Effect of Olive Pomace Oil on Cardiovascular Health and Associated Pathologies
Abstract
:1. Introduction
2. Materials and Methods
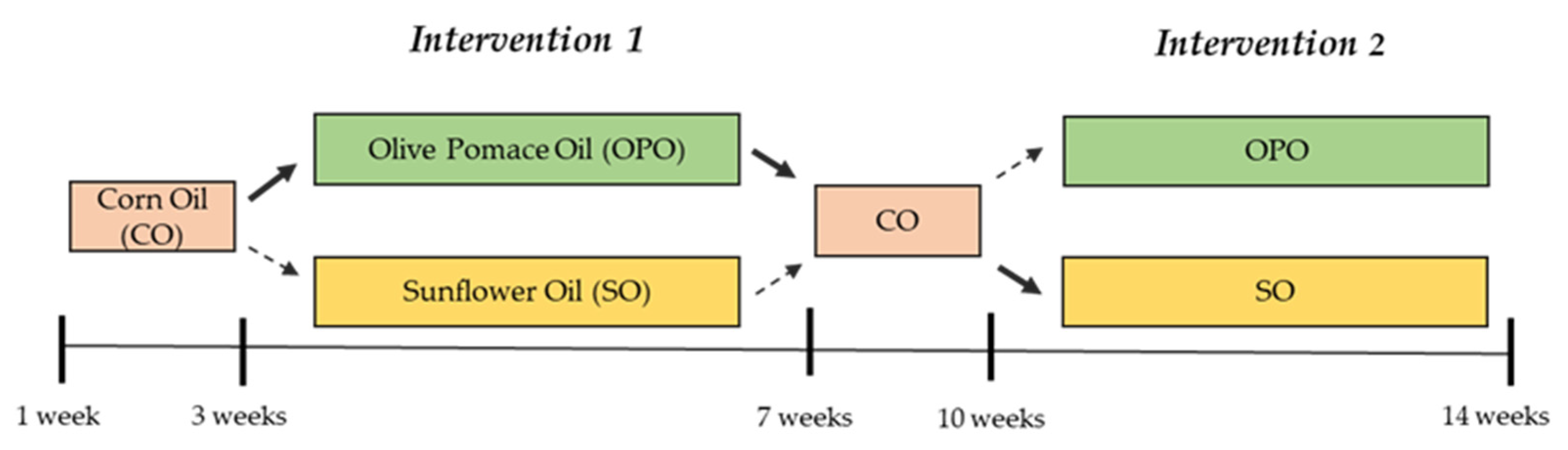
2.1. Study Design
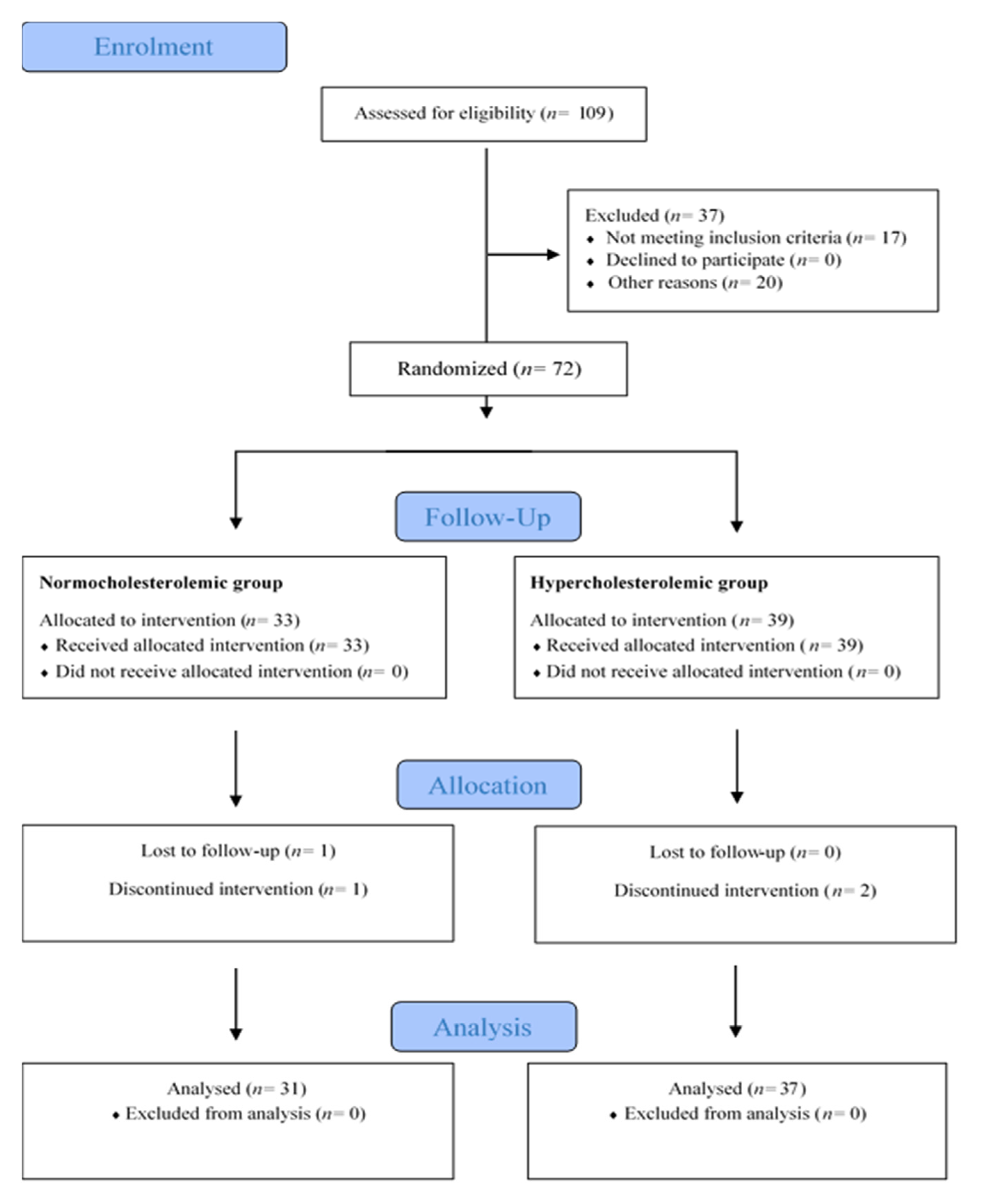
2.2. Participants and Setting
2.3. Intervention
2.4. Chemical Characterization of the Study Oils
2.5. Dietary Assessment and Compliance
2.6. Blood Sample Collection
2.7. Primary Outcomes and Other Outcomes Measures
2.7.1. Biochemical Analysis
2.7.2. Blood Pressure
2.7.3. Endothelial Function
2.7.4. Diabetes and Obesity Biomarkers Analysis
2.7.5. Anthropometry and Body Composition
2.7.6. Inflammatory Biomarker Analysis
2.7.7. Antioxidant Capacity and Oxidation Biomarkers
2.8. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Chemical Composition of the Study Oils
3.2. Baseline Characteristics of Participants and Dietary Control
3.3. Blood Pressure
3.4. Blood Biochemistry: Lipid Profile and Liver Function
3.5. Inflammatory Biomarkers
3.6. Biomarkers of Endothelial Function
3.7. Diabetes Markers: Glycaemia, Insulin Levels, Glycosylated Haemoglobin Concentration, Insulin Resistance/Sensitivity Indices (HOMA-IR/QUICKI), and Pancreatic Beta-Cell Function (HOMA-β)
3.8. Obesity Biomarkers
3.9. Anthropometric Measurements and Body Composition
3.10. Antioxidant Capacity and Oxidation Biomarkers
4. Discussion
4.1. Chemical Composition of the Study Oils
4.2. Effect of Nutritional Intervention on Lipid Profile, Blood Pressure, and Markers of Liver Function and Endothelial Function
4.3. Effect of Nutritional Intervention on Inflammation Markers
4.4. Effect of Nutritional Intervention on Diabetes and Obesity Markers
4.5. Effect of Nutritional Intervention on Anthropometric Measurements
4.6. Effect of Nutritional Intervention on Oxidative Status
4.7. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A review of evidence of the role and sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; PREDIMED investigators. Benefits of the Mediterranean Diet: Insights from the PREDIMED study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Sarriá, B.; Bravo, L. Nutritional and other health properties of olive pomace oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 3506–3521. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; Freitas, K.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The secrets of the Mediterranean Diet. Does [only] olive oil matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [PubMed]
- ANIERAC: Asociación Nacional de Industriales Envasadores y Refinadores de Aceites Comestibles. Available online: https://anierac.org/consumo-en-espana/?lang=es (accessed on 1 January 2022).
