Abstract
Genetic background is estimated to play >50% in common obesity etiology. FTO single nucleotide polymorphisms (SNPs) are strongly associated with BMI, typically in European cohorts. We investigated the interaction of common FTO SNPs with actionable environmental factors, namely physical activity, sugar-sweetened beverages (SSB) and wine consumption, and verified FTO common SNPs predisposition to obesity in the Israeli population. Adults’ (>18 years old, n = 1720) FTO common SNPs data and lifestyle and nutrition habits questionnaires were analyzed using binary logistic regression models, adjusted for confounding variables (age, sex) assuming dominant, recessive and additive genetic models. Eighteen FTO SNPs were associated with significant increased obesity risk and interacted with physical activity (p < 0.001), wine consumption (p < 0.014) and SSB consumption (p < 0.01). Inactive rs9939609 risk-allele carriers had significantly higher obesity risk compared to their active counterparts (OR = 2.54, 95% CI 1.91–3.39 and OR = 3.77, 95% CI 2.47–5.75; p < 0.001 with 3.1 and 3.5 BMI increment for heterozygotes and homozygotes, respectively). SSB consumption (≥1 serving/day) significantly raised obesity risk and wine consumption (1–3 drinks/weekly) significantly lowered obesity risk for rs9939609 risk-allele carriers (OR = 1.54, 95% CI 1.05–2.27; p = 0.028 and OR = 0.61, 95% CI 0.47–0.79; p < 0.001, respectively). Our findings demonstrate that actionable lifestyle factors modify the common FTO obesity risk in predisposed carriers, and they have personal and public health implications.
1. Introduction
The etiology of common obesity involves both environmental and genetic factors. It has been estimated that body mass index (BMI) heritability is high, ranging between 40% and 70% [1]. Common polygenic obesity is attributed to several hundreds of genetic polymorphisms, each with a relatively small effect [2]. The genetic predisposition to common obesity is phenotypically expressed with exposure to an obesogenic and sedentary environment. An obesogenic environment, shared by developed and developing countries worldwide [3], is characterized by calorie-dense food, pollutants, speed eating, large and increasing food portion size, high consumption of sugar-sweetened beverages (SSBs), and excessive intake of simple carbohydrates and sugars. Additionally, sedentary lifestyle, characterized by elevation in screen-related immobility, insufficient sleep and lack of adequate physical activity, contribute to the sharp elevation in obesity rates [4,5].
The fat mass and obesity associated (FTO) gene, located on chromosome 16, was identified through mutant mouse studies [6]. FTO is expressed in many tissues, both in peripheral and central-feeding brain regions such as arcuate, paraventricular and ventromedial nuclei [7]. The FTO gene encodes an alpha-ketoglutarate-dependent dioxygenase that through the methylation of DNA/RNA regulates transcription and translation [8]. Although FTO’s role in the development of obesity is not entirely clear, common polymorphisms in the FTO gene are strongly associated with obesity [9], BMI [10,11], increased energy and dietary fat intake [12,13], increased appetite and reduced satiety [14] as well as poor food choices and eating habits [15]. In fact, common FTO gene polymorphisms have the largest effect known on BMI and obesity risk [16] across ancestries [17,18,19], among adults, children and adolescents [10,11,16]. Of the 21 identified FTO single nucleotide polymorphisms (SNPs), 18 have shown strong association and have been replicated, mostly in European descent cohorts [20] with FTO SNP rs9939609 being the most studied [21]. It was suggested that FTO SNPs’ predisposition to obesity is race-specific, as the relationships between rs9939609 and obesity is inconsistent [22,23]. Previous studies of the interaction of FTO common SNPs with lifestyle factors, focusing mainly on rs9939609, found that obesity predisposition due to FTO SNPs can be modified by physical activity, attenuating increase in BMI [24,25]. Evidence for interaction was also found with SSBs in relation to BMI [26] and for dietary intake. However, reports in this regard are controversial and need further investigation [27,28].
Given that genetic predisposition to obesity is inherited and thus unchangeable, identifying actionable environmental factors that interact with this disposition is essential for treatment. Furthermore, different common FTO SNPs show variable association with obesity in different populations, thus challenging the inclusion of obesity risk estimates in other world populations. This study aims to investigate the interaction of common FTO SNPs with actionable environmental factors, including physical activity and drinking habits (SSB and wine consumption), in determining and modifying the risk of obesity through studies of the Israeli population.
2. Materials and Methods
2.1. Participants
Healthy adults (n = 1972), 1377 (79% females), mean aged 55.22 ± 14.36 data (21 December 2021–26 July 2022) were studied. The analysis in this work is based on information listed in Israeli registry database (#700068969) of Lev Hai Genetics LTD—MyGenes. Non-identifiable genetic data were used. The study was approved by the ethical committee of Ariel university (#AU-HEA-RB-20220214). Participants under the age of 18 years, having a genetic disease, diabetes or missing anthropometric data were excluded from the study (n = 40).
