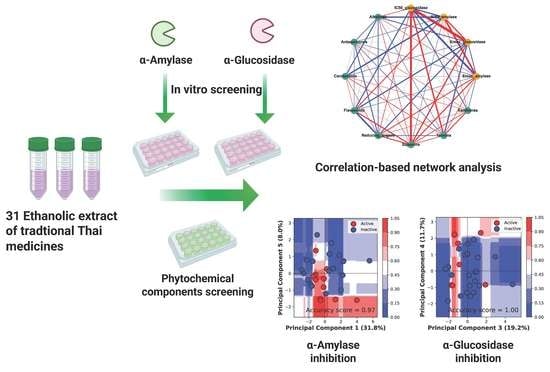
Machine Learning and In Vitro Chemical Screening of Potential α-Amylase and α-Glucosidase Inhibitors from Thai Indigenous Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extraction
2.3. Phytochemical Identification
2.4. α-Amylase Inhibitory Activity
2.5. α-Glucosidase Inhibitory Activity
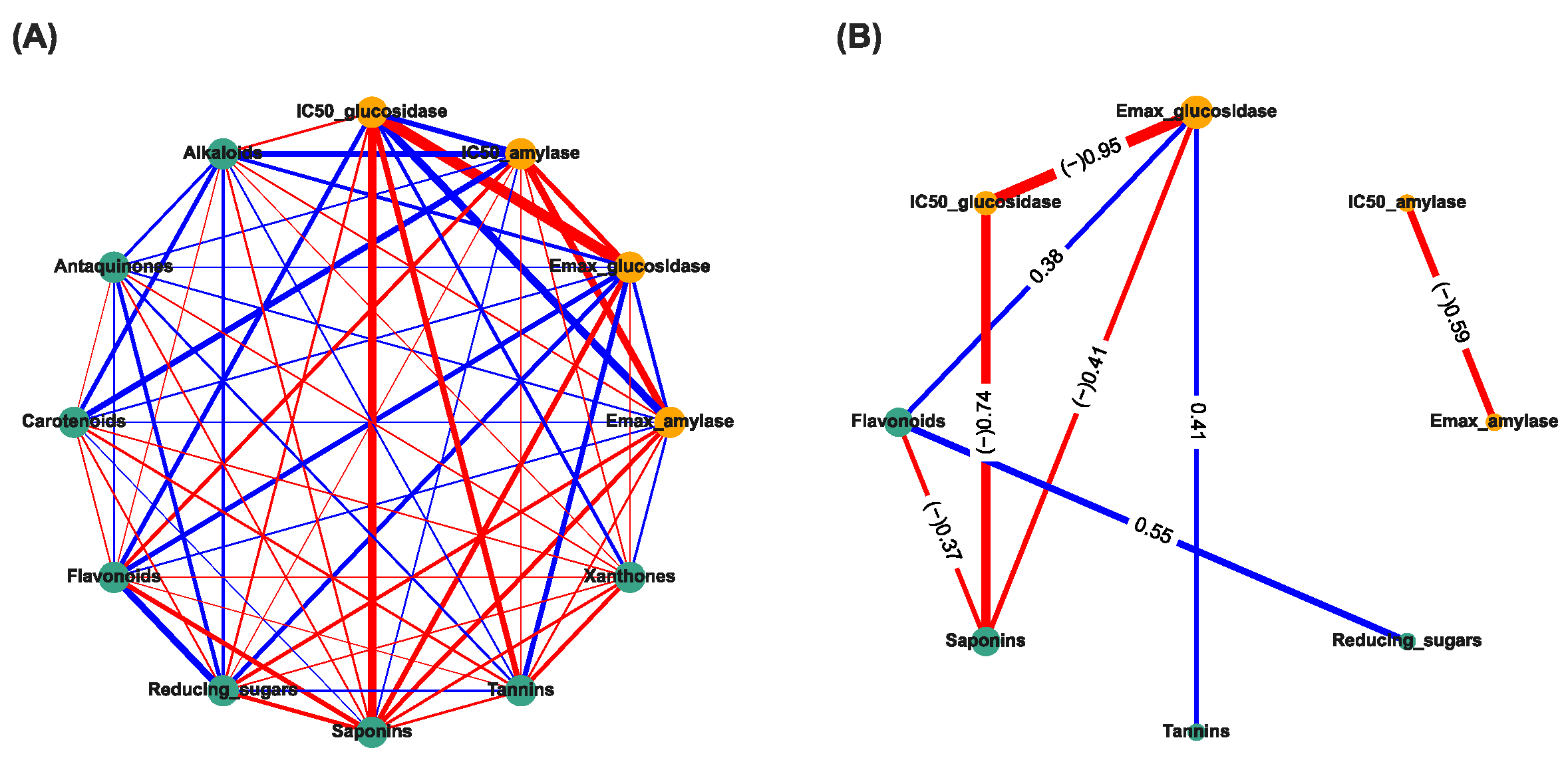
2.6. Correlation-Based Network Analysis
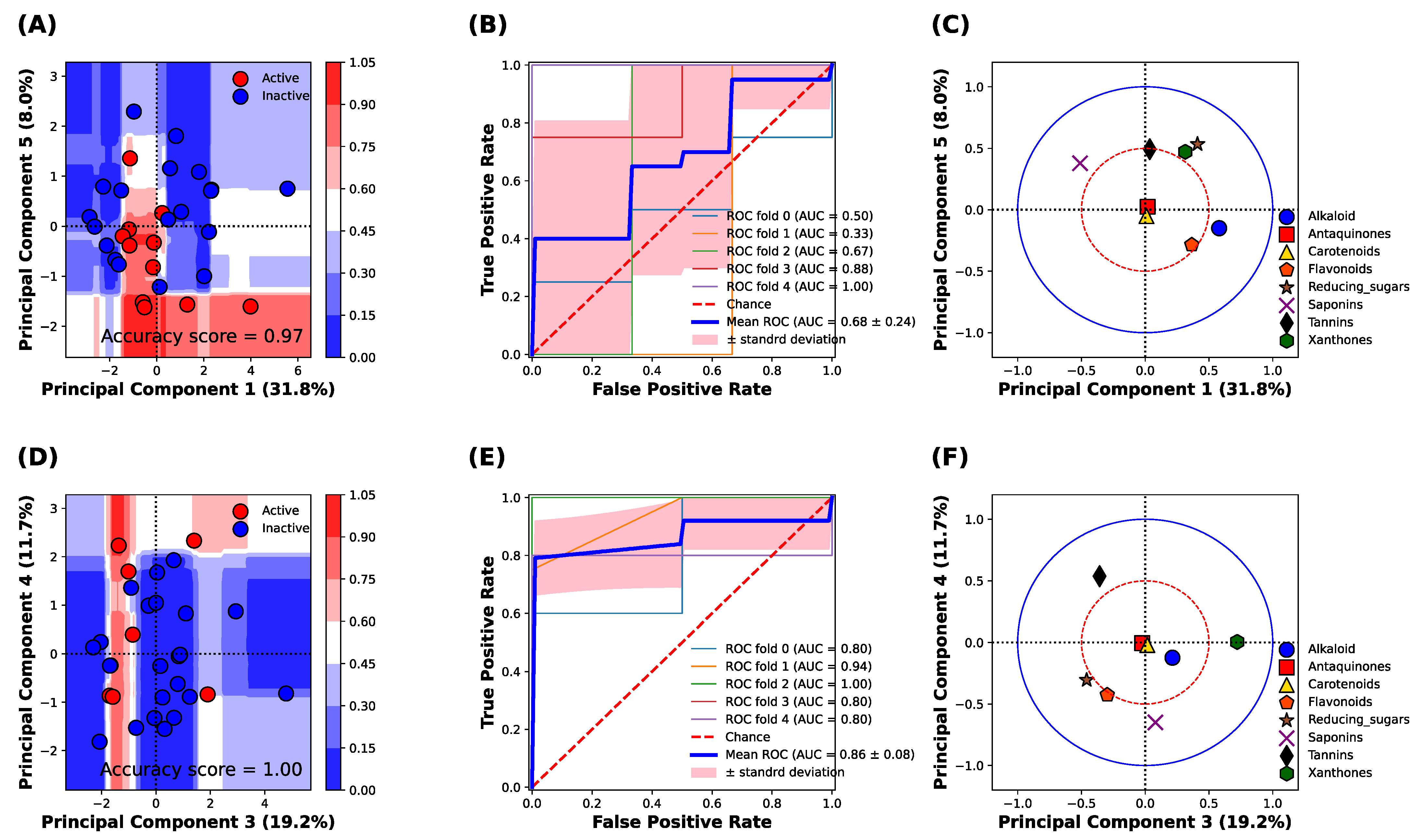
2.7. Multivariate Classification Analysis
2.8. Statistical Analysis
3. Results
3.1. Screening of Phytochemical Constituents
3.2. α-Amylase and α-Glucosidase Inhibitory Activities
3.3. Correlation-Based Network Analysis
3.4. Classification Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Jialal, I. Diabetes Mellitus Type 2. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, T.; Fujiwara, M.; Yao, Z. Postprandial Hyperglycemia and Postprandial Hypertriglyceridemia in Type 2 Diabetes. J. Biomed. Res. 2019, 33, 1–16. [Google Scholar] [CrossRef]
- Ceriello, A. Postprandial Hyperglycemia and Diabetes Complications: Is It Time to Treat? Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Ding, D.; Byun, R.; Comino, E.; Bauman, A.; Jalaludin, B. Lifestyle Changes After a Diagnosis of Type 2 Diabetes. Diabetes Spectr. 2017, 30, 43–50. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-Amylase and α-Glucosidase: Potential Linkage for Whole Cereal Foods on Prevention of Hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Ribeiro, D.; Freitas, M.; Fernandes, E. Flavonoids as Potential Agents in the Management of Type 2 Diabetes through the Modulation of α-Amylase and α-Glucosidase Activity: A Review. Crit. Rev. Food Sci. Nutr. 2021, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Rosak, C.; Mertes, G. Critical Evaluation of the Role of Acarbose in the Treatment of Diabetes: Patient Considerations. Diabetes Metab. Syndr. Obes. 2012, 5, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Thengyai, S.; Thiantongin, P.; Sontimuang, C.; Ovatlarnporn, C.; Puttarak, P. α-Glucosidase and α-Amylase Inhibitory Activities of Medicinal Plants in Thai Antidiabetic Recipes and Bioactive Compounds from Vitex Glabrata R. Br. Stem Bark. J. Herb. Med. 2020, 19, 100302. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Jugran, A.K.; Rawat, S.; Devkota, H.P.; Bhatt, I.D.; Rawal, R.S. Diabetes and Plant-derived Natural Products: From Ethnopharmacological Approaches to Their Potential for Modern Drug Discovery and Development. Phytother. Res. 2021, 35, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.M.; Freitas, M.; Fernandes, E. A Comprehensive Review on Xanthone Derivatives as α-Glucosidase Inhibitors. Eur. J. Med. Chem. 2018, 157, 1460–1479. [Google Scholar] [CrossRef]
- Guo, T.; Wu, S.; Guo, S.; Bai, L.; Liu, Q.; Bai, N. Synthesis and Evaluation of a Series of Oleanolic Acid Saponins as α-Glucosidase and α-Amylase Inhibitors: Oleanolic Acid Saponins as α-Glucosidase Inhibitors. Arch. Pharm. Chem. Life Sci. 2015, 348, 615–628. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The Inhibitory Effects of Flavonoids on α-Amylase and α-Glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chai, W.-M.; Ma, Z.-Y.; Ou-Yang, C.; Wei, Q.-M.; Song, S.; Zou, Z.-R.; Peng, Y.-Y. Inhibition of α-Glucosidase Activity and Non-Enzymatic Glycation by Tannic Acid: Inhibitory Activity and Molecular Mechanism. Int. J. Biol. Macromol. 2019, 141, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Gomes, N.G.M.; Duangsrisai, S.; Andrade, P.B.; Pereira, D.M.; Valentão, P. Medicinal Plants Utilized in Thai Traditional Medicine for Diabetes Treatment: Ethnobotanical Surveys, Scientific Evidence and Phytochemicals. J. Ethnopharmacol. 2020, 263, 113177. [Google Scholar] [CrossRef] [PubMed]
- Suksri, S.; Premcharoen, S.; Thawatphan, C.; Sangthongprow, S. Ethnobotany in Bung Khong Long Non-Hunting Area, Northeast Thailand. Agric. Nat. Resour. 2005, 39, 519–533. [Google Scholar]