- García-Aloy, M.; Hulshof, P.J.M.; Estruel-Amades, S.; Osté, M.C.J.; Lankinen, M.; Geleijnse, J.M.; de Goede, J.; Ulaszewska, M.; Mattivi, F.; Bakker, S.J.L.; et al. Biomarkers of food intake for nuts and vegetable oils: An extensive literature search. Genes Nutr. 2019, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Aceites de Semillas: Palma, Colza, Soja y Girasol Lideran la Producción y el Consumo Mundial. Available online: https://www.mercasa.es/media/publicaciones/58/pag_065-070_Murcia.pdf (accessed on 2 February 2022).
- Ruiz-Méndez, M.V.; Márquez-Ruiz, G.; Holgado, F.; Velasco, J. Stability of bioactive compounds in olive-pomace oil at frying temperature and incorporation into fried foods. Foods 2021, 10, 2906. [Google Scholar] [CrossRef]
- Olimerca. Available online: https://www.olimerca.com/precios/tipoInforme/1 (accessed on 2 February 2022).
- Sánchez-Moral, P.; Ruíz-Méndez, M.V. Production of pomace olive oil. Grasas y Aceites 2006, 57, 47–55. [Google Scholar] [CrossRef]
- Lee, W.; Yang, E.J.; Ku, S.K.; Song, K.S.; Bae, J.S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 2013, 36, 94–102. [Google Scholar] [CrossRef]
- Valero-Muñoz, M.; Martín-Fernández, B.; Ballesteros, S.; de la Fuente, E.; Quintela, J.C.; Lahera, V.; de las Heras, N. Protective effect of a pomace olive oil concentrated in triterpenic acids in alterations related to hypertension in rats: Mechanisms involved. Mol. Nutr. Food Res. 2014, 58, 376–383. [Google Scholar] [CrossRef]
- Claro-Cala, C.M.; Quintela, J.C.; Pérez-Montero, M.; Miñano, J.; de Sotomayor, M.A.; Herrera, M.D.; Rodríguez-Rodríguez, A.R. Pomace olive oil concentrated in triterpenic acids restores vascular function, glucose tolerance and obesity progression in mice. Nutrients 2020, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Moruno, R.; Martinez-Force, E.; Montero, E.; Perona, J.S. Minor components of olive oil facilitate the triglyceride clearance from postprandial lipoproteins in a polarity-dependent manner in healthy men. Nutr. Res. 2014, 34, 40–47. [Google Scholar] [CrossRef] [PubMed]
- González-Rámila, S.; Sarriá, B.; Seguido, M.A.; García-Cordero, J.; Mateos, R.; Bravo, L. Olive pomace oil can improve blood lipid profile: A randomized, blind, crossover, controlled clinical trial in healthy and at-risk volunteers. Eur. J. Nutr. 2022, in press.
- González-Rámila, S.; Mateos, R.; García-Cordero, J.; Seguido, M.A.; Bravo-Clemente, L.; Sarriá, B. Olive pomace oil versus high oleic sunflower oil and sunflower oil: A comparative study in healthy and cardiovascular risk humans. Foods 2022, 11, 2186. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Camino, M.C.; Cert, A. Quantitative determination of hydroxy pentacyclic triterpene acids in vegetable oils. J. Agric. Food Chem. 1999, 47, 1558–1562. [Google Scholar] [CrossRef]
- Giacometti, J. Determination of aliphatic alcohols, squalene, alpha-tocopherol and sterols in olive oils: Direct method involving gas chromatography of the unsaponifiable fraction following silylation. Analyst 2001, 126, 472–475. [Google Scholar] [CrossRef]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- Manual de Nutrición y Dietética. Available online: https://eprints.ucm.es/id/eprint/22755/1/Manual-nutricion-dietetica-CARBAJAL.pdf (accessed on 20 January 2022).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2012, 50, 4437–4444. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Mateos, R.; Lecumberri, E.; Ramos, S.; Goya, L.; Bravo, L. Determination of malondialdehyde (mda) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress: Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J. Chromatogr. B 2005, 827, 76–82. [Google Scholar]
- Martínez-López, S.; Sarriá, B.; Mateos, R.; Bravo-Clemente, L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: Results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur. J. Nutr. 2019, 58, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Ingestas diarias recomendadas de energía y nutrientes para la población española. In Tabla de Composición de Alimentos, 18th ed.; Moreiras, O., Carbajal, A., Cabrera, L., Cuadrado, C., Eds.; Ediciones Pirámides (Grupo Anaya, SA): Madrid, Spain, 2016; pp. 127–131. [Google Scholar]
- Holgado, F.; Ruiz-Méndez, M.V.; Velasco, J.; Márquez-Ruiz, G. Performance of olive-pomace oils in discontinuous and continuous frying. Comparative behavior with sunflower oils and high-oleic sunflower oils. Foods 2021, 10, 3081. [Google Scholar] [CrossRef]
- Güneşer, B.A.; Yılmaz, E.; Ok, S. Cold pressed versus refined winterized corn oils: Quality, composition and aroma. Grasas y Aceites 2017, 68, e194. [Google Scholar] [CrossRef]
- Food and Drugs Administration (FDA). Available online: https://www.fda.gov/ (accessed on 9 June 2022).
