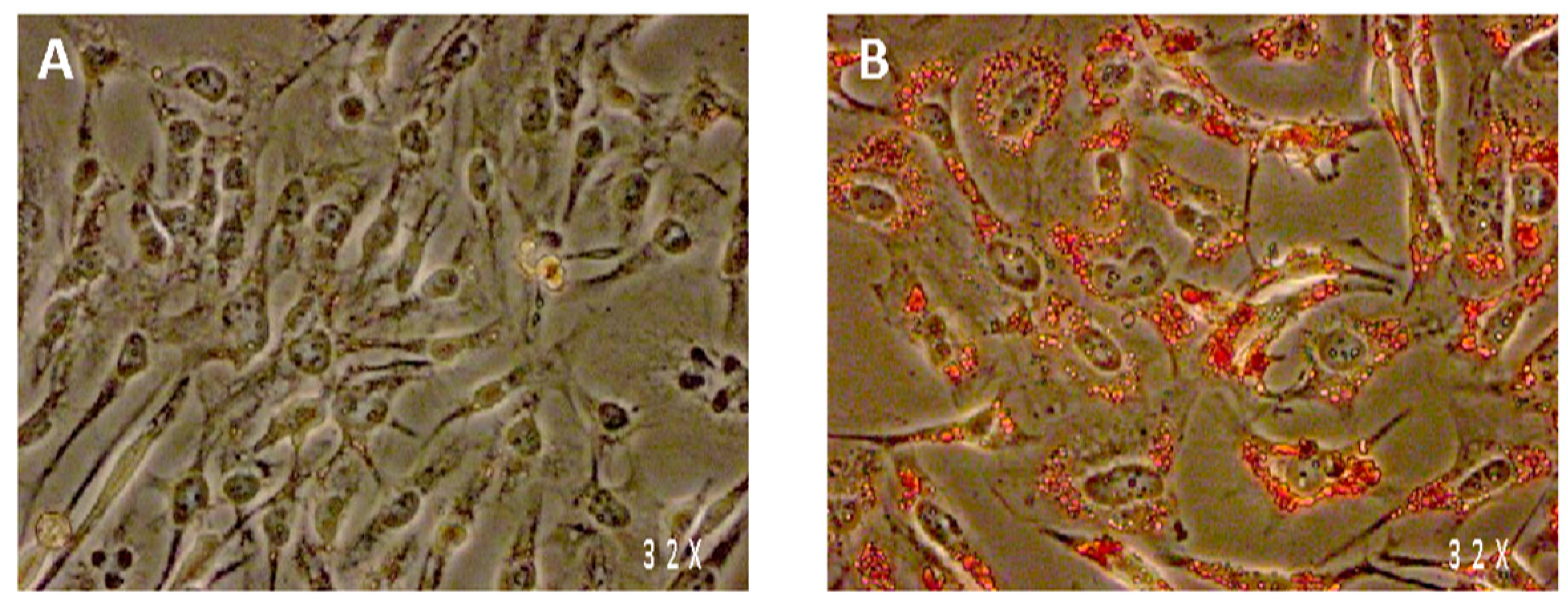
2.4. Oil-Red-O Staining
2.5. MTS (3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) Cell Viability Assay
2.6. Morphological Analysis
2.7. Interleukin-6 and Interleukin-8 Measurement
2.8. Triglyceride Content Measurement
2.9. Measurement of ROS Production
2.10. Glucose Uptake FACS Analysis
2.11. Western Blotting Analysis
2.12. Statistical Analysis
3. Results
3.1. Spice Extracts up to 25 μg/mL Do Not Affect SW 872 Cell Viability and Morphology
3.2. All Spice Extracts Reduce Triglyceride Accumulation in Differentiated Adipocytes
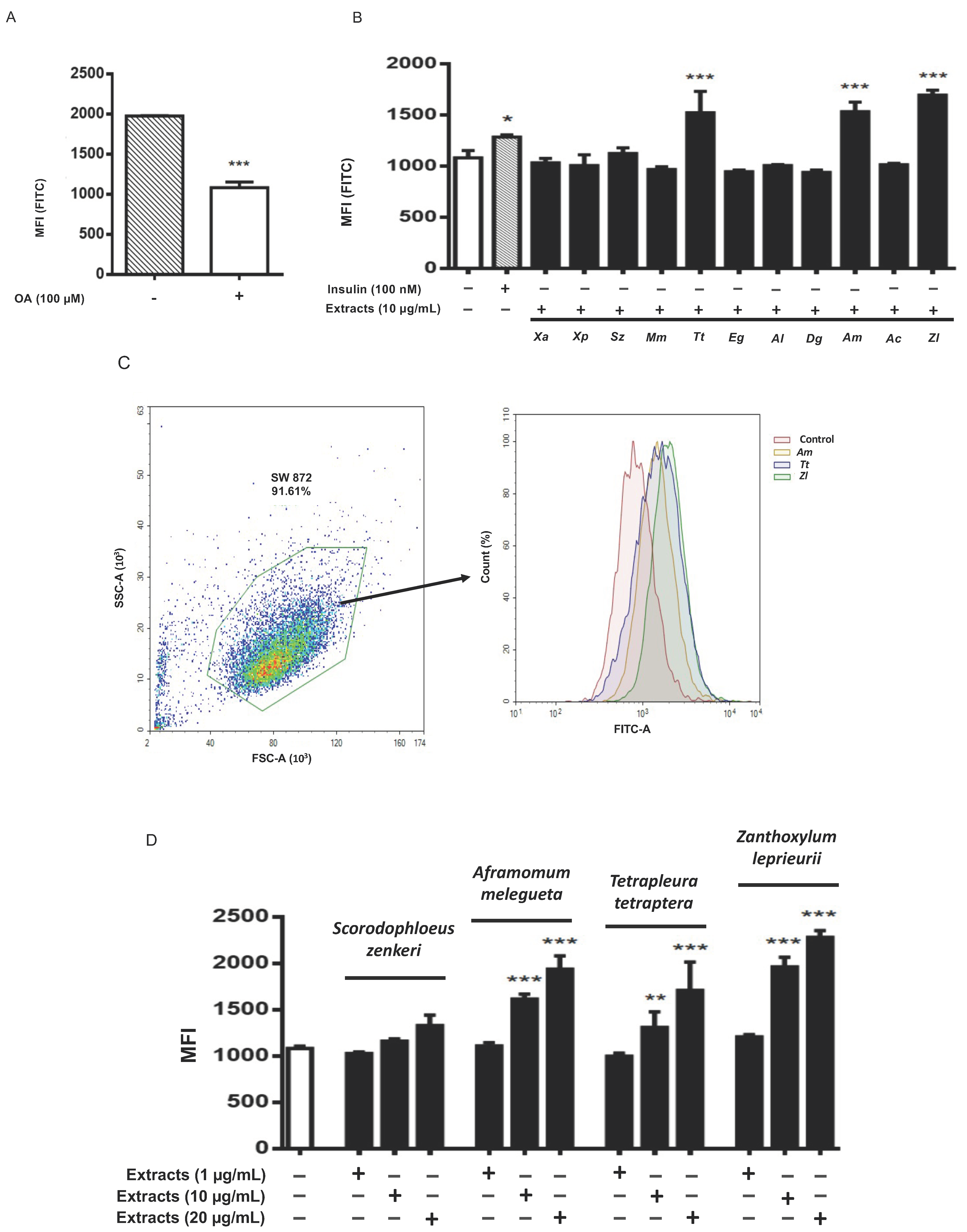
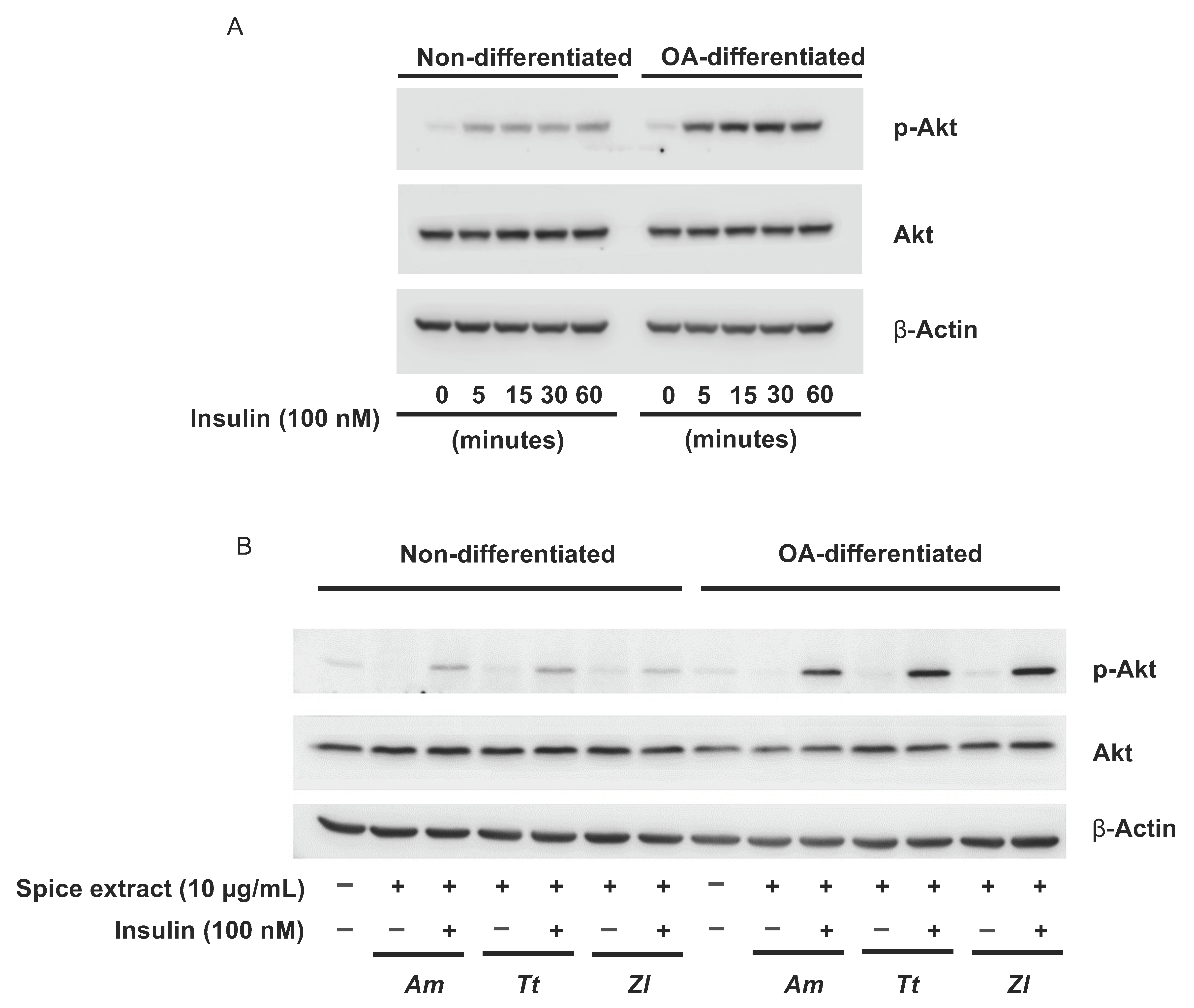
3.3. Tetrapleura tetraptera, Aframomum melegueta and Zanthoxylum leprieurii Increase Glucose Uptake in Differentiated Adipocytes
3.4. Spice Extracts Modulate IL-6 and IL-8 Release from Differentiated Adipocytes
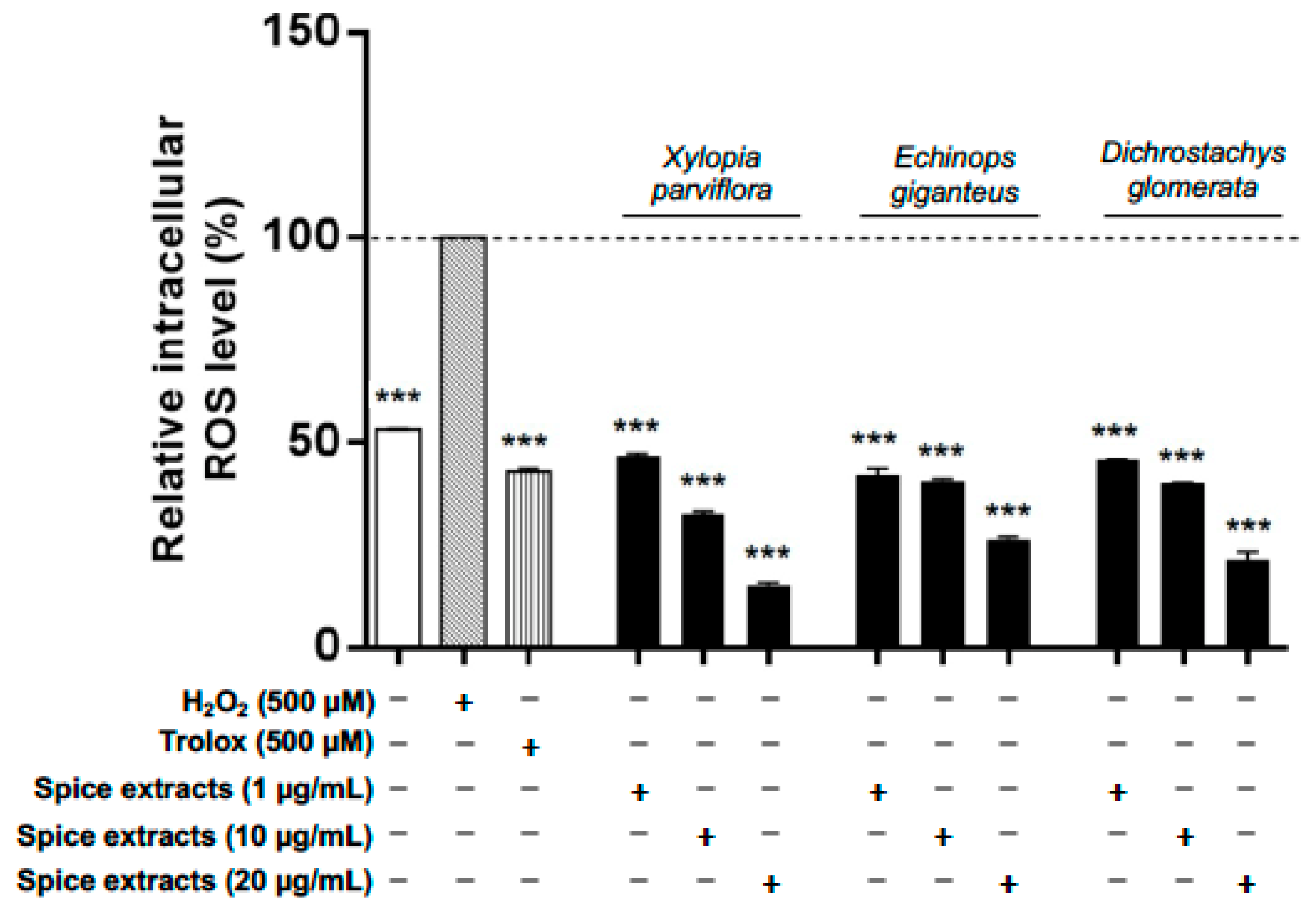