- Yadav, R.; Agarwala, M. Phytochemical Analysis of Some Medicinal Plants. J. Phytol. 2011, 3, 10–14. [Google Scholar]
- Manosroi, J.; Boonpisuttinant, K.; Manosroi, W.; Manosroi, A. Anti-Proliferative Activities on HeLa Cancer Cell Line of Thai Medicinal Plant Recipes Selected from MANOSROI II Database. J. Ethnopharmacol. 2012, 142, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Ukoha, P.O.; Cemaluk, E.A.C.; Nnamdi, O.L.; Madus, E.P. Tannins and Other Phytochemical of the Samanaea Saman Pods and Their Antimicrobial Activities. Afr. J. Pure Appl. Chem. 2011, 5, 237–244. [Google Scholar]
- Ghadage, D.M.; Kshirsagar, P.R.; Pai, S.R.; Chavan, J.J. Extraction Efficiency, Phytochemical Profiles and Antioxidative Properties of Different Parts of Saptarangi (Salacia Chinensis L.)—An Important Underutilized Plant. Biochem. Biophys. Rep. 2017, 12, 79–90. [Google Scholar] [CrossRef]
- Hagberg, A.A.; Schult, D.A.; Swart, P.J. Exploring Network Structure, Dynamics, and Function Using NetworkX; Varoquaux, G., Vaught, T., Millman, J., Eds.; Los Alamos National Lab. (LANL): Los Alamos, NM, USA, 2008.
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-First Century? Int. J. Health Sci. 2007, 1, V–VIII. [Google Scholar]
- Brown, G.C.; Brown, M.M.; Sharma, S.; Brown, H.; Gozum, M.; Denton, P. Quality of Life Associated with Diabetes Mellitus in an Adult Population. J. Diabetes Its Complicat. 2000, 14, 18–24. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxidative Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Oral and Injectable (Non-Insulin) Pharmacological Agents for the Treatment of Type 2 Diabetes. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Shabab, S.; Gholamnezhad, Z.; Mahmoudabady, M. Protective Effects of Medicinal Plant against Diabetes Induced Cardiac Disorder: A Review. J. Ethnopharmacol. 2021, 265, 113328. [Google Scholar] [CrossRef]
- Fernandes, R. The Controversial Role of Glucose in the Diabetic Kidney. Porto. Biomed. J. 2021, 6, e113. [Google Scholar] [CrossRef]