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Vasudevan, D.M.; Sundaram, K.R.; Krishnan, S.; Vaidyanathan, K.; Nandakumar, S.; Chandrasekhar, R.; Mathew, N. A randomized study of coconut oil versus sunflower oil on cardiovascular risk factors in patients with stable coronary heart disease. Indian Heart J. 2016, 68, 498–506. [Google Scholar] [CrossRef]
- Jandacek, R.J. Linoleic acid: A nutritional quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef]
- Weiß, J.; Rau, M.; Geier, A. Non-alcoholic fatty liver disease: Epidemiology, clinical course, investigation, and treatment. Dtsch. Arztebl. Int. 2014, 111, 447–452. [Google Scholar]
- de la Sierra, A. New American and European hypertension guidelines, reconciling the differences. Cardiol. Ther. 2019, 8, 157–166. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: Molecular mechanisms and therapeutic perspectives. Curr. Med. Chem. 2015, 22, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, R.; Herrera, M.D.; de Sotomayor, M.A.; Ruiz-Gutiérrez, V. Pomace olive oil improves endothelial function in spontaneously hypertensive rats by increasing endothelial nitric oxide synthase expression. Am. J. Hypertens. 2007, 20, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.X.; Yang, Q.D.; Liu, Y.H.; Xie, X.Y.; Wang, M.; Niu, R.C. Palmitic and linoleic acids impair endothelial progenitor cells by inhibition of Akt/eNOS pathway. Arch. Med. Res. 2008, 39, 434–442. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 signalling in allergic airway disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef]
- Gonçalves, F.; Freitas, E.; Torres, T. Selective IL-13 inhibitors for the treatment of atopic dermatitis. Drugs Context 2021, 10, 2021-1-7. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; López-Biedma, A.; Toledo, E.; Gaforio, J.J. Squalene stimulates a key innate immune cell to foster wound healing and tissue repair. Evid. Based Complement. Alternat. Med. 2018, 2018, 9473094. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Lee, Y.C. Oleanolic acid suppresses ovalbumin-induced airway inflammation and Th2-mediated allergic asthma by modulating the transcription factors T-bet, GATA-3, RORγt and Foxp3 in asthmatic mice. Int. Immunopharmacol. 2014, 18, 311–324. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Lee, Y.C. Ursolic acid, a potential PPARγ agonist, suppresses ovalbumin-induced airway inflammation and Penh by down-regulating IL-5, IL-13, and IL-17 in a mouse model of allergic asthma. Eur. J. Pharmacol. 2013, 701, 131–143. [Google Scholar] [CrossRef]
- Sun, N.; Han, Z.; Wang, H.; Guo, Z.; Deng, C.; Dong, W.; Zhuang, G.; Zhang, R. Effects of ursolic acid on the expression of Th1-Th2-related cytokines in a rat model of allergic rhinitis after PM2.5 exposure. Am. J. Rhinol. Allergy 2020, 34, 587–596. [Google Scholar] [CrossRef]
- Aryaeian, N.; Djalali, M.; Shahram, F.; Djazayery, A.; Eshragian, M.R. Effect of conjugated linoleic acid, vitamin E, alone or combined on immunity and inflammatory parameters in adults with active rheumatoid arthritis: A randomized controlled trial. Int. J. Prev. Med. 2014, 5, 1567–1577. [Google Scholar] [PubMed]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B.; American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009, 52, 17–30. [Google Scholar] [CrossRef]
- Akrami, A.; Nikaein, F.; Babajafari, S.; Faghih, S.; Yarmohammadi, H. Comparison of the effects of flaxseed oil and sunflower seed oil consumption on serum glucose, lipid profile, blood pressure, and lipid peroxidation in patients with metabolic syndrome. J. Clin. Lipidol. 2018, 12, 70–77. [Google Scholar] [CrossRef]
- Lambert, E.V.; Goedecke, J.H.; Bluett, K.; Heggie, K.; Claassen, A.; Rae, D.E.; West, S.; Dugas, J.; Dugas, L.; Meltzeri, S.; et al. Conjugated linoleic acid versus high-oleic acid sunflower oil: Effects on energy metabolism, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br. J. Nutr. 2007, 97, 1001–1011. [Google Scholar] [CrossRef]
- Santos-Lozano, J.M.; Rada, M.; Lapetra, J.; Guinda, Á.; Jiménez-Rodríguez, M.C.; Cayuela, J.A.; Ángel-Lugo, A.; Vilches-Arenas, Á.; Gómez-Martín, A.M.; Ortega-Calvo, M.; et al. Prevention of type 2 diabetes in prediabetic patients by using functional olive oil enriched in oleanolic acid: The PREDIABOLE study, a randomized controlled trial. Diabetes Obes. Metab. 2019, 21, 2526–2534. [Google Scholar] [CrossRef]
- de Melo, C.L.; Queiroz, M.G.; Fonseca, S.G.; Bizerra, A.M.; Lemos, T.L.; Melo, T.S.; Santos, F.A.; Rao, V.S. Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. Chem. Biol. Interact. 2010, 185, 59–65. [Google Scholar] [CrossRef]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. The effects of plant-derived oleanolic acid on selected parameters of glucose homeostasis in a diet-induced pre-diabetic rat model. Molecules 2018, 23, 794. [Google Scholar] [CrossRef]