Data on physical activity and drinking habits were obtained as a part of an online lifestyle questionnaire filled by 1796 (90%) of study participants. Questions regarding physical activity habits included: “Are you physically active” (possible answers: yes; no); “How many days a week do you engage in physical activity” (answer: number of days participant is physically activity); “What is your physical activity duration” (possible answers: up to 30 min; at least 30 min; more than 60 min); Physically activity was defined as engaging ≥ 30 min/day in physical activity. Questions regarding drinking habits included; “How many cups of sugar-sweetened beverages do you drink on a daily basis?” (possible answers: none; 1–3 daily cups; more than 3 daily cups); “How many drinks of wine do you drink on a weekly basis” (possible answers: none; 1–3 weekly drinks; more than 3 weekly drinks)”. SSBs consumption was defined as having at least 1 daily serving equals 12 fluid ounces of SSB and regular wine consumption was defined as having 1–3 weekly drinks of wine; one drink equals 5 fluid ounces of wine.
Weight and height were self-reported. Height was reported in centimeters and weight in kilograms. BMI was calculated as the ratio of weight/(height)2 (kg/m2). Subjects were classified as obese (BMI ≥ 30) or not obese (BMI < 30) according to BMI cutoff points [29].
2.2. SNP Selection and Hardy–Weinberg Equilibrium (HWE)
SNPs that were previously shown to be significantly associated with obesity were selected. These SNPs were prioritized based on their minor allele frequency (MAF) (>0.01) in at least two GWAS populations [10,11,16,17,20,30,31,32,33,34] and based on the validated catalog of published genome-wide association studies [35]. The SNPs included: rs9939609, rs1421085, rs8050136, rs8051591, rs3751812, rs9935401, rs11075989, rs9923233, rs9936385, rs17817964, rs8043757, rs1121980, rs17817449, rs62033400, rs7202116, rs7193144, rs11075990, rs6499640, rs13333228, rs1558902 and rs9302652. Hardy–Weinberg equilibrium (HWE) was tested for each SNP using a 1 degree of freedom χ2-test, and all studied common FTO SNPs were in HWE.
2.3. Statistical Analyses
An a priori power analysis was conducted using G*Power 3.1.9.7 software [36] in order to determine the study sample size. The analysis revealed that using a sample of 80 participants across two groups was sufficient to observe the association between SNP and obesity (α = 0.05, with an odds ratio (OR) = 2 and a power of 0.80). We used a chi-square statistical test to compare dichotomic characteristic variables between obese and non- obese participants and Mann–Whitney for independent groups for continuous variables that were not normally distributed. Continuous variables are presented as mean values ± standard deviation. Binary logistic regression was performed to test the hypothesis of association between polymorphisms as predictors and their effect on the relative likelihood of obesity (BMI ≥ 30) after adjustment for potential confounders (age, gender). Logistic regression with interaction was used to determine the OR of gene–environment interaction with obesity. We determined whether the association of genotype with obesity risk was modified by physical activity and drinking habits by constructing a regression model that included the following independent variables: sex, age, physical activity (yes/no; yes regards for at least 30 min/a day regularly), regular SSBs consumption (≥1 daily serving/not consuming) and regular wine consumption (1–3 weekly drinks/not consuming) × genotype. The presence of an interaction between actionable lifestyle variables (physical activity and drinking habits) and SNPs genotype on obesity risk was assessed by a likelihood ratio test, in which we compared genotypes SNPs model with the interaction term. Statistical analyses were performed using SPSS 27.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Participants Characteristics
This cross-sectional study included 1972 (79% female) Israeli adult participants 55.22 ± 14.36. Population characteristics are shown in Table 1. Men were significantly more obese than women (p < 0.001). Risk of obesity for men was 1.5-fold higher compared to women. There was a significant difference in being physically active (≥30 min/day), consuming SSB (≥1 serving/day) and regularly drinking wine (1–3 drinks/week) between participants defined as obese (BMI ≥ 30) and participants defined as non-obese. Specifically, participants defined as obese were significantly less active compared to participants defined as non-obese (p < 0.001). Among the obese group, there were significantly less participants who consumed wine and more participants who consumed SSBs (p < 0.001 and p = 0.002), respectively. Age, height and smoking status did not differ between participants defined as obese and participants defined as non-obese.

Table 1.
Descriptive characteristics of study population by obesity status.
3.2. FTO SNPs Association and Obesity Risk
We analyzed twenty-one FTO SNPs with previously reported association to obesity. Three of the SNPs were found to be non-significant in relation to obesity, and all other eighteen studied SNPs were significantly related to elevated obesity risk with OR ranging from 1.2 to 1.35 and exhibited strong linkage disequilibrium (r2 = ~1) corresponding to CEU population. Thus, we focused on FTO rs9939609 as a representative of all SNPs. The distribution of FTO rs9939609 genotypes and OR for dominant, recessive additive and codominant genetic models adjusted for age and gender are shown in Table 2.

Table 2.
Participants’ FTO SNPs genotype frequency and obesity risk.
Among all individuals tested for the FTO rs9939609 variant, 24.8% were homozygous (AA), 46.9% were heterozygous (TA), and 28.2% were wild type (TT). Follow-up regression analysis of FTO SNPs homozygous individuals compared to heterozygotes variants carriers indicated that the additional allele did not contribute to a higher OR had a very weak contribution, indicating that the SNP association to obesity follows a dominant model. Compared to wild type, a 0.65 BMI increment was found for rs9939609 variant heterozygous and 1.2 BMI increment for rs9939609 variant homozygous (p < 0.001).