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts. Antioxidants 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-Amylase as Molecular Target for Treatment of Diabetes Mellitus: A Comprehensive Review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, Y.; Kim, J.-E.; Kim, Y.; Paek, N.-S.; Kang, C.-H. Anti-Obesity Potential of Lactobacillus Spp. Isolated from Infant Feces. Biotechnol. Bioproc. E 2021, 26, 575–585. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Price, M.J.; Greenfield, S.M.; Paudyal, V. Global Prevalence and Types of Complementary and Alternative Medicines Use amongst Adults with Diabetes: Systematic Review and Meta-Analysis. Eur. J. Clin. Pharmacol. 2021, 77, 1259–1274. [Google Scholar] [CrossRef]
- Eawsakul, K.; Panichayupakaranant, P.; Ongtanasup, T.; Warinhomhoun, S.; Noonong, K.; Bunluepuech, K. Computational Study and in Vitro Alpha-Glucosidase Inhibitory Effects of Medicinal Plants from a Thai Folk Remedy. Heliyon 2021, 7, e08078. [Google Scholar] [CrossRef]
- Rosi, I.; Vinella, M.; Domizio, P. Characterization of β-Glucosidase Activity in Yeasts of Oenological Origin. J. Appl. Bacteriol. 1994, 77, 519–527. [Google Scholar] [CrossRef]
- Tizon, R.U.; Serrano, A.E.; Traifalgar, R.F. Effects of pH on Amylase, Cellulase and Protease of the Angelwing Clam, Pholas orientalis. Euro. J. Exp. Bio. 2012, 2, 2280–2285. [Google Scholar]
- Narita, Y.; Inouye, K. Kinetic Analysis and Mechanism on the Inhibition of Chlorogenic Acid and Its Components against Porcine Pancreas α-Amylase Isozymes I and II. J. Agric. Food Chem. 2009, 57, 9218–9225. [Google Scholar] [CrossRef]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic Activity Screening of Some Indigenous Thai Plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Nonpunya, A.; Sripanidkulchai, B.; Thitimetharoch, T. Cytotoxic and Apoptotic Effects of Six Herbal Plants against the Human Hepatocarcinoma (HepG2) Cell Line. Chin. Med. 2011, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Thumanu, K.; Tanthanuch, W. Synergistic Anticancer Effect of the Extracts from Polyalthia Evecta Caused Apoptosis in Human Hepatoma (HepG2) Cells. Asian Pac. J. Trop. Biomed. 