- Jia, Y.; Kim, S.; Kim, J.; Kim, B.; Wu, C.; Lee, J.H.; Jun, H.J.; Kim, N.; Lee, D.; Lee, S.J. Ursolic acid improves lipid and glucose metabolism in high-fat-fed C57BL/6J mice by activating peroxisome proliferator-activated receptor alpha and hepatic autophagy. Mol. Nutr. Food Res. 2015, 59, 344–354. [Google Scholar] [CrossRef]
- Rao, V.S.; de Melo, C.L.; Queiroz, M.G.; Lemos, T.L.; Menezes, D.B.; Melo, T.S.; Santos, F.A. Ursolic acid, a pentacyclic triterpene from Sambucus australis, prevents abdominal adiposity in mice fed a high-fat diet. J. Med. Food. 2011, 14, 1375–1382. [Google Scholar] [CrossRef]
- Nigam, P.; Bhatt, S.; Misra, A.; Chadha, D.S.; Vaidya, M.; Dasgupta, J.; Pasha, Q.M. Effect of a 6-month intervention with cooking oils containing a high concentration of monounsaturated fatty acids (olive and canola oils) compared with control oil in male Asian Indians with nonalcoholic fatty liver disease. Diabetes Technol. Ther. 2014, 16, 255–261. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef]
- Casado-Díaz, A.; Túnez-Fiñana, I.; Mata-Granados, J.M.; Ruiz-Méndez, M.V.; Dorado, G.; Romero-Sánchez, M.C.; Navarro-Valverde, C.; Quesada-Gómez, J.M. Serum from postmenopausal women treated with a by-product of olive-oil extraction process stimulates osteoblastogenesis and inhibits adipogenesis in human mesenchymal stem-cells (MSC). Exp. Gerontol. 2017, 90, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Conterno, L.; Martinelli, F.; Tamburini, M.; Fava, F.; Mancini, A.; Sordo, M.; Pindo, M.; Martens, S.; Masuero, D.; Vrhovsek, U.; et al. Measuring the impact of olive pomace enriched biscuits on the gut microbiota and its metabolic activity in mildly hypercholesterolaemic subjects. Eur. J. Nutr. 2019, 58, 63–81. [Google Scholar] [CrossRef]
- Iskender, H.; Dokumacioglu, E.; Terim Kapakin, K.A.; Yenice, G.; Mohtare, B.; Bolat, I.; Hayirli, A. Effects of oleanolic acid on inflammation and metabolism in diabetic rats. Biotech. Histochem. 2021, 97, 269–276. [Google Scholar] [CrossRef]
- Madlala, H.P.; Van Heerden, F.R.; Mubagwa, K.; Musabayane, C.T. Changes in renal function and oxidative status associated with the hypotensive effects of oleanolic acid and related synthetic derivatives in experimental animals. PLoS ONE 2015, 10, e0128192. [Google Scholar] [CrossRef]
- Liu, B.; Piao, X.; Guo, L.; Liu, S.; Chai, F.; Gao, L. Ursolic acid protects against ulcerative colitis via anti-inflammatory and antioxidant effects in mice. Mol. Med. Rep. 2016, 13, 4779–4785. [Google Scholar] [CrossRef]
- Mkhwanazi, B.N.; Serumula, M.R.; Myburg, R.B.; Van Heerden, F.R.; Musabayane, C.T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: Effects on kidney function. Ren. Fail. 2014, 36, 419–431. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, Y.; Zhao, H.; Zhang, H.; Zhou, Y. Effects of graded levels of dietary squalene supplementation on the growth performance, plasma biochemical parameters, antioxidant capacity, and meat quality in broiler chickens. Poult. Sci. 2020, 99, 5915–5924. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 432/2012. Off. J. Eur. Union 2012, L136, 1–40. [Google Scholar]
| OPO | SO | CO | |
|---|---|---|---|
| Fatty acids (%) | |||
| C 12:0 (Lauric acid) | - | <0.01 | 0.01 |
| C 14:0 (Myristic acid) | 0.02 | 0.08 | 0.04 |
| C 16:0 (Palmitic acid) | 10.78 | 6.43 | 10.60 |
| C 16:1 (Palmitoleic acid) | 0.84 | 0.14 | 0.15 |
| C 17:0 (Margaric acid) | 0.06 | 0.03 | 0.07 |
| C 17:1 (Margaroleic acid) | 0.10 | 0.03 | 0.05 |
| C 18:0 (Stearic acid) | 3.10 | 3.63 | 2.03 |
| C 18:1 (Oleic acid) | 74.32 | 29.76 | 35.36 |
| C 18:2 (Linoleic acid) | 9.02 | 58.46 | 50.52 |
| C 20:0 (Arachidic acid) | 0.48 | 0.26 | 0.48 |
| C 18:3 (Linolenic acid) | 0.68 | 0.09 | 0.04 |
| C 20:1 (Eicosenoic acid) | 0.33 | 0.16 | 0.31 |
| C 22:0 (Behenic acid) | 0.19 | 0.69 | 0.17 |
| C 22:1 (Erucic acid) | - | <0.01 | <0.01 |
| C 24:0 (Lignoceric acid) | 0.08 | 0.24 | 0.17 |
| Trans Oleic (t-C18:1) | 0.28 | 0.03 | 0.02 |
| Trans Linoleic + Trans Linolenic (t-C18:2 + t-C18:3) | 0.15 | 0.19 | 1.15 |
| Squalene (ppm) | |||
| Squalene (ppm) | 675 | 314 | 548 |
| Tocopherols (mg/kg) | |||
| α-Tocopherol (Vit. E) | 195 | 217 | 33 |
| β-Tocopherol | <2 | 9 | <2 |
| γ-Tocopherol | <2 | <2 | 205 |
| δ -Tocopherol | <2 | <2 | 6 |
| Tocotrienols (mg/kg) | |||
| A-Tocotrienol | - | - | 10 |
| β-Tocotrienol | - | - | <2 |
| Υ-Tocotrienol | - | - | 5 |
| Δ-Tocotrienol | - | - | <2 |
| Sterols (%) | |||
| Cholesterol | 0.19 | 0.12 | 0.21 |
| Brassicasterol | <0.10 | <0.1 | 0.62 |
| 24-Methylcholesterol | <0.10 | 0.12 | 0.92 |
| Campesterol | 3.06 | 8.40 | 20.75 |
| Campestanol | 0.16 | 0.07 | 0.92 |
| Stigmasterol | 1.23 | 6.90 | 6.85 |
| Δ7-Campesterol | <0.10 | 2.70 | <0.10 |
| Δ5,23-Stigmastadienol | 0.32 | 0.34 | <0.10 |
| Clerosterol | 1.08 | 0.66 | 0.70 |
| β-Sitosterol | 88.64 | 53.67 | 61.09 |
| Sitostanol | 1.44 | 0.64 | 2.11 |
| Δ5-Avenasterol | 1.82 | 3.08 | 3.90 |
| Δ5,24-Stigmastadienol | 1.35 | 1.08 | 0.51 |
| Δ7-Stigmastenol | 0.50 | 16.29 | 0.63 |
| Δ7-Avenasterol | 0.19 | 5.93 | 0.78 |