3.5. Spice Extracts Affect Reactive Oxygen Species Production in Differentiated Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Ruscica, M.; Gomaraschi, M.; Mombelli, G.; Macchi, C.; Bosisio, R.; Pazzucconi, F.; Pavanello, C.; Calabresi, L.; Arnoldi, A.; Sirtori, C.R.; et al. Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 2014, 8, 61–68. [Google Scholar] [CrossRef]
- Cicolari, S.; Pavanello, C.; Olmastroni, E.; del Puppo, M.; Bertolotti, M.; Mombelli, G.; Catapano, A.L.; Calabresi, L.; Magni, P. Interactions of Oxysterols with Atherosclerosis Biomarkers in Subjects with Moderate Hypercholesterolemia and Effects of a Nutraceutical Combination (Bifidobacterium longum BB536, Red Yeast Rice Extract) (Randomized, Double-Blind, Placebo-Controlled Study). Nutrients 2021, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Atchan Nwakiban, A.P.; Sokeng, A.J.; Dell’Agli, M.; Bossi, L.; Beretta, G.; Gelmini, F.; Deutou Tchamgoue, A.; Agbor Agbor, G.; Kuiate, J.R.; Daglia, M.; et al. Hydroethanolic plant extracts from Cameroon positively modulate enzymes relevant to carbohydrate/lipid digestion and cardio-metabolic diseases. Food Funct. 2019, 10, 6533–6542. [Google Scholar] [CrossRef]