2012, 2, 589–596. [Google Scholar] [CrossRef]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Thumanu, K.; Tanthanuch, W. FTIR Microspectroscopy Discriminates Anticancer Action on Human Leukemic Cells by Extracts of Pinus Kesiya; Cratoxylum Formosum Ssp. Pruniflorum and Melphalan. Talanta 2012, 93, 371–382. [Google Scholar] [CrossRef]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S. Anticancer Effect of the Extracts from Polyalthia Evecta against Human Hepatoma Cell Line (HepG2). Asian Pac. J. Trop. Biomed. 2012, 2, 368–374. [Google Scholar] [CrossRef]
- Pocasap, P.; Nonpunya, A.; Weerapreeyakul, N. Pinus Kesiya Royle Ex Gordon Induces Apoptotic Cell Death in Hepatocellular Carcinoma HepG2 Cell via Intrinsic Pathway by PARP and Topoisomerase I Suppression. Biomed. Pharmacother. 2021, 139, 111628. [Google Scholar] [CrossRef] [PubMed]
- Nonpunya, A.; Sethabouppha, B.; Rufini, S.; Weerapreeyakul, N. Cratoxylum Formosum Ssp. Pruniflorum Activates the TRAIL Death Receptor Complex and Inhibits Topoisomerase I. S. Afr. J. Bot. 2018, 114, 150–162. [Google Scholar] [CrossRef]
- Weerapreeyakul, N.; Nonpunya, A.; Barusrux, S.; Thitimetharoch, T.; Sripanidkulchai, B. Evaluation of the Anticancer Potential of Six Herbs against a Hepatoma Cell Line. Chin. Med. 2012, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Vlietinck, A.; Pieters, L.; Apers, S. Legal Requirements for the Quality of Herbal Substances and Herbal Preparations for the Manufacturing of Herbal Medicinal Products in the European Union. Planta Med. 2009, 75, 683–688. [Google Scholar] [CrossRef]
- Bureau of Drug and Narcotic. Thai Herbal Pharmacopoeia. 2021. Available online: https://bdn.go.th/thp/ebook/qQIcZatkpR9gC3q0GT5gMJq0qT5co3uw (accessed on 5 January 2022).