3.3. Gene Environment Interactions
3.3.1. Physical Activity Interaction with FTO on Obesity Risk
All eighteen FTO SNPs had a significant interaction with physical activity (p < 0.001) (Table S1). The frequency of FTO rs9939609 genotypes did not differ significantly between physically active and inactive participants (p = 0.38). Specifically, the distribution of FTO rs9939609 genotypes regarding physical activity was 240 (53.7%) homozygous (AA), 413 (49%) heterozygous (TA), and 255 (50.4%) wild type (TT). Although there was no significant difference in genotype frequency between active and inactive individuals, there was a significant difference in mean weight, BMI and obesity status between FTO rs9939609 genotypes (p = 0.008, p = 0.004 and p = 0.013, respectively) (Table 3).

Table 3.
FTO rs9939609 SNP population characteristics.
As shown in Table 4, obesity risk was significantly higher among FTO rs9939609 risk-allele carriers that were physically inactive as compared to non-risk inactive counterparts, both in the dominant, recessive and codominant models. Obesity risk was higher in inactive individuals homozygous to FTO rs9939609 compared to inactive individuals heterozygous to FTO rs9939609 (OR = 1.78, 95% CI 1.23–2.57; p = 0.002 and OR = 1.4, 95% CI 1–1.93; p = 0.04), respectively. There was no significant difference in obesity risk between physically active risk carriers, both heterozygous or homozygous to FTO rs9939609 variant, compared to physically active wild types.

Table 4.
Physical activity effect on FTO rs9939609 obesity risk.
We further analyzed effects of gender in relation to obesity risk interaction with physical activity. We found a significant effect of gender on obesity risk interaction with physical activity. Obesity risk for inactive FTO rs9939609 homozygous men was significantly higher compared to inactive homozygous women (OR = 3, 95% CI 1.2–7.52; p = 0.019 and OR = 1.58, 95% CI 1.05–2.38; p = 0.03, respectively).
Table 5 shows the effect of potential lifestyle variables on FTO rs9939609 risk-allele carriers and obesity risk. After adjusting for potential confounders (age and gender), inactive individuals FTO rs9939609 risk-allele carriers (TA and AA) demonstrated higher obesity risk compared to their physically active counterparts (β = 1.048, p < 0.001). Inactive non-risk carriers had 2.29 times higher obesity risk compared to active counterparts. Inactive FTO rs9939609 risk-allele homozygotes had 3.77 times higher obesity risk compared to active homozygotes. Inactivity significantly increased risk of obesity even in wild-type individuals (TT carriers).

Table 5.
Physical inactivity and wine consumption effect on FTO rs9939609 obesity risk.
3.3.2. Wine Consumption Interaction with FTO Polymorphism in Determining Obesity Risk
Wine consumption significantly interacted with all eighteen FTO SNPs in determining FTO SNPs-related obesity, assuming a dominant model. Regardless of genotype, the regular consumption of one to three weekly drinks of wine reduced obesity risk by 36% (p < 0.001) in our sample (not shown in the table).
Weekly wine consumption reduced the risk of obesity by 44% (OR = 0.56) for the wild type (TT) (p = 0.009) and by 32% (OR = 0.68) for those who carried at least one FTO rs9939609 risk allele (p = 0.005) compared to their non-drinking counterparts (n = 494 and n = 1260, respectively) (Table 5). Thus, the risk of obesity among risk-allele carriers regularly drinking wine was reduced to that of non-risk carriers.
3.3.3. SSBs Consumption Interaction with FTO on Obesity Risk
SSB consumption of ≥1 serving/day significantly increased obesity risk (OR = 1.5, 95% CI 1.1–2.06; p = 0.011).
Consuming SSBs significantly interacted with FTO rs8050136, rs1421085, rs9939609 and rs1121980 in risk-allele carriers to effect obesity (p = 0.013, p = 0.015 p = 0.049 and p = 0.017), respectively.
FTO rs9939609 A allele carriers who consumed ≥1 serving of SSB on a daily basis had a 1.5-fold increased risk of obesity compared to A allele carriers who did not consume any SSBs (p = 0.028). Interestingly, when stratified to gender, obesity risk was higher but remained significant only for rs9939609 heterozygous women (OR = 2.23, 95% CI 1.33–3.76; p = 0.002). The same results were shown for the other three SNP’s (Table 6).

Table 6.
Interaction between SSBs consumption and FTO SNPs on obesity risk (BMI > 30) in women.
BMI levels were significantly different between active and inactive participants and between SSB consumers and non-consumers across all FTO rs9939609 genotype groups. Significant differences in BMI levels were found between wine consumers and non-consumers within the wild-type (TT) and heterozygote (TA) genotypes (Figure 1).
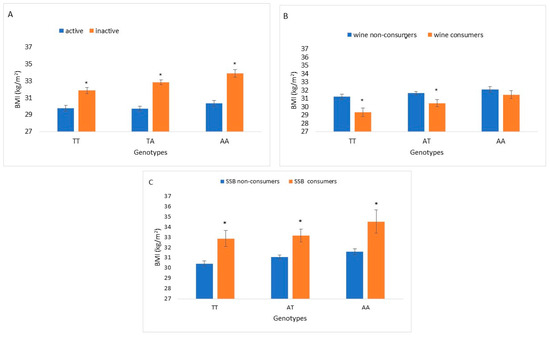
Figure 1.