| Δ-Sitosterol apparent | 94.65 | 59.47 | 68.31 |
| Total Sterols (ppm) | 3344.2 | 2820.5 | 8962.0 |
| Triterpenic alcohols (mg/kg) | |||
| Erythrodiol + Uvaol | 745 | <1.0 | <1.0 |
| Phenols (mg/kg) | |||
| Total phenols | <1.0 | <1.0 | <1.0 |
| Triterpenic acids (mg/kg) | |||
| Oleanolic acid | 187 | <2.0 | <2.0 |
| Ursolic acid | <2.0 | <2.0 | <2.0 |
| Maslinic acid | <2.0 | <2.0 | <2.0 |
| Aliphatic alcohols (mg/kg) | |||
| C22 + C24 + C26 + C28 | 1681 | 38 | 29 |
| Normocholesterolemic (n = 31) | Hypercholesterolemic (n = 37) | |
|---|---|---|
| Men, n | 8 | 19 |
| Women, n | 23 | 18 |
| Age (years) | 30 ± 2 | 41 ± 2 |
| BMI (kg/m2) | 23 ± 2 | 26 ± 1 |
| Waist circumference (cm) | 78 ± 2 | 85 ± 2 |
| Total cholesterol (mg/dL) | 172 ± 3 | 239 ± 5 |
| LDL-cholesterol (mg/dL) | 93 ± 3 | 148 ± 5 |
| Systolic blood pressure (mmHg) | 110 ± 2 | 121 ± 2 |
| Diastolic blood pressure (mmHg) | 74 ± 1 | 81 ± 2 |
| Normocholesterolemic n = 31 | p Value ** | Hypercholesterolemic n = 37 | p Value ** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPO | SO | OPO | SO | |||||||||
| Baseline | Initial | Final | Initial | Final | Oil | Baseline | Initial | Final | Initial | Final | Oil | |
| Energy (kcal/day) | 1976 ± 70 | 1906 ± 77 | 1949 ± 78 | 1996 ± 85 | 1982 ± 82 | 0.314 | 2081 ± 79 | 2061 ± 78 | 2042 ± 72 | 2025 ± 72 | 2043 ± 74 | 0.455 |
| Proteins (g/day) | 84 ± 4 | 78 ± 3 | 80 ± 3 | 84 ± 5 | 81 ± 4 | 0.621 | 92 ± 4 | 88 ± 4 | 83 ± 4 | 88 ± 4 | 81 ± 3 | 0.103 |
| Carbohydrates (g/day) | 187 ± 10 | 182 ± 12 | 181 ± 11 | 186 ± 11 | 182 ± 10 | 0.942 | 195 ± 12 | 199 ± 10 | 195 ± 9 | 188 ± 10 | 187 ± 10 | 0.427 |
| Lipids (g/day) | 89 ± 4 | 88 ± 4 | 90 ± 4 | 93 ± 4 | 93 ± 5 | 0.400 | 91 ± 4 | 90 ± 4 | 93 ± 4 | 94 ± 4 | 98 ± 4 | 0.124 |
| SFA (g/day) | 28 ± 2 | 28 ± 2 | 28 ± 2 | 31 ± 2 | 28 ± 2 | 0.287 | 29 ± 2 | 28 ± 2 | 28 ± 2 | 28 ± 2 | 28 ± 2 | 0.773 |
| MUFA (g/day) | 38 ± 2 a | 30 ± 2 b | 44 ± 3 a | 31 ± 1 b | 28 ± 2 b | 0.000 | 41 ± 2 a | 30 ± 1 b | 45 ± 2 a | 31 ± 1 b | 31 ± 2 b | 0.000 |
| PUFA (g/day) | 15 ± 1 a | 22 ± 2 b | 11 ± 1 c | 23 ± 2 bd | 29 ± 2 d | 0.000 | 12 ± 1 a | 23 ± 1 b | 13 ± 1 a | 27 ± 1 bc | 31 ± 2 d | 0.000 |
| Cholesterol (mg/day) | 325 ± 23 | 308 ± 23 | 337 ± 23 | 319 ± 19 | 340 ± 29 | 0.668 | 372 ± 21 | 311 ± 22 | 317 ± 24 | 312 ± 21 | 351 ± 23 | 0.067 |
| Dietary fibre (g/day) | 20 ± 2 | 17 ± 1 | 17.5 ± 0.1 | 18 ± 1 | 20 ± 1 | 0.137 | 22 ± 2 | 22 ± 1 | 22 ± 1 | 22 ± 1 | 22 ± 1 | 0.964 |
| Vitamin E (mg/day) | 9.7 ± 0.8 a | 14 ± 1 b | 17 ± 1 b | 14 ± 1 b | 22 ± 2 c | 0.000 | 120 ± 14 a | 121 ± 13 b | 137 ± 18 c | 146 ± 13 c | 114 ± 11 d | 0.000 |
| Normocholesterolemic n = 31 | Hypercholesterolemic n = 37 | p Value | |||||
|---|---|---|---|---|---|---|---|
| OPO | SO | OPO | SO | Oil | N/H | N/H × Oil | |
| Total-cholesterol (mg/dL) | |||||||
| Initial | 168 ± 5 | 168 ± 5 | 221 ± 5 | 221 ± 6 | 0.923 | 0.000 | 0.868 |
| Final | 164 ± 5 | 169 ± 5 | 220 ± 5 | 220 ± 6 | 0.642 | 0.000 | 0.679 |
| Rate of change | −1.4 ± 2.0 | 1.1 ± 1.8 | −0.3 ± 1.6 | −0.1 ± 1.6 | 0.425 | 0.907 | 0.813 |
| Triglycerides (mg/dL) | |||||||
| Initial | 73 ± 5 | 76 ± 6 | 111 ± 9 | 111 ± 11 | 0.778 | 0.000 | 0.884 |
| Final | 70 ± 5 | 72 ± 6 | 109 ± 9 | 114 ± 11 | 0.694 | 0.000 | 0.975 |
| Rate of change | −2.6 ± 0.04 | −1.8 ± 5.0 | 1.2 ± 4.0 | 6.9 ± 5.7 | 0.486 | 0.215 | 0.784 |
| HDL-cholesterol (mg/dL) | |||||||
| Initial | 60 ± 2 | 60 ± 2 | 65 ± 3 | 64 ± 3 | 0.857 | 0.146 | 0.814 |
| Final | 59 ± 2 | 60 ± 2 | 64 ± 2 | 63 ± 2 | 0.676 | 0.179 | 0.405 |
| Rate of change | −1.2 ± 1.9 | 0.8± 2.2 | 0.9 ± 2.0 | −2.0 ± 1.9 | 0.838 | 0.754 | 0.342 |
| LDL-cholesterol (mg/dL) | |||||||
| Initial | 93 ± 4 | 92 ± 4 | 133 ± 5 | 134 ± 5 | 0.919 | 0.000 | 0.859 |
| Final | 91 ± 4 | 94 ± 4 | 134 ± 4 | 134 ± 5 | 0.622 | 0.000 | 0.801 |
| Rate of change | −0.3 ± 2.8 | 3.3 ± 2.9 | 1.1 ± 1.7 | 1.0 ± 2.2 | 0.547 | 0.733 | 0.657 |
| VLDL-cholesterol (mg/dL) | |||||||
| Initial | 15 ± 1 | 15 ± 1 | 24 ± 3 | 23 ± 3 | 0.942 | 0.000 | 0.713 |
| Final | 14 ± 1 | 14 ± 1 | 22 ± 2 | 23 ± 3 | 0.766 | 0.000 | 0.902 |
| Rate of change | −3.0± 4.3 | −2.8 ± 4.7 | 0.2 ± 4.5 | 5.7 ± 5.8 | 0.267 | 0.267 | 0.783 |
| LDL-cholesterol/HDL-cholesterol | |||||||
| Initial | 1.6 ± 0.1 | 1.6 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 0.852 | 0.000 | 0.823 |
| Final | 1.6 ± 0.1 | 1.6 ± 0.1 | 2.3 ± 0.1 | 2.2 ± 0.1 | 0.788 | 0.000 | 0.874 |
| Rate of change | 1.7 ± 3.2 | 4.3 ± 3.7 | 1.4 ± 2.3 | 4.4 ± 3.0 | 0.315 | 0.945 | 0.968 |
| Total Cholesterol/HDL-cholesterol | |||||||
| Initial | 2.9 ± 0.1 | 2.9 ± 0.1 | 3.6 ± 0.1 | 3.7 ± 0.2 | 0.992 | 0.000 | 0.990 |
| Final | 2.9 ± 0.1 | 2.9 ± 0.1 | 3.7 ± 0.2 | 3.6 ± 0.2 | 0.778 | 0.000 | 0.866 |
| Rate of change | 0.26 ± 1.8 | 1.32 ± 2.2 | −0.3 ± 1.8 | 2.8 ± 2.0 | 0.942 | 0.262 | 0.615 |
| Apolipoprotein (Apo A1) (mg/dL) | |||||||
| Initial | 161 ± 3 | 163 ± 3 | 175 ± 4 | 177 ± 4 | 0.635 | 0.000 | 0.963 |
| Final | 165 ± 4 | 167 ± 3 | 183 ± 4 | 179 ± 5 | 0.397 | 0.001 | 0.333 |