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila Aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-Amylase by Flavonoids: Structure Activity Relationship (SAR). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Du, J.; Deng, X.; Li, C. Persimmon Tannin Decreased the Glycemic Response through Decreasing the Digestibility of Starch and Inhibiting α-Amylase, α-Glucosidase, and Intestinal Glucose Uptake. J. Agric. Food Chem. 2018, 66, 1629–1637. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase Inhibition by Flavonoids: An In Vitro and In Silico Structure–Activity Relationship Study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, B.; Mo, H.; Liang, G. Comparative Evaluation of Tannic Acid Inhibiting α-Glucosidase and Trypsin. Food Res. Int. 2015, 76, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Ou-Yang, C.; Ma, Z.; Song, S.; Huang, Q.; Wei, Q.; Peng, Y. Anti-α-Glucosidase and Antityrosinase Activity of Condensed Tannins from the Bark of Clausena Lansium (Lour.) Skeels with Antiproliferative and Apoptotic Properties in B16 Mouse Melanoma Cells. Process Biochem. 2019, 86, 205–214. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J. CARBOHYDRATES | Classification and Properties. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 862–875. ISBN 978-0-12-227055-0. [Google Scholar]
- Phukhatmuen, P.; Raksat, A.; Laphookhieo, S.; Charoensup, R.; Duangyod, T.; Maneerat, W. Bioassay-Guided Isolation and Identification of Antidiabetic Compounds from Garcinia Cowa Leaf Extract. Heliyon 2020, 6, e03625. [Google Scholar] [CrossRef]
- Thiantongin, P.; Thammaporn, C. Wisdom of Diabetes Mellitus Treatment by Folk Healers in Ubon Ratchathani Province. J. Humanit. Soc. Sci. Surin Rajabhat Univ. 2020, 22, 1–18. [Google Scholar]
- Wanniarachchi, K.K.; Peiris, L.D.C.; Ratnasooriya, W.D. Antihyperglycemic and Hypoglycemic Activities of Phyllanthus Debilis Aqueous Plant Extract in Mice. Pharm. Biol. 2009, 47, 260–265. [Google Scholar] [CrossRef][Green Version]
- Li, Z.P.; Song, Y.H.; Uddin, Z.; Wang, Y.; Park, K.H. Inhibition of Protein Tyrosine Phosphatase 1B (PTP1B) and α-Glucosidase by Xanthones from Cratoxylum Cochinchinense, and Their Kinetic Characterization. Bioorganic Med. Chem. 2018, 26, 737–746. [Google Scholar] [CrossRef]
- Tiwari, P.; Kaur, M.; Kaur, H.; Kaur, G.; Kaur, H. Phytochemical Screening and Extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Shaikh, J.R.; Patil, M. Qualitative Tests for Preliminary Phytochemical Screening: An Overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Josse, R.G.; Chiasson, J.-L.; Ryan, E.A.; Lau, D.C.W.; Ross, S.A.; Yale, J.-F.; Leiter, L.A.; Maheux, P.; Tessier, D.; Wolever, T.M.S.; et al. Acarbose in the Treatment of Elderly Patients with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2003, 59, 37–42. [Google Scholar] [CrossRef]