BMI increments of FTO rs9939609 genotypes interaction with actionable environmental factors: physical activity (n = 1735), wine consumption (n = 1754) and SSB consumption (n = 1796). (A): BMI of physical activity stratified by FTO rs9939609 genotypes. Bars indicate BMI levels of active and inactive FTO rs9939609 wild type (TT), heterozygous (TA) and homozygous (AA). (B): BMI of wine consumption stratified by FTO rs9939609 genotypes. Bars indicate BMI levels of participants consuming 1–3 drinks/week and non-wine consumers among FTO rs9939609 genotypes. (C): BMI of SSBs (≥1 serving/day) consumption stratified by FTO rs9939609 genotypes. Bars indicate BMI levels of participants consuming ≥1 serving of SSB/day and non-SSB consumers among FTO rs9939609 genotypes. Differences between BMI levels within each genotype group (TT, TA and AA) were tested by Mann–Whitney for 2 independent samples. Asterisks represent statistically significant difference p < 0.05.
4. Discussion
We demonstrate that FTO common obesity SNPs interact with actionable environmental factors and are associated with obesity in the Israeli population. All 18 studied SNPs were significantly associated with elevated obesity risk and interacted with physical activity and wine consumption as obesity risk modulators. Four SNPs interacted with SSBs consumption to enhance obesity risk.
We show a significant modulating interaction between physical activity and FTO rs9939609 on obesity risk. To date, publications regarding FTO rs9939609 obesity risk-allele carriers’ interaction with physical activity show inconsistency in different populations [37,38,39,40], stressing the importance of studying this interaction in different populations. In our study, we found no difference in obesity risk between physically active rs9939609 variant carriers and physically active non-carriers. FTO rs9939609 risk-allele carriers who were physically active had 25% lower risk for obesity compared to risk-allele carriers who were not physically active, where homozygous risk carriers had a 2.03 BMI increment compared to active counterparts (p = 0.004). This increment is higher than previous reports showing 0.44 and 1.95 BMI increment per risk allele in inactive rs9939609 A-allele homozygotes carriers compared with T-allele homozygotes [41,42]. The mechanism underlying the effect of physical activity on FTO might be related to FTO’s potential role in the regulation of energy homeostasis [8]. Our results suggest that physical activity is an important key factor that might attenuate and prevent the adverse effect of FTO SNPs on obesity for susceptible individuals, reinforcing the importance of physical activity as a strategy for the treatment and prevention of obesity in general and for genetically designated individuals in particular. The appropriate activity and duration should be further investigated for the precise protocol for different genetic architecture.
Further investigating actionable interactions between lifestyle factor and FTO polymorphisms, we studied the interaction between wine consumption and FTO rs9939609 variation on obesity risk. Previous studies mostly investigated interactions between FTO variants and alcohol consumption and risk for alcohol dependence but not the risk of obesity [43]. Several studies showed that FTO SNPs (including rs8062891, rs1108086, rs1420318, rs12597786 and rs7204609) had significant associations with alcohol dependence [44]. Our study is to the best of our knowledge the first to provide evidence for a possible interaction between wine consumption and FTO risk predisposition to obesity. We show that wine consumption (1–3 weekly drinks) reduced the risk of obesity by 32% for rs9939609 A carriers who had at least one risk allele, suggesting that wine has a protective effect against obesity when consumed in moderation. The consumption of 1–3 wine drinks/week are in line with the 2015–2020 dietary guidelines for Americans. recommending alcohol consumption of up to one alcoholic drink per day for women and up to two alcoholic drinks per day for men (one drink of wine = 150 mL) [45]. The effect of wine on obesity parameters has been inconsistent, with reports of either beneficial or adverse effects [46,47,48]. However, regardless of genetic predisposition, several epidemiological studies and clinical trials have reported a beneficial effect of wine consumption on human health with anti-obesity properties [49,50]. Wine consumption had the greatest inverse association for risk of overweight among other alcoholic beverages (hazard ratio: 0.75, 95% CI 0.68–0.84) [51]. In a large cohort (n = 280,183 participants), red-wine drinkers had −0.75 kg/m2 lower BMI compared to non-drinkers [52]. In our study, FTO rs9939609 wild type and heterozygotes who drank between one and three weekly drinks of wine had ≈−1.9 kg/m2 and 1.2 kg/m2 BMI reduction compared to their non-drinking wine counterparts, respectively. To date, the functional mechanisms explaining the interaction between risk of obesity, moderate wine consumption and genetic polymorphisms (including FTO rs9939609) have not been fully resolved due to the complexity of the factors involved. Yet, several studies suggested that the presence of resveratrol, a natural polyphenol found in grape skin with antioxidant properties, has beneficial effects on health, including body weight, BMI, adipocyte size and inflammatory cytokines reduction [53,54]. Nevertheless, the precise functional relation between wine consumption, FTO variants and obesity risk should be further studied.
SSB consumption has been strongly linked to obesity [55]. We found an interaction between FTO rs9939609, rs8050136, rs1421085 and rs1121980 polymorphism carriers and SSB on obesity risk in women. Female carriers of the rs9939609 risk allele consuming daily SSB (≥1 serving) had significantly increased obesity risk compared to non-consumers. In concordance with our results, a recent study has shown that rs9939609 A risk-allele carriers are prone to higher scores of food cravings and resistance to age-related decline in cravings [56]. Moreover, a significant association between FTO rs11642841 (correlated with FTO rs9939609) and total sugar intake was reported [57]. A potential mechanism related to the interaction of these four particular FTO SNPs with SSBs may be explained by their association to sugar metabolism and insulin resistance [58,59], although reports on the role of rs1421085 in glucose metabolism were inconclusive [60,61]. Our results reinforce previous findings, but more importantly, they complete a bigger picture of a potential intervention strategy to prevent the effect of SSB consumption by targeting guidelines in this genetic group predisposed to obesity, specifically in women.