| Rate of change | 2.98 ± 1.9 | 3.0 ± 1.7 | 4.6 ± 1.1 | 1.0 ± 1.3 | 0.195 | 0.849 | 0.298 |
| Apo B (mg/dL) | |||||||
| Initial | 72 ± 3 | 73 ± 3 | 101 ± 3 | 100 ± 3 | 0.974 | 0.000 | 0.772 |
| Final | 77 ± 3 | 79 ± 3 | 108 ± 3 | 108 ± 4 | 0.833 | 0.000 | 0.580 |
| Rate of change | 8.8 ± 2.4 | 9.5 ± 2.3 | 8.3 ± 1.9 | 9.0 ± 1.9 | 0.768 | 0.846 | 0.953 |
| Apo B/Apo A1 | |||||||
| Initial | 0 46 ± 0.02 | 0.45 ± 0.02 | 0.59 ± 0.03 | 0.56 ± 0.02 | 0.458 | 0.000 | 0.757 |
| Final | 0.47 ± 0.02 | 0.48 ± 0.02 | 0.61 ± 0.03 | 0.63 ± 0.03 | 0.875 | 0.000 | 0.970 |
| Rate of change | 4.3 ± 3.2 | 6.3 ± 2.7 | 5.6 ± 2.4 | 11.3 ± 2.6 | 0.126 | 0.420 | 0.578 |
| ALAT (UI/L) | |||||||
| Initial | 21 ± 2 | 21 ± 2 | 24 ± 3 | 27 ± 3 | 0.504 | 0.052 | 0.608 |
| Final | 19 ± 2 | 20 ± 2 | 26 ± 3 | 28 ± 3 | 0.498 | 0.005 | 0.868 |
| Rate of change | 107.7 ± 5.0 | 109.41 ± 7.5 | 119.8 ± 5.7 | 117.0 ± 7.1 | 0.921 | 0.119 | 0.782 |
| ASAT (UI/L) | |||||||
| Initial | 20.6 ± 0.9 | 21.0 ± 1.2 | 22.7 ± 1.3 | 24.1 ± 1.3 | 0.536 | 0.033 | 0.322 |
| Final | 18.5 ± 0.8 | 19.2 ± 0.7 | 22.5 ± 1.2 | 24.2 ± 1.3 | 0.275 | 0.000 | 0.635 |
| Rate of change | 101.7 ± 3.1 | 106.9 ± 4.7 | 113.5 ± 4.6 | 112.4 ± 4.7 | 0.559 | 0.045 | 0.418 |
| Normocholesterolemic n = 31 | Hypercholesterolemic n = 35 ** | p Value | |||||
|---|---|---|---|---|---|---|---|
| OPO | SO | OPO | SO | Oil | N/H | N/H × Oil | |
| CRP (mg/dL) | |||||||
| Initial | 0.23 ± 0.07 | 0.25 ± 0.10 | 0.14 ± 0.03 | 0.34 ± 0.17 | 0.222 | 0.823 | 0.860 |
| Final | 0.17 ± 0.05 | 0.11 ± 0.03 | 0.17 ± 0.04 | 0.16 ± 0.04 | 0.311 | 0.579 | 0.438 |
| Rate of change * | 39.1 ± 3.1 | 32.1 ± 0.3 | 15.1 ± 0.7 | 17.5 ± 1.1 | 0.476 | 0.410 | 0.815 |
| IL-1β (pg/mL) | |||||||
| Initial | 0.97 ± 0.06 | 0.95 ± 0.03 | 0.89 ± 0.02 | 0.91 ± 0.03 | 0.785 | 0.097 | 0.691 |
| Final | 0.92 ± 0.03 | 0.91 ± 0.03 | 0.89 ± 0.02 | 1.05 ± 0.15 | 0.311 | 0.980 | 0.335 |
| Rate of change | −2.4 ± 2.8 | −3.2 ± 2.8 | 4.6 ± 2.3 | 0.8 ± 14.1 | 0.449 | 0.202 | 0.259 |
| IL-2 (pg/mL) | |||||||
| Initial | 16.4 ± 0.7 | 16.6 ± 0.5 | 15.4 ±0.3 | 15.9 ± 0.5 | 0.308 | 0.147 | 0.766 |
| Final | 16.0 ± 0.5 | 15.6 ± 0.5 | 15.3 ± 0.4 | 15.6 ± 0.5 | 0.573 | 0.488 | 0.433 |
| Rate of change | 0.1 ± 2.3 | −5.2 ± 2.3 | −0.4 ± 1.8 | −1.4 ± 2.3 | 0.193 | 0.544 | 0.370 |
| IL-4 (pg/mL) | |||||||
| Initial | 2.68 ± 0.06 | 2.64 ± 0.06 | 2.57 ± 0.05 | 2.61 ± 0.05 | 0.907 | 0.217 | 0.430 |
| Final | 2.64 ± 0.06 | 2.59 ± 0.07 | 2.55 ± 0.04 | 2.62 ± 0.07 | 0.515 | 0.762 | 0.137 |
| Rate of change | 0.7 ± 2.3 | −3.0 ± 2.3 | 2.5 ± 1.6 | −1.6 ± 2.1 | 0.778 | 0.229 | 0.021 |
| IL-6 (pg/mL) | |||||||
| Initial | 5.2 ± 0.2 | 5.4 ± 0.3 | 5.1 ± 0.2 | 5.0 ± 0.2 | 0.947 | 0.206 | 0.258 |
| Final | 5.3 ± 0.3 | 5.1 ± 0.3 | 5.0 ± 0.2 | 5.2 ± 0.2 | 0.986 | 0.571 | 0.668 |
| Rate of change | 3.5 ± 4.4 | −2.1 ± 4.4 | −3.0 ± 4.3 | 5.0 ± 3.3 | 0.816 | 0.885 | 0.169 |
| IL-7 (pg/mL) | |||||||
| Initial | 33 ± 1 | 34 ± 1 | 33 ± 1 | 34 ± 1 | 0.388 | 0.613 | 0.782 |
| Final | 34 ± 1 | 34 ± 1 | 33 ± 1 | 33 ± 1 | 0.692 | 0.610 | 0.856 |
| Rate of change | 2.3 ± 2.5 | −1.2 ± 2.5 | 0.6 ± 1.7 | −1.0 ± 2.3 | 0.252 | 0.777 | 0.641 |
| IL-8 (pg/mL) | |||||||
| Initial | 22 ± 2 | 24 ± 2 | 20 ± 1 | 21 ± 1 | 0.634 | 0.057 | 0.861 |
| Final | 21 ± 1 | 21 ± 1 | 20 ± 1 | 20 ± 1 | 0.871 | 0.633 | 0.874 |
| Rate of change | −5.6 ± 3.8 | −3.2 ± 3.8 | 3.0 ± 2.5 | −3.0 ± 2.2 | 0.204 | 0.113 | 0.568 |
| IL-10 (pg/mL) | |||||||
| Initial | 10.9 ± 0.2 | 11.1 ± 0.3 | 10.8 ± 0.3 | 10.7 ± 0.2 | 0.319 | 0.260 | 0.677 |
| Final | 11.0 ± 0.2 | 10.8 ± 0.2 | 10.9 ± 0.3 | 10.6 ± 0.2 | 0.883 | 0.552 | 0.233 |
| Rate of change | 1.9 ± 1.6 | −2.6 ± 1.6 | −0.3 ± 1.1 | 0.9 ± 1.6 | 0.310 | 0.686 | 0.080 |
| IL-12 (p70) (pg/mL) | |||||||
| Initial | 12.1 ± 0.3 | 12.4 ± 0.3 | 11.7 ± 0.3 | 11.9 ± 0.2 | 0.287 | 0.091 | 0.838 |
| Final | 12.2 ± 0.3 | 11.9 ± 0.3 | 11.7 ± 0.2 | 12.0 ± 0.3 | 0.957 | 0.565 | 0.253 |
| Rate of change | 1.5 ± 2.5 | −4.1 ± 2.5 | 0.9 ± 1.7 | 1.0 ± 1.7 | 0.200 | 0.293 | 0.182 |
| IL-13 (pg/mL) | |||||||
| Initial | 3.0 ± 0.1 | 3.2 ± 0.2 | 2.8 ± 0.1 | 2.9 ± 0.1 | 0.345 | 0.045 | 0.992 |
| Final | 3.1 ± 0.1 | 3.0 ± 0.1 | 2.8 ± 0.1 | 2.9 ± 0.1 | 0.826 | 0.142 | 0.437 |
| Rate of change | 2.8 ± 2.8 | −5.2 ± 2.8 | 1.0 ± 1.9 | −0.7 ± 3.0 | 0.023 | 0.527 | 0.159 |
| IL-17 (pg/mL) | |||||||
| Initial | 13.9 ± 0.4 | 14.0 ± 0.3 | 13.4 ± 0.3 | 13.5 ± 0.2 | 0.475 | 0.199 | 0.875 |
| Final | 14.0 ± 0.4 | 13.8 ± 0.4 | 13.3 ± 0.2 | 13.5 ± 0.3 | 0.936 | 0.143 | 0.489 |
| Rate of change | 0.8 ± 2.1 | −0.7 ± 2.1 | −0.2 ± 1.6 | 0.3 ± 1.6 | 0.773 | 0.997 | 0.570 |
| G-CSF (pg/mL) | |||||||
| Initial | 137 ± 3 | 140 ± 2 | 134 ± 2 | 134 ± 2 | 0.456 | 0.041 | 0.453 |
| Final | 137 ± 2 | 137 ± 3 | 133 ± 2 | 135 ± 2 | 0.875 | 0.150 | 0.547 |
| Rate of change | 0.9 ± 1.7 | −1.7 ± 1.7 | −0.4 ± 1.0 | 0.8 ± 1.2 | 0.618 | 0.711 | 0.185 |
| GM-CSF (pg/mL) | |||||||
| Initial | 4.7 ± 0.2 | 4.7 ± 0.2 | 4.7 ± 0.3 | 4.6 ± 0.2 | 0.726 | 0.929 | 0.911 |