- Schuster, D.; Laggner, C.; Langer, T. Why Drugs Fail–A Study on Side Effects in New Chemical Entities. Curr. Pharm. Des. 2005, 11, 3545–3559. [Google Scholar]

| Sample No. | Scientific Names | Family | Part Used |
|---|---|---|---|
| 1 | Abrus pulchellus ssp. mollis | Fabaceae | Stem |
| 2 | Aganosma marginata (Roxb.) G. Don | Apocynaceae | Leaf |
| 3 | Artabotrys harmandii Finet & Gagnep. | Annonaceae | Vine |
| 4 | Bombax anceps | Bombaceae | Stem |
| 5 | Bridelia ovata Decne. | Euphorbiaceae | Stem |
| 6 | Cassytha filiformis L. | Lauraceae | Vine |
| 7 | Catunaregam tomentosa | Rubiaceae | Stem |
| 8 | Cratoxylum formosum spp. pruniflorum | Hypericaceae | Stem |
| 9 | Croton oblongifolius | Euphorbiaceae | Stem |
| 10 and 11 | Diospyros castanea Fletcher | Ebenaceae | Twig and Leaf |
| 12 | Diospyros winitii | Ebenaceae | Stem |
| 13 | Ellipeiopsis cherrevensis (Pierre ex Finet & Gagnep.) R.E.Fr. | Annonaceae | Twig |
| 14 | Erythrophleum succirubrum | Fabaceae | Stem |
| 15 | Erythroxylum cuneatum (Miq.) Kurz | Erythroxylaceae | Twig |
| 16 | Flacourtia indica (Burm.f.) Merr. | Flacourtiaceae | Twig |
| 17 | Garcinia cowa Roxb. ex DC. | Guttiferae | Leaf |
| 18 | Glochidion daltonii (MÜll. Arg.) Kurz | Euphorbiaceae | Stem |
| 19 | Harrisonia perforata (Blanco) Merr. | Simaroubaceae | Twig |
| 20 | Lannea coromandelica | Anacardiaceae | Stem |
| 21 | Parinari anamense Hance | Chrysobalanaceae | Twig |
| 22 | Pinus kesiya Royle | Pinaceae | Twig |
| 23 | Polyalthia debilis (Pierre) Finet & Gagnep. | Annonaceae | Leaf |
| 24 and 25 | Polyalthia evecta (Pierre) Finet & Gagnep. | Annonaceae | Rhizome and Leaf |
| 26 | Rhodamnia dumetorum (DC.) Merr. & L.M. Perry | Myrtaceae | Twig |
| 27 | Rhus javanica | Anacardiaceae | Stem |
| 28 | Rhus succedanea | Anacardiaceae | Twig |
| 29 | Terminalia mucronata | Combretaceae | Stem |
| 30 | Terminalia triptera | Combretaceae | Stem |
| 31 | Tetracera loureiri (Finet. & Gagnep.) Pierre ex Craib | Dilleniaceae | Stem |
| Scientific Names | α-Amylase | α-Glucosidase | ||
|---|---|---|---|---|
| Emax | IC50 (µg/mL) | Emax | IC50 (µg/mL) | |
| Abrus pulchellus ssp. mollis | 24.7 ± 0.3 e | ND | 15.9 ± 0.1 o | ND |
| Aganosma marginata | 16 ± 0.1 c | ND | 37.6 ± 0.2 h,i | ND |
| Artabotrys harmandii | 27.2 ± 0.2 f | ND | 30.8 ± 0.2 l | ND |
| Bombax anceps | 13.2 ± 0.2 b | ND | 39.1 ± 0.6 e,f,g | ND |
| Bridelia ovata | 92.3 ± 0.4 q | 0.16 ± 0.004 g,h | 32.7 ± 1.4 j,k | ND |
| Cassytha filiformis | 35.6 ± 0.3 h,i | c | 36.7 ± 0.4 i | ND |
| Catunaregam tomentosa | 21.3 ± 2.2 p | ND | 33.9 ± 0.4 j | ND |
| Cratoxylum formosum spp. pruniflorum | 87 ± 0.2 f | 0.17 ± 0.005 g,h,i | 50.3 ± 0.4 c,d | 0.65 ± 0.03 b,c |
| Croton oblongifolius | 12.6 ± 0.4 a | ND | 53.0 ± 0.7 a,b | 0.49 ± 0.05 a |