- Nwakiban, A.P.A.; Fumagalli, M.; Piazza, S.; Magnavacca, A.; Martinelli, G.; Beretta, G.; Magni, P.; Tchamgoue, A.D.; Agbor, G.A.; Kuiaté, J.R.; et al. Dietary Cameroonian Plants Exhibit Anti-Inflammatory Activity in Human Gastric Epithelial Cells. Nutrients 2020, 12, 3787. [Google Scholar] [CrossRef]
- Nwakiban, A.P.A.; Cicolari, S.; Piazza, S.; Gelmini, F.; Sangiovanni, E.; Martinelli, G.; Bossi, L.; Carpentier-Maguire, E.; Tchamgoue, A.D.; Agbor, G.; et al. Oxidative Stress Modulation by Cameroonian Spice Extracts in HepG2 Cells: Involvement of Nrf2 and Improvement of Glucose Uptake. Metabolites 2020, 10, 182. [Google Scholar] [CrossRef]
- Cicolari, S.; Dacrema, M.; Tsetegho Sokeng, A.J.; Xiao, J.; Atchan Nwakiban, A.P.; Di Giovanni, C.; Santarcangelo, C.; Magni, P.; Daglia, M. Hydromethanolic Extracts from Adansonia digitata L Edible Parts Positively Modulate Pathophysiological. Molecules 2020, 25, 2858. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, A.; Kazantzis, M.; Thomas, R.; Wabitsch, M.; Tews, D.; Seetharama Sastry, K.; Abdelkarim, M.; Zilberfarb, V.; Strosberg, A.D.; Chouchane, L. Comprehensive molecular characterization of human adipocytes reveals a transient brown phenotype. J. Transl. Med. 2015, 13, 135. [Google Scholar] [CrossRef]
- Carmel, J.F.; Tarnus, E.; Cohn, J.S.; Bourdon, E.; Davignon, J.; Bernier, L. High expression of apolipoprotein E impairs lipid storage and promotes cell proliferation in human adipocytes. J. Cell Biochem. 2009, 106, 608–617. [Google Scholar] [CrossRef]
- Butterweck, V.; Nahrstedt, A. What is the best strategy for preclinical testing of botanicals? A critical perspective. Planta Med. 2012, 78, 747–754. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jang, M.S. Anti-obesity effects of Laminaria japonica fermentation on 3T3-L1 adipocytes are mediated by the inhibition of C/EBP-alpha/beta and PPAR-gamma. Cell Mol. Biol. 2018, 64, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Di Lorenzo, C.; Colombo, E.; Colombo, F.; Fumagalli, M.; Frigerio, G.; Restani, P.; Dell’Agli, M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. leaves. Food Funct. 2015, 6, 2453–2463. [Google Scholar] [CrossRef]
- Piazza, S.; Pacchetti, B.; Fumagalli, M.; Bonacina, F.; Dell’Agli, M.; Sangiovanni, E. Comparison of Two Ginkgo biloba L. Extracts on Oxidative Stress and Inflammation Markers in Human Endothelial Cells. Mediat. Inflamm. 2019, 2019, 6173893. [Google Scholar] [CrossRef]
- Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Évaluation Biologique des Dispositifs Médicaux—Partie 5: Essais Concernant la Cytotoxicité In Vitro, Third Edition. International Organization for Standardization. Posted June 1. 2009. Available online: https://nhiso.com/wp-content/uploads/2018/05/ISO-10993-5-2009.pdf (accessed on 25 November 2021).