| Final | 4.7 ± 0.2 | 4.5 ± 0.2 | 4.5 ± 0.2 | 4.8 ± 0.3 | 0.746 | 0.772 | 0.329 |
| Rate of change | 0.9 ± 2.6 | −2.4 ± 2.6 | −1.6 ± 2.3 | 4.4 ± 3.3 | 0.651 | 0.472 | 0.114 |
| MCP-1 (pg/mL) | |||||||
| Initial | 39 ± 3 | 37 ± 4 | 33 ± 2 | 33 ± 2 | 0.909 | 0.088 | 0.949 |
| Final | 35 ± 6 | 34 ± 3 | 31 ± 1 | 32 ± 2 | 0.574 | 0.243 | 0.416 |
| Rate of change | −2.9 ± 3.8 | −6.5 ± 3.8 | −1.7 ± 3.2 | 0.3 ± 3.2 | 0.816 | 0.273 | 0.497 |
| MIP-1β (ng/mL) | |||||||
| Initial | 69 ± 6 | 73 ± 5 | 71 ± 8 | 73 ± 8 | 0.572 | 0.790 | 0.793 |
| Final | 67 ± 4 | 66 ± 4 | 71 ± 8 | 71 ± 9 | 0.834 | 0.453 | 0.845 |
| Rate of change | 4.9 ± 3.9 | −8.3 ± 3.9 | −1.3 ± 4.1 | 1.4 ± 6.3 | 0.361 | 0.774 | 0.133 |
| TNF-α (pg/mL) | |||||||
| Initial | 23.1 ± 0.9 | 23.7 ± 0.8 | 23.3 ± 1.4 | 23.9 ± 1.4 | 0.633 | 0.897 | 0.978 |
| Final | 23.0 ± 0.8 | 22.4 ± 0.8 | 23.3 ± 1.5 | 23.4 ± 1.5 | 0.888 | 0.584 | 0.644 |
| Rate of change | 0.5 ± 1.8 | −5.0 ± 1.8 | 0.0 ± 1.4 | −1.5 ± 2.0 | 0.083 | 0.567 | 0.382 |
| Normocholesterolemic n = 31 | Hypercholesterolemic n = 37 | p Value | |||||
|---|---|---|---|---|---|---|---|
| OPO | SO | OPO | SO | Oil | N/H | N/H × Oil | |
| eNOS (ng/mL) | |||||||
| Initial | 0.21 ± 0.03 | 0.25 ± 0.05 | 0.22 ± 0.03 | 0.21 ± 0.03 | 0.926 | 0.647 | 0.597 |
| Final | 0.22 ± 0.04 | 0.20 ± 0.03 | 0.19 ± 0.03 | 0.20 ± 0.03 | 0.944 | 0.708 | 0.644 |
| Rate of change | 16.03 ± 0.14 | −6.67 ± 0.09 | 15.98 ± 0.14 | −4.76 ± 0.12 | 0.083 | 0.903 | 0.912 |
| E-selectin (ng/mL) | |||||||
| Initial | 11 ± 2 | 15 ± 2 | 12 ± 2 | 13 ± 2 | 0.260 | 0.939 | 0.540 |
| Final | 11 ± 2 | 14 ± 3 | 11 ± 2 | 10 ± 1 | 0.588 | 0.193 | 0.045 |
| Rate of change | 18.0 ± 0.1 | 14.4 ± 0.1 | 5.4 ± 0.1 | 16.5 ± 0.2 | 0.552 | 0.342 | 0.960 |
| P-selectin (ng/mL) | |||||||
| Initial | 214 ± 18 | 190 ± 12 | 195 ± 11 | 195 ± 11 | 0.412 | 0.537 | 0.469 |
| Final | 206 ± 15 | 202 ± 16 | 179 ± 12 | 196 ± 11 | 0.635 | 0.226 | 0.448 |
| Rate of change | 0.60 ± 0.05 | 6.86 ± 0.05 | −1.72 ± 0.06 | 2.85 ± 0.04 | 0.289 | 0.519 | 0.871 |
| ICAM-1 (pg/mL) | |||||||
| Initial | 3093 ± 495 | 3332 ± 502 | 2954 ± 439 | 3291 ± 579 | 0.546 | 0.854 | 0.553 |
| Final | 2900 ± 452 | 3302 ± 566 | 3270 ± 557 | 3311 ± 643 | 0.705 | 0.770 | 0.601 |
| Rate of change | −0.09 ± 0.05 | −1.84 ± 0.04 | 9.30 ± 0.06 | −1.88 ± 0.05 | 0.120 | 0.742 | 0.473 |
| VCAM-1 (pg/mL) | |||||||
| Initial | 11,049 ± 2375 | 11,961 ± 2422 | 10,716 ± 2081 | 12,911 ± 2894 | 0.426 | 0.909 | 0.822 |
| Final | 11,280 ± 2523 | 13,101 ± 3152 | 12,271 ± 2480 | 13,917 ± 3574 | 0.522 | 0.822 | 0.621 |
| Rate of change | 5.44 ± 0.07 | 10.51 ± 0.08 | 19.90 ± 0.08 | 6.11 ± 0.09 | 0.373 | 0.856 | 0.073 |
| Normocholesterolemic n = 31 | Hypercholesterolemic n = 37 | p Value | |||||
|---|---|---|---|---|---|---|---|
| OPO | SO | OPO | SO | Oil | N/H | N/H × Oil | |
| Glucose (mg/dL) | |||||||
| Initial | 80 ± 1 | 82 ± 1 | 83 ± 2 | 81 ± 1 | 0.938 | 0.259 | 0.130 |
| Final | 81 ± 1 | 79 ± 2 | 83 ± 1 | 83 ± 1 | 0.433 | 0.403 | 0.944 |
| Rate of change | 1.3 ± 1.2 a | −2.6 ± 1.3 b | −0.3 ± 1.2 | 3.1 ± 1.3 | 0.825 | 0.073 | 0.007 |
| Insulin (µUI/mL) | |||||||
| Initial | 7.3 ± 0.8 | 8.3 ± 0.8 | 10.2 ± 1.5 | 8.5 ± 0.9 | 0.977 | 0.243 | 0.579 |
| Final | 6.2 ± 0.6 | 6.8 ± 0.7 | 9.1 ± 0.9 | 9.1 ± 1.2 | 0.918 | 0.007 | 0.449 |
| Rate of change | −5.6 ± 6.5 | −12.5 ± 5.6 | 4.7 ± 7.6 | 8.8 ± 5.9 | 0.077 | 0.802 | 0.825 |
| HbA1c (%) | |||||||
| Initial | 5.30 ± 0.05 | 5.33 ± 0.04 | 5.33 ± 0.04 | 5.36 ± 0.04 | 0.433 | 0.403 | 0.944 |
| Final | 5.30 ± 0.04 | 5.29 ± 0.04 | 5.33 ± 0.03 | 5.32 ± 0.04 | 0.837 | 0.488 | 0.949 |
| Rate of change | 0.1 ± 0. 5 | −0.6 ± 0. 6 | 0.0 ± 0. 4 | −0.8 ± 0. 4 | 0.994 | 0.021 | 0.258 |
| HOMA-IR | |||||||
| Initial | 1.4 ± 0.2 | 1.7 ± 0.2 | 2.2 ± 0.4 | 1.7 ± 0.2 | 0.886 | 0.186 | 0.674 |
| Final | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.9 ± 0.2 | 1.9 ± 0.3 | 0.978 | 0.006 | 0.560 |
| Rate of change | −3.1 ± 7.4 | −14.3 ± 5.8 | 6.0 ± 8.6 | 12.4 ± 6.2 | 0.952 | 0.020 | 0.102 |
| HOMA-β | |||||||
| Initial | 174 ± 21 | 191 ± 27 | 188 ± 20 | 140 ± 36 | 0.503 | 0.619 | 0.767 |
| Final | 143 ± 14 | 185 ± 43 | 186 ± 19 | 166 ± 15 | 0.669 | 0.607 | 0.185 |
| Rate of change | −8.5 ± 5.3 | 4.9 ± 5.2 | 7.1 ± 7.2 | −1.7 ± 6.9 | 0.665 | 0.633 | 0.218 |
| QUICKI | |||||||
| Initial | 0.371 ± 0.005 | 0.363 ± 0.005 | 0.358 ± 0.006 | 0.365 ± 0.006 | 0.931 | 0.348 | 0.200 |
| Final | 0.379 ± 0.005 | 0.376 ± 0.006 | 0.359 ± 0.005 | 0.363 ± 0.006 | 0.943 | 0.005 | 0.658 |
| Rate of change | 2.2 ± 1.3 | 3.9 ± 1.2 | 0.7 ± 0.9 | −0.7 ± 1.1 | 0.891 | 0.011 | 0.096 |
| Normocholesterolemic n = 31 | Hypercholesterolemic n = 37 | p Value | |||||
|---|---|---|---|---|---|---|---|
| OPO | SO | OPO | SO | Oil | N/H | N/H × Oil | |
| C-peptide (pg/mL) | |||||||
| Initial | 548 ± 45 | 546 ± 43 | 687 ± 63 | 634 ± 43 | 0.582 | 0.030 | 0.788 |
| Final | 489 ± 33 | 504 ± 38 | 639 ± 46 | 630 ± 43 | 0.937 | 0.001 | 0.873 |
| Rate of change | −6.2 ± 4.7 | −3.3 ± 4.7 | 1.9 ± 5.8 | 1.8 ± 3.7 | 0.697 | 0.259 | 0.723 |