We have demonstrated that eighteen studied FTO common SNPs are significantly associated with obesity in the Israeli population. In all eighteen FTO SNPs, carrying at least one risk variant allele elevated the odds of being obese (BMI ≥ 30 kg/m2). The FTO rs9939609 minor allele frequency (MAF) in our studied population was 0.48 (with similar MAF of the other SNPs), which is close to the previously reported European (0.41) and African (0.47) MAF (in line with the fact that the Israeli population originated mostly from European and African countries) [62], but it was higher than the Asian allele frequency (0.13) [11,63]. Carrying the FTO rs9939609 risk allele was associated with BMI increments of 0.65 kg/m2 (p = 0.015) and 1.2 kg/m2 (p = 0.002) for heterozygotes and homozygotes, respectively. These findings are in agreement with studies in Caucasian populations [11,64,65]. Our findings replicate and verify the association between FTO SNPs and elevated risk of obesity.
Our study has several limitations: due to the cross-sectional nature of this investigation, we cannot infer causality regarding the FTO SNPs and obesity, as functional variants are still unknown. Furthermore, BMI is a convenient surrogate measure for obesity, but it may be influenced by changes in bone mass and lean mass as well as adiposity. Another limitation of the study is the use of self-reported questionnaires and anthropometric measurements which may not be always accurate and to some degree rely on the weight and emotional status of the participants compared to measurement taken by professional stuff. Furthermore, self-reported weight usually tends to underestimation, especially in overweight and obese participants than those of normal weight [66]. This research studied the Israeli population, and thus, the findings may not be relevant to other populations. Our study has several strengths, including a large sample cohort, providing more reliable results with smaller margins of error. Additionally, we analyzed both several common FTO genetic variations and lifestyle habits. Furthermore, this is, to the best of our knowledge, the first nutrigenetics report regarding FTO and obesity in the Israeli population.
5. Conclusions
We found a significant association of eighteen common FTO obesity-predisposition SNPs with the risk of obesity in the Israeli population. Significant gene–environmental interaction was found for the rs9939609 FTO, where physical activity significantly reduces the FTO genetic obesity predisposition. Furthermore, consuming SSB was found to significantly elevate the FTO genetic obesity predisposition risk, while wine consumption was found to significantly reduce the FTO genetic obesity predisposition risk. Our results demonstrate actionable treatments effective in reducing elevated genetic predisposition for obesity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14194202/s1, Table S1: FTO SNP’s interaction with physical activity.
Author Contributions
D.C. and R.B. Conceptualization, methodology formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing. R.B. supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Ariel university (#AU-HEA-RB-20220214).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge Lev Hai Genetics LTD—MyGenes for the data.
Conflicts of Interest
R.B. is a scientific consultant of MyGenes. D.C. declares no conflict of interest.
References
- Elks, C.E.; den Hoed, M.; Zhao, J.H.; Sharp, S.J.; Wareham, N.J.; Loos, R.J.; Ong, K.K. Variability in the heritability of body mass index: A systematic review and meta-regression. Front. Endocrinol. 2012, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Rankinen, T.; Zuberi, A.; Chagnon, Y.C.; Weisnagel, S.J.; Argyropoulos, G.; Walts, B.; Pérusse, L.; Bouchard, C. The Human Obesity Gene Map: The 2005 Update. Obesity 2006, 14, 529–644. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Obes. Lipotoxicity 2017, 960, 1–17. [Google Scholar]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P., Jr. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidis, S. Environment and obesity. Metab. Clin. Exp. 2019, 100, 153942. [Google Scholar] [CrossRef]
- Peters, T.; Ausmeier, K.; Dildrop, R.; Rüther, U. The mouse Fused toes (Ft) mutation is the result of a 1.6-Mb deletion including the entire Iroquois B gene cluster. Mamm. Genome 2002, 13, 186–188. [Google Scholar] [CrossRef]
- Fredriksson, R.; Hägglund, M.; Olszewski, P.K.; Stephansson, O.; Jacobsson, J.A.; Olszewska, A.M.; Levine, A.S.; Lindblom, J.; Schiöth, H.B. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 2008, 149, 2062–2071. [Google Scholar] [CrossRef]