- Krycer, J.R.; Quek, L.E.; Francis, D.; Zadoorian, A.; Weiss, F.C.; Cooke, K.C.; Nelson, M.E.; Diaz-Vegas, A.; Humphrey, S.J.; Scalzo, R.; et al. Insulin signaling requires glucose to promote lipid anabolism in adipocytes. J. Biol. Chem. 2020, 295, 13250–13266. [Google Scholar] [CrossRef]
- Shoshani, O.; Livne, E.; Armoni, M.; Shupak, A.; Berger, J.; Ramon, Y.; Fodor, L.; Gilhar, A.; Peled, I.J.; Ullmann, Y. The effect of interleukin-8 on the viability of injected adipose tissue in nude mice. Plast. Reconstr. Surg. 2005, 115, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chang, X.; Yu, J.; Xu, W. Cherry Polyphenol Reduces High-Fat Diet-Induced Obesity in C57BL/6 Mice by Mitigating Fat Deposition, Inflammation, and Oxidation. J. Agric. Food Chem. 2020, 68, 4424–4436. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Krusche, B.; Youns, M.; Voukeng, I.; Fankam, A.G.; Tankeo, S.; Lacmata, S.; Efferth, T. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J. Ethnopharmacol. 2011, 134, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Mori, T.; Shibata, T.; Kita, M.; Mitsunaga, T. 6-Paradol Acts as a Potential Anti-obesity Vanilloid from Grains of Paradise. Mol. Nutr. Food Res. 2021, 65, e2100185. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Wassef, H.; Bernier, L.; Davignon, J.; Cohn, J.S. Synthesis and secretion of apoC-I and apoE during maturation of human SW872 liposarcoma cells. J. Nutr. 2004, 134, 2935–2941. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lehrke, M.; Lazar, M.A. The many faces of PPARgamma. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Rayalam, S.; Yang, J.Y.; Ambati, S.; Della-Fera, M.A.; Baile, C.A. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother. Res. 2008, 22, 1367–1371. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Hosoya, Y.; Yamashita, H.; Fujita, H.; Ohsugi, M.; Tobe, K.; Kadowaki, T.; Nagai, R.; et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 2007, 56, 1517–1526. [Google Scholar] [CrossRef]
- Kim, M.R.; Kim, J.W.; Park, J.B.; Hong, Y.K.; Ku, S.K.; Choi, J.S. Anti-obesity effects of yellow catfish protein hydrolysate on mice fed a 45% kcal high-fat diet. Int. J. Mol. Med. 2017, 40, 784–800. [Google Scholar] [CrossRef]
- Kuate, D.; Kengne, A.P.; Biapa, C.P.; Azantsa, B.G.; Abdul Manan Bin Wan Muda, B. Tetrapleura tetraptera spice attenuates high-carbohydrate, high-fat diet-induced obese and type 2 diabetic rats with metabolic syndrome features. Lipids Health Dis. 2015, 14, 50. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Sethi, J.K. TNF-alpha and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Lee, J.J.; Kim, I.S.; Kim, Y.; Myung, C.S. Stimulation of glucose uptake and improvement of insulin resistance by aromadendrin. Pharmacology 2011, 88, 266–274. [Google Scholar] [CrossRef]