| Diospyros castanea (leaf) | 84.1 ± 0.2 m | 0.16 ± 0.0007 g,h | 20.9 ± 0.2 n | ND |
| Diospyros castanea (twig) | 42.1 ± 0.4 i | ND | 29.7 ± 0.2 m | ND |
| Diospyros winitii | 93.3 ± 0.1 r | 0.12 ± 0.0004 b | 39.7 ± 0.2 e,f | ND |
| Ellipeiopsis cherrevensis | 77.8 ± 0.2 k | 0.18 ± 0.005 a | 39.8 ± 0.3 e,f | ND |
| Erythrophleum succirubrum | 35.4 ± 0.2 h,i | ND | 32.5 ± 0.2 j,k | ND |
| Erythroxylum cuneatum | 86.5 ± 0.1 o | 0.17 ± 0.011 h,i | 39.6 ± 0.1 e,f | ND |
| Flacourtia indica | 84.4 ± 0.1 n | 0.16 ± 0.004 f,g,h | 39.1 ± 0.2 f,g | ND |
| Garcinia cowa | 99.6 ± 0.2 u | 0.13 ± 0.001 c | 51.1 ± 0.4 c | 0.63 ± 0.02 b,c |
| Glochidion daltonii | 27.3 ± 2.1 p,q,r | ND | 37.6 ± 0.2 g,h,i | ND |
| Harrisonia perforata | 35.2 ± 0.3 h,i | ND | 39.9 ± 0.7 d,e,f | ND |
| Lannea coromandelica | 29.9 ± 0.2 f,g | ND | 31.4 ± 0.2 k | ND |
| Parinari anamense | 35.1 ± 0.3 h,i | ND | 54.5 ± 0.5 a | 0.52 ± 0.01 a |
| Pinus kesiya | 94.9 ± 0.1 t | 0.11 ± 0.001 a | 52.3 ± 0 a,b | 0.60 ± 0.01 b,c |
| Polyalthia debilis | 99.5 ± 0.2 u | 0.14 ± 0.0001 d,e,f | 50.0 ± 0.1 c,d,e | 0.72 ± 0.02 c |
| Polyalthia evecta (leaf) | 93.9 ± 0 s | 0.14 ± 0.0013 c,d | 52.0 ± 0.2 b | 0.57 ± 0.00 a,b |
| Polyalthia evecta (rhizome) | 35.8 ± 0.6 h,i | ND | 50.6 ± 0.3 c | 0.63 ± 0.03 b,c |
| Rhodamnia dumetorum | 30.3 ± 0.2 g | ND | 10.5 ± 0.8 p | ND |
| Rhus javanica | 82.1 ± 0.0 l | 0.14 ± 0.0009 c,d,e | 38.9 ± 0.1 f,g,h | ND |
| Rhus succedanea | 29.9 ± 0.1 f,g | ND | 31.6 ± 0.2 j,k | ND |
| Tetracera loureiri | 47.3 ± 0.1 j | ND | 20.5 ± 0.2 n | ND |
| Terminalia mucronata | 95.7 ± 0.1 u | 0.16 ± 0.001 e,f,g | 9.7 ± 0.5 p | ND |
| Terminalia triptera | 33.4 ± 0.1 h | ND | 32.6 ± 0.2 j,k | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisongkram, T.; Waithong, S.; Thitimetharoch, T.; Weerapreeyakul, N. Machine Learning and In Vitro Chemical Screening of Potential α-Amylase and α-Glucosidase Inhibitors from Thai Indigenous Plants. Nutrients 2022, 14, 267. https://doi.org/10.3390/nu14020267
Srisongkram T, Waithong S, Thitimetharoch T, Weerapreeyakul N. Machine Learning and In Vitro Chemical Screening of Potential α-Amylase and α-Glucosidase Inhibitors from Thai Indigenous Plants. Nutrients. 2022; 14(2):267. https://doi.org/10.3390/nu14020267
Chicago/Turabian StyleSrisongkram, Tarapong, Sasisom Waithong, Thaweesak Thitimetharoch, and Natthida Weerapreeyakul. 2022. "Machine Learning and In Vitro Chemical Screening of Potential α-Amylase and α-Glucosidase Inhibitors from Thai Indigenous Plants" Nutrients 14, no. 2: 267. https://doi.org/10.3390/nu14020267
APA StyleSrisongkram, T., Waithong, S., Thitimetharoch, T., & Weerapreeyakul, N. (2022). Machine Learning and In Vitro Chemical Screening of Potential α-Amylase and α-Glucosidase Inhibitors from Thai Indigenous Plants. Nutrients, 14(2), 267. https://doi.org/10.3390/nu14020267