- Gerken, T.; Girard, C.A.; Tung, Y.L.; Webby, C.J.; Saudek, V.; Hewitson, K.S.; Yeo, G.S.; McDonough, M.A.; Cunliffe, S.; McNeill, L.A.; et al. The Obesity-Associated FTO Gene Encodes a 2-Oxoglutarate–Dependent Nucleic Acid Demethylase. Science 2007, 318, 1469–1472. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Sun, B.; Zhao, Y.; Yang, Y. FTO and obesity: Mechanisms of association. Curr. Diab. Rep. 2014, 14, 486. [Google Scholar] [CrossRef]
- Meyre, D.; Delplanque, J.; Chèvre, J.; Lecoeur, C.; Lobbens, S.; Gallina, S.; Durand, E.; Vatin, V.; Degraeve, F.; Proença, C.; et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat. Genet. 2009, 41, 157–159. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A Common Variant in the FTO Gene Is Associated with Body Mass Index and Predisposes to Childhood and Adult Obesity. Science 2007, 316, 889. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.E.; Tavendale, R.; Watt, P.; Hetherington, M.M.; Palmer, C.N.A. An obesity-associated FTO gene variant and increased energy intake in children. N. Engl. J. Med. 2008, 359, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Cheng, I.; Pendergrass, S.A.; Kucharska-Newton, A.M.; Lim, U.; Ambite, J.L.; Caberto, C.P.; Monroe, K.R.; Schumacher, F.; Hindorff, L.A.; et al. Association of the FTO Obesity Risk Variant rs8050136 With Percentage of Energy Intake from Fat in Multiple Racial/Ethnic Populations: The PAGE Study. Am. J. Epidemiol. 2013, 178, 780. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Llewellyn, C.; Sanderson, S.; Plomin, R. The FTO gene and measured food intake in children. Int. J. Obes. 2009, 33, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Ericson, U.; Hellstrand, S.; Gullberg, B.; Orho-Melander, M.; Sonestedt, E. Genetic variation in the fat mass and obesity-associated gene (FTO) in association with food preferences in healthy adults. Food Nutr. Res. 2013, 57, 20028. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F.; Yeo, G.S.H. The bigger picture of FTO—The first GWAS-identified obesity gene. Nature reviews. Endocrinology 2014, 10, 51–61. [Google Scholar] [PubMed]
- Croteau-Chonka, D.C.; Marvelle, A.F.; Lange, E.M.; Lee, N.R.; Adair, L.S.; Lange, L.A.; Mohlke, K.L. Genome-wide association study of anthropometric traits and evidence of interactions with age and study year in Filipino women. Obesity 2011, 19, 1019–1027. [Google Scholar] [CrossRef]
- Hotta, K.; Nakata, Y.; Matsuo, T.; Kamohara, S.; Kotani, K.; Komatsu, R.; Itoh, N.; Mineo, I.; Wada, J.; Masuzaki, H.; et al. Variations in the FTO gene are associated with severe obesity in the Japanese. J. Hum. Genet. 2008, 53, 546–553. [Google Scholar] [CrossRef]
- Tan, J.T.; Dorajoo, R.; Seielstad, M.; Sim, X.L.; Ong, R.T.; Chia, K.S.; Wong, T.Y.; Saw, S.M.; Chew, S.K.; Aung, T.; et al. FTO Variants Are Associated With Obesity in the Chinese and Malay Populations in Singapore. Diabetes 2008, 57, 2851–2857. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, H.; He, H.; Wu, K.; Li, J.; Chen, X.; Zhang, J.G.; Shen, H.; Tian, Q.; Krousel-Wood, M.; et al. Replication of 6 obesity genes in a meta-analysis of genome-wide association studies from diverse ancestries. PLoS ONE 2014, 9, e96149. [Google Scholar] [CrossRef]
- Dastgheib, S.A.; Bahrami, R.; Setayesh, S.; Salari, S.; Mirjalili, S.R.; Noorishadkam, M.; Sadeghizadeh-Yazdi, J.; Akbarian, E.; Neamatzadeh, H. Evidence from a meta-analysis for association of MC4R rs17782313 and FTO rs9939609 polymorphisms with susceptibility to obesity in children. Diabetes Metab. Syndr. 2021, 15, 102234. [Google Scholar] [CrossRef] [PubMed]
- Hester, J.M.; Wing, M.R.; Li, J.; Palmer, N.D.; Xu, J.; Hicks, P.J.; Roh, B.H.; Norris, J.M.; Wagenknecht, L.E.; Langefeld, C.D.; et al. Implication of European-derived adiposity loci in African Americans. Int. J. Obes. 2012, 36, 465–473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Wu, Y.; Loos, R.J.F.; Hu, F.B.; Liu, Y.; Wang, J.; Yu, Z.; Lin, X. Variants in the fat mass- and obesity-associated (FTO) gene are not associated with obesity in a Chinese Han population. Diabetes 2008, 57, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, T.O.; Qi, L.; Brage, S.; Sharp, S.J.; Sonestedt, E.; Demerath, E.; Ahmad, T.; Mora, S.; Kaakinen, M.; Sandholt, C.H.; et al. Physical activity attenuates the influence of FTO variants on obesity risk: A meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011, 8, e1001116. [Google Scholar] [CrossRef]
- Kim, J.Y.; DeMenna, J.T.; Puppala, S.; Chittoor, G.; Schneider, J.; Duggirala, R.; Mandarino, L.J.; Shaibi, G.Q.; Coletta, D.K. Physical activity and FTO genotype by physical activity interactive influences on obesity. BMC Genet. 2016, 17, 47. [Google Scholar] [CrossRef][Green Version]