- Kobashi, C.; Asamizu, S.; Ishiki, M.; Iwata, M.; Usui, I.; Yamazaki, K.; Tobe, K.; Kobayashi, M.; Urakaze, M. Inhibitory effect of IL-8 on insulin action in human adipocytes via MAP kinase pathway. J. Inflamm. 2009, 6, 25. [Google Scholar] [CrossRef]
- Issa, N.; Lachance, G.; Bellmann, K.; Laplante, M.; Stadler, K.; Marette, A. Cytokines promote lipolysis in 3T3-L1 adipocytes through induction of NADPH oxidase 3 expression and superoxide production. J. Lipid Res. 2018, 59, 2321–2328. [Google Scholar] [CrossRef]
- Suh, S.J.; Kwak, C.H.; Chung, T.W.; Park, S.J.; Cheeeei, M.; Park, S.S.; Seo, C.S.; Son, J.K.; Chang, Y.C.; Park, Y.G.; et al. Pimaric acid from Aralia cordata has an inhibitory effect on TNF-α-induced MMP-9 production and HASMC migration via down-regulated NF-κB and AP-1. Chem. Biol. Interact. 2012, 199, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-κB signaling. Cancer Prev. Res. (Phila) 2014, 7, 627–638. [Google Scholar] [CrossRef]
- Liang, N.; Sang, Y.; Liu, W.; Yu, W.; Wang, X. Anti-Inflammatory Effects of Gingerol on Lipopolysaccharide-Stimulated RAW 264.7 Cells by Inhibiting NF-κB Signaling Pathway. Inflammation 2018, 41, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Ruifeng, G.; Yunhe, F.; Zhengkai, W.; Ershun, Z.; Yimeng, L.; Minjun, Y.; Xiaojing, S.; Zhengtao, Y.; Naisheng, Z. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur. J. Pharm. 2014, 729, 54–58. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Roy, A.; Dinda, S. Dietary plant flavonoids in prevention of obesity and diabetes. Adv. Protein Chem. Struct. Biol. 2020, 120, 159–235. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]

| Oleic Acid (100 µM) | Triglyceride (% of Oleic Acid-Treated Cells) | ||
|---|---|---|---|
| +24 h | +48 h | ||
| undifferentiated control | − | 49.6 ± 4.1 *** | 64 ± 0.7 *** |
| differentiated control | + | 100 | 100 |
| resveratrol (10 µM) | + | 94.9 ± 5.9 | 69.6 ± 3.0 *** |
| Xylopia aethiopica | + | 96.5 ± 7.6 | 85.5 ± 2.3 * |
| Xylopia parviflora | + | 88.2 ± 1.0 | 86.2 ± 1.6 * |
| Scorodophloeus zenkeri | + | 97.9 ± 9.6 | 81.5 ± 4.9 * |
| Monodora myristica | + | 90.4 ± 9.0 | 84.7 ± 3.2 * |
| Tetrapleura tetraptera | + | 90.9 ± 4.5 | 86.2 ± 2.2 * |
| Echinops giganteus | + | 104.2 ± 5.9 | 88.7 ± 0.9 * |
| Afrostyrax lepidophyllus | + | 102.5 ± 6.4 | 83.5 ± 10.1 * |
| Dichrostachys glomerata | + | 102.6 ± 2.5 | 82.6 ± 6.8 * |
| Aframomum melegueta | + | 104 ± 5.2 | 87.0 ± 4.7 * |
| Aframomum citratum | + | 98.3 ± 4.7 | 84 ± 1.8 * |
| Zanthoxylum leprieurii | + | 98.5 ± 8.8 | 86.6 ± 5.9 * |
| H2O2 (500 µM) | Relative Intracellular ROS Level (%) | |
|---|---|---|
| Control | − | 35.9 ± 0.2 *** |
| H2O2 (500 µM) | + | 100 |
| Trolox (500 µM) | + | 61.8 ± 8.6 *** |
| Xylopia aethiopica | + | 255.7 ± 9.3 *** |
| Xylopia parviflora | + | 49.6 ± 3.53 ** |