- Qi, Q.; Chu, A.Y.; Kang, J.H.; Jensen, M.K.; Curhan, G.C.; Pasquale, L.R.; Ridker, P.M.; Hunter, D.J.; Willett, W.C.; Rimm, E.B.; et al. Sugar-Sweetened Beverages and Genetic Risk of Obesity. N. Engl. J. Med. 2012, 367, 1387–1396. [Google Scholar] [CrossRef]
- Haupt, A.; Thamer, C.; Staiger, H.; Tschritter, O.; Kirchhoff, K.; Machicao, F.; Häring, H.U.; Stefan, N.; Fritsche, A. Variation in the FTO gene influences food intake but not energy expenditure. Exp. Clin. Endocrinol. Diabetes 2009, 117, 194–197. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, H.; Pan, A.; Patel, B.; Xiang, G.; Qi, L.; Kaplan, R.C.; Hu, F.; Wylie-Rosett, J.; Qi, Q. FTO genotype and weight loss in diet and lifestyle interventions: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 10, 1162–1170. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Thorleifsson, G.; Walters, G.B.; Gudbjartsson, D.F.; Steinthorsdottir, V.; Sulem, P.; Helgadottir, A.; Styrkarsdottir, U.; Gretarsdottir, S.; Thorlacius, S.; Jonsdottir, I.; et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009, 41, 18–24. [Google Scholar] [CrossRef]
- Hinney, A.; Nguyen, T.T.; Scherag, A.; Friedel, S.; Brönner, G.; Müller, T.D.; Grallert, H.; Illig, T.; Wichmann, H.E.; Rief, W.; et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE 2007, 2, e1361. [Google Scholar] [CrossRef]
- Scuteri, A.; Sanna, S.; Chen, W.; Uda, M.; Albai, G.; Strait, J.; Najjar, S.; Nagaraja, R.; Orrú, M.; Usala, G.; et al. Genome-Wide Association Scan Shows Genetic Variants in the FTO Gene Are Associated with Obesity-Related Traits. PLoS Genet. 2007, 3, e115. [Google Scholar] [CrossRef] [PubMed]
- Willer, C.J.; Speliotes, E.K.; Loos, R.J.F.; Li, S.; Lindgren, C.M.; Heid, I.M.; Berndt, S.I.; Elliott, A.L.; Jackson, A.U.; Lamina, C.; et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 2009, 41, 25–34. [Google Scholar] [PubMed]
- Qi, Q.; Kilpeläinen, T.O.; Downer, M.K.; Tanaka, T.; Smith, C.E.; Sluijs, I.; Sonestedt, E.; Chu, A.Y.; Renström, F.; Lin, X.; et al. FTO genetic variants, dietary intake and body mass index: Insights from 177 330 individuals. Hum. Mol. Genet. 2014, 23, 6961–6972. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, J.; Bowler, E.; Cerezo, M.; Gil, L.; Hall, P.; Hastings, E.; Junkins, H.; McMahon, A.; Milano, A.; Morales, J.; et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Rampersaud, E.; Mitchell, B.D.; Pollin, T.I.; Fu, M.; Shen, H.; O’Connell, J.R.; Ducharme, J.L.; Hines, S.; Sack, P.; Naglieri, R.; et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch. Intern. Med. 2008, 168, 1791–1797. [Google Scholar] [CrossRef]
- Liaw, Y.; Liaw, Y.; Lan, T. Physical Activity Might Reduce the Adverse Impacts of the FTO Gene Variant rs3751812 on the Body Mass Index of Adults in Taiwan. Genes 2019, 10, 354. [Google Scholar] [CrossRef]
- Jonsson, A.; Renström, F.; Lyssenko, V.; Brito, E.C.; Isomaa, B.; Berglund, G.; Nilsson, P.M.; Groop, L.; Franks, P.W. Assessing the effect of interaction between an FTO variant (rs9939609) and physical activity on obesity in 15,925 Swedish and 2,511 Finnish adults. Diabetologia 2009, 52, 1334–1338. [Google Scholar] [CrossRef][Green Version]
- Leońska-Duniec, A.; Jastrzębski, Z.; Zarębska, A.; Maciejewska, A.; Ficek, K.; Cięszczyk, P. Assessing effect of interaction between the FTO A/T polymorphism (rs9939609) and physical activity on obesity-related traits. J. Sport Health Sci. 2018, 7, 459–464. [Google Scholar] [CrossRef]
- Andreasen, C.H.; Stender-Petersen, K.L.; Mogensen, M.S.; Torekov, S.S.; Wegner, L.; Andersen, G.; Nielsen, A.L.; Albrechtsen, A.; Borch-Johnsen, K.; Rasmussen, S.S.; et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes 2008, 57, 95–101. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Li, S.; Zhao, J.H.; Luan, J.; Bingham, S.A.; Khaw, K.; Ekelund, U.; Wareham, N.J.; Loos, R.J. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am. J. Clin. Nutr. 2009, 90, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Hubacek, J.A.; Adamkova, V.; Dlouha, D.; Jirsa, M.; Šperl, J.; Tönjes, A.; Kovacs, P.; Pikhart, H.; Peasey, A.; Bobak, M. Fat mass and obesity-associated (fto) gene and alcohol intake. Addiction 2012, 107, 1185–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Luo, X.; Zeng, M.; Zuo, L.; Wang, K. Genetic variants in the fat mass- and obesity-associated (FTO) gene are associated with alcohol dependence. J. Mol. Neurosci. 2013, 51, 416–424. [Google Scholar] [CrossRef] [PubMed]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary Guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef]
- Duncan, B.B.; Chambless, L.E.; Schmidt, M.I.; Folsom, A.R.; Szklo, M.; Crouse, J.R.; Carpenter, M.A. Association of the waist-to-hip ratio is different with wine than with beer or hard liquor consumption. Atherosclerosis Risk in Communities Study Investigators. Am. J. Epidemiol. 1995, 142, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, L.; Bengtsson, C.; Hällström, T.; Björntorp, P. Obesity, adipose tissue distribution and health in women--results from a population study in Gothenburg, Sweden. Appetite 1989, 13, 25–35. [Google Scholar] [CrossRef]
- Slattery, M.L.; McDonald, A.; Bild, D.E.; Caan, B.J.; Hilner, J.E.; Jacobs, D.R.; Liu, K. Associations of body fat and its distribution with dietary intake, physical activity, alcohol, and smoking in blacks and whites. Am. J. Clin. Nutr. 1992, 55, 943–949. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef]
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health-New Evidence. Eur. J. Clin. Nutr. 2019, 72, 55–59. [Google Scholar] [CrossRef]
- Thomson, C.A.; Wertheim, B.C.; Hingle, M.; Wang, L.; Neuhouser, M.L.; Gong, Z.; Garcia, L.; Stefanick, M.L.; Manson, J.E. Alcohol consumption and body weight change in postmenopausal women: Results from the Women’s Health Initiative. Int. J. Obes. 2012, 36, 1158–1164. [Google Scholar] [CrossRef]
- Inan-Eroglu, E.; Powell, L.; Hamer, M.; O’Donovan, G.; Duncan, M.J.; Stamatakis, E. Is There a Link between Different Types of Alcoholic Drinks and Obesity? An Analysis of 280,183 UK Biobank Participants. Int. J. Environ. Res. Public Health 2020, 17, 5178. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.C.; Samanez-Larkin, G.R.; Smith, C.T.; Castrellon, J.J.; Perkins, S.F.; Cowan, R.L.; Claassen, D.O.; Zald, D.H. FTO affects food cravings and interacts with age to influence age-related decline in food cravings. Physiol. Behav. 2018, 192, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.; Lin, C.; Gharahkhani, P.; Cuellar-Partida, G.; Ong, J.; An, J.; Gordon, S.D.; Zhu, G.; MacGregor, S.; Lawlor, D.A.; et al. New insight into human sweet taste: A genome-wide association study of the perception and intake of sweet substances. Am. J. Clin. Nutr. 2019, 109, 1724–1737. [Google Scholar] [CrossRef]
- Kong, X.; Hong, J.; Chen, Y.; Chen, L.; Zhao, Z.; Li, Q.; Ge, J.; Chen, G.; Guo, X.; Lu, J.; et al. Association of Genetic Variants with Isolated Fasting Hyperglycaemia and Isolated Postprandial Hyperglycaemia in a Han Chinese Population. PLoS ONE 2013, 8, e71399. [Google Scholar] [CrossRef]
- Shimaoka, I.; Kamide, K.; Ohishi, M.; Katsuya, T.; Akasaka, H.; Saitoh, S.; Sugimoto, K.; Oguro, R.; Congrains, A.; Fujisawa, T.; et al. Association of gene polymorphism of the fat-mass and obesity-associated gene with insulin resistance in Japanese. Hypertens. Res. 2010, 33, 214–218. [Google Scholar] [CrossRef]
- Chauhan, G.; Tabassum, R.; Mahajan, A.; Dwivedi, O.P.; Mahendran, Y.; Kaur, I.; Nigam, S.; Dubey, H.; Varma, B.; Madhu, S.V.; et al. Common variants of FTO and the risk of obesity and type 2 diabetes in Indians. J. Hum. Genet. 2011, 56, 720–726. [Google Scholar] [CrossRef]
- Grzeszczak, W.; Molsa, M.; Tłuczykont, M.; Markowicz, A.; Swoboda, R.; Biedak, M.; Kałuża, A.; Sirek, S.; Strojek, K. The age of developing diabetes and FTO polymorphisms (rs9939609, rs1421085, and rs9930506). Endokrynol. Pol. 2017, 68, 402–406. [Google Scholar] [CrossRef]
- Lewin-Epstein, N.; Cohen, Y. Ethnic origin and identity in the Jewish population of Israel. J Ethn Migr Stud 2018, 45, 2118–2137. [Google Scholar] [CrossRef]
- Sentinelli, F.; Incani, M.; Coccia, F.; Capoccia, D.; Cambuli, V.M.; Romeo, S.; Cossu, E.; Cavallo, M.G.; Leonetti, F.; Baroni, M.G. Association of FTO Polymorphisms with Early Age of Obesity in Obese Italian Subjects. Exp. Diabetes Res. 2012, 2012, 872176. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Carrasco, P.; Sorlí, J.V.; Coltell, O.; Ortega-Azorín, C.; Guillén, M.; González, J.I.; Sáiz, C.; Estruch, R.; Ordovas, J.M. Education modulates the association of the FTO rs9939609 polymorphism with body mass index and obesity risk in the Mediterranean population. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhu, Y.; Xu, F.; Ren, X.; Li, X.; Lai, M. FTO gene polymorphisms and obesity risk: A meta-analysis. BMC Med. 2011, 9, 71. [Google Scholar] [CrossRef]
- Maukonen, M.; Männistö, S.; Tolonen, H. A comparison of measured versus self-reported anthropometrics for assessing obesity in adults: A literature review. Scand. J. Public Health 2018, 46, 565–579. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).