| Scorodophloeus zenkeri | + | 94.7 ± 1.6 |
| Monodora myristica | + | 60.0 ± 8.9 *** |
| Tetrapleura tetraptera | + | 72.7 ± 1.5 ** |
| Echinops giganteus | + | 56.4 ± 1.2 *** |
| Afrostyrax lepidophyllus | + | 75.4 ± 0.9 * |
| Dichrostachys glomerata | + | 66.6 ± 0.6 |
| Aframomum melegueta | + | 122.5 ± 9.9 ** |
| Aframomum citratum | + | 112.8 ± 0.4 |
| Zanthoxylum leprieurii | + | 89.9 ± 3.3 |
| Triglyceride Reduction | Glucose Uptake Stimulation | ROS Production | IL-6 Reduction | IL-8 Reduction | |
|---|---|---|---|---|---|
| Xylopia aethiopica | −14.5% | +55.8% | −21.1% | ||
| Xylopia parviflora | −13.8% | −50.5% | −36.8% | ||
| Scorodophloeus zenkeri | −18.5% | ||||
| Monodora myristica | −15.3% | −40% | −24.3% | ||
| Tetrapleura tetraptera | −13.8% | +40.8% | −27.4% | −29.7% | |
| Echinops giganteus | −11.3% | −43.6% | −29% | ||
| Afrostyrax lepidophyllus | −16.5% | −24.6% | |||
| Dichrostachys glomerata | −17.4% | −40% | |||
| Aframomum melegueta | −13% | +41.7% | −43.1% | ||
| Aframomum citratum | −16% | −58.6% | |||
| Zanthoxylum leprieurii | −13.4% | +56.6% | −32.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atchan Nwakiban, A.P.; Passarelli, A.; Da Dalt, L.; Olivieri, C.; Demirci, T.N.; Piazza, S.; Sangiovanni, E.; Carpentier-Maguire, E.; Martinelli, G.; Shivashankara, S.T.; et al. Cameroonian Spice Extracts Modulate Molecular Mechanisms Relevant to Cardiometabolic Diseases in SW 872 Human Liposarcoma Cells. Nutrients 2021, 13, 4271. https://doi.org/10.3390/nu13124271
Atchan Nwakiban AP, Passarelli A, Da Dalt L, Olivieri C, Demirci TN, Piazza S, Sangiovanni E, Carpentier-Maguire E, Martinelli G, Shivashankara ST, et al. Cameroonian Spice Extracts Modulate Molecular Mechanisms Relevant to Cardiometabolic Diseases in SW 872 Human Liposarcoma Cells. Nutrients. 2021; 13(12):4271. https://doi.org/10.3390/nu13124271
Chicago/Turabian StyleAtchan Nwakiban, Achille Parfait, Anna Passarelli, Lorenzo Da Dalt, Chiara Olivieri, Tugba Nur Demirci, Stefano Piazza, Enrico Sangiovanni, Eugénie Carpentier-Maguire, Giulia Martinelli, Shilpa Talkad Shivashankara, and et al. 2021. "Cameroonian Spice Extracts Modulate Molecular Mechanisms Relevant to Cardiometabolic Diseases in SW 872 Human Liposarcoma Cells" Nutrients 13, no. 12: 4271. https://doi.org/10.3390/nu13124271
APA StyleAtchan Nwakiban, A. P., Passarelli, A., Da Dalt, L., Olivieri, C., Demirci, T. N., Piazza, S., Sangiovanni, E., Carpentier-Maguire, E., Martinelli, G., Shivashankara, S. T., Manjappara, U. V., Tchamgoue, A. D., Agbor, G. A., Kuiate, J.-R., Daglia, M., Dell’Agli, M., & Magni, P. (2021). Cameroonian Spice Extracts Modulate Molecular Mechanisms Relevant to Cardiometabolic Diseases in SW 872 Human Liposarcoma Cells. Nutrients, 13(12), 4271. https://doi.org/10.3390/nu13124271