| Ghrelin (pg/mL) | |||||||
| Initial | 319 ± 42 | 297 ± 26 | 271 ± 26 | 252 ± 23 | 0.642 | 0.159 | 0.971 |
| Final | 299 ± 38 | 309 ± 30 | 250 ± 23 | 296 ± 26 | 0.161 | 0.330 | 0.543 |
| Rate of change | 3.8 ± 7.9 | 6.0 ± 5.8 | 2.3 ± 7.4 | 41.2 ± 20.7 | 0.031 | 0.348 | 0.166 |
| GIP (pg/mL) | |||||||
| Initial | 807 ± 168 | 840 ± 188 | 667 ± 29 | 635 ± 36 | 0.639 | 0.281 | 0.549 |
| Final | 770 ± 162 | 837 ± 188 | 657 ± 24 | 647 ± 38 | 0.847 | 0.581 | 0.571 |
| Rate of change | −1.9 ± 5.7 | 0.2 ± 2.9 | 0.4 ± 3.0 | 3.1 ± 2.9 | 0.554 | 0.513 | 0.975 |
| GLP-1 (pg/mL) | |||||||
| Initial | 255 ± 12 | 252 ± 13 | 259 ± 9 | 251 ± 10 | 0.624 | 0.852 | 0.656 |
| Final | 261 ± 11 | 257 ± 10 | 256 ± 9 | 256 ± 10 | 0.842 | 0.775 | 0.896 |
| Rate of change | −3.1 ± 7.7 | −4.4 ± 7.8 | 0.1 ± 2.8 | 4.0 ± 3.4 | 0.992 | 0.902 | 0.274 |
| Glucagon (pg/mL) | |||||||
| Initial | 865 ± 71 | 807 ± 64 | 833 ± 54 | 858 ± 62 | 0.806 | 0.871 | 0.512 |
| Final | 906 ±74 | 824 ± 71 | 903 ± 55 | 869 ± 53 | 0.351 | 0.751 | 0.679 |
| Rate of change | 16.8 ± 14.7 | 5.5 ± 7.1 | 14.54 ± 6.1 | 7.97 ± 5.6 | 0.956 | 0.480 | 0.627 |
| Insulin (pg/mL) | |||||||
| Initial | 176 ± 25 | 200 ± 23 | 246 ± 33 | 211 ± 24 | 0.739 | 0.114 | 0.198 |
| Final | 150 ± 16 | 162 ± 20 | 235 ± 32 | 234 ± 32 | 0.841 | 0.005 | 0.818 |
| Rate of change | −0.6 ± 9.1 | −9.3 ± 9.2 | 12.0 ± 10.3 | 15.0 ± 8.1 | 0.886 | 0.065 | 0.807 |
| Leptin (ng/mL) | |||||||
| Initial | 3.3 ± 0.4 | 3.2 ± 0.4 | 3.6 ± 0.5 | 3.0 ± 0.4 | 0.535 | 0.940 | 0.645 |
| Final | 2.9 ± 0.4 | 3.2 ± 0.4 | 3.2 ± 0.4 | 3.4 ± 0.4 | 0.593 | 0.519 | 0.911 |
| Rate of change | −8.0± 5.4 | 1.7 ± 6.3 | 8.6 ± 6.8 | 18.6 ± 98.3 | 0.017 | 0.180 | 0.947 |
| PAI-1 (ng/mL) | |||||||
| Initial | 6.9 ± 0.3 | 7.0 ± 0.3 | 7.2 ± 0.5 | 6.9 ± 0.4 | 0.815 | 0.853 | 0.608 |
| Final | 7.1 ± 0.3 | 6.9 ± 0.3 | 7.1 ± 0.4 | 7.2 ± 0.4 | 0.847 | 0.565 | 0.590 |
| Rate of change | 4.5 ± 3.3 | −1.5 ± 2.8 | 5.7 ± 6.3 | 9.3 ± 8.1 | 0.805 | 0.459 | 0.444 |
| Resistin (ng/mL) | |||||||
| Initial | 6.0 ± 0.6 | 6.6 ± 0.7 | 6.5 ± 0.6 | 7.2 ± 1.0 | 0.179 | 0.641 | 0.377 |
| Final | 6.1 ± 0.4 | 6.4 ± 0.6 | 6.1 ± 0.6 | 6.3 ± 0.5 | 0.744 | 0.969 | 0.858 |
| Rate of change | 22.1 ± 0.217.5 | 8.1 ± 10.4 | 5.5 ± 11.7 | 11.7 ± 24.7 | 0.826 | 0.221 | 0.719 |
| Visfatin (ng/mL) | |||||||
| Initial | 1.53 ± 0.09 | 1.36 ± 0.08 | 1.63 ± 0.06 | 1.56 ± 0.07 | 0.100 | 0.097 | 0.596 |
| Final | 1.44 ± 0.07 | 1.48 ± 0.16 | 1.62 ± 0.07 | 1.62 ± 0.07 | 0.764 | 0.050 | 0.971 |
| Rate of change | −5.3 ± 2.2 | 3.9 ± 20.6 | 0.5 ± 3.0 | 5.7 ± 4.2 | 0.724 | 0.091 | 0.470 |
| Adiponectin (ng/mL) | |||||||
| Initial | 39 ± 8 | 56 ± 12 | 71 ± 17 | 78 ± 21 | 0.157 | 0.264 | 0.461 |
| Final | 49 ± 9 | 61 ± 20 | 91 ± 29 | 63 ± 15 | 0.029 | 0.793 | 0.916 |
| Rate of change | 20.4 ± 33.1 | 63.1 ± 28.2 | 54.4 ± 28.8 | −69.8 ± 36.9 | 0.205 | 0.081 | 0.098 |
| Adipsin (pg/mL) | |||||||
| Initial | 660 ± 68 | 677 ± 107 | 853 ± 92 | 815 ± 91 | 0.866 | 0.072 | 0.739 |
| Final | 619 ± 73 | 600 ± 65 | 716 ± 64 | 664 ± 61 | 0.523 | 0.190 | 0.619 |
| Rate of change | 4.6 ± 13.2 | 9.6 ± 11.1 | −6.0 ± 1.0 | −6.5 ± 9.1 | 0.969 | 0.260 | 0.621 |
| Normocholesterolemic n = 31 | Hypercholesterolemic n = 37 | p Value | |||||
|---|---|---|---|---|---|---|---|
| OPO | SO | OPO | SO | Oil | N/H | N/H × Oil | |
| Weight (kg) | |||||||
| Initial | 64.1 ± 2.1 | 64.0 ± 2.1 | 74.1 ± 2.6 | 74.1 ± 2.7 | 0.990 | 0.000 | 0.967 |
| Final | 63.9 ± 2.1 | 64.0 ± 2.1 | 74.2 ± 2.7 | 74.2 ± 2.7 | 0.945 | 0.000 | 0.967 |
| Rate of change | −0.3± 0.3 | 0.0 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.660 | 0.272 | 0.545 |
| BMI (kg/m2) | |||||||
| Initial | 23.9 ± 0.7 | 23.7 ± 0.7 | 26.0 ± 0.7 | 26.0 ± 0.7 | 0.893 | 0.002 | 0.976 |
| Final | 23.8 ± 0.7 | 23.8 ± 0.7 | 26.1 ± 0.7 | 26.1 ± 0.7 | 0.930 | 0.001 | 0.980 |
| Rate of change | −0.4 ± 0. 3 | 0.3 ± 0.5 | 0.2 ± 0.2 | 0.4 ± 0.3 | 0.365 | 0.050 | 0.805 |
| Body fat (%) | |||||||
| Initial | 25.5 ± 1.5 | 24.8 ± 1.5 | 26.2 ± 0.7 | 26.0 ± 0.7 | 0.803 | 0.512 | 0.845 |
| Final | 25.9 ± 1.3 | 25.2 ± 1.5 | 26.1 ± 0.7 | 25.8 ± 0.7 | 0.844 | 0.805 | 0.818 |
| Rate of change | 1.1 ± 2.3 | −1.0 ± 1.1 | 3.3 ± 2.4 | 3.8 ± 2.2 | 0.848 | 0.058 | 0.714 |
| Visceral fat (%) | |||||||
| Initial | 3.5 ± 0.5 | 3.3 ± 0.5 | 7.3 ± 0.7 | 7.0 ± 0.7 | 0.794 | 0.000 | 0.935 |
| Final | 3.4 ± 0.5 | 3.4 ± 0.5 | 7.2 ± 0.7 | 7.1 ± 0.7 | 0.822 | 0.000 | 0.867 |
| Rate of change | −4.5 ± 2.7 | 2.2 ± 4.0 | −1.4 ± 1.5 | 6.6 ± 6.1 | 0.028 | 0.490 | 0.636 |
| Waist circumference (cm) | |||||||
| Initial | 75.8 ± 1.8 | 76.6 ± 2.2 | 84.5 ± 2.3 | 85.6 ± 2.5 | 0.545 | 0.000 | 0.863 |
| Final | 74.7 ± 1.8 | 76.0 ± 2.0 | 83.7 ± 2.3 | 85.1 ± 2.3 | 0.548 | 0.000 | 0.997 |
| Rate of change | −1.2 ± 0. 9 | −0.7 ± 0. 9 | −1.0± 0. 4 | −0.5 ± 0. 8 | 0.467 | 0.935 | 0.884 |
| Hip circumference (cm) | |||||||
| Initial | 96.1 ± 1.5 | 96.0 ± 1.7 | 99.7 ± 1.2 | 98.8 ± 1.6 | 0.973 | 0.053 | 0.886 |
| Final | 97.6 ± 1.4 | 96.7 ± 1.7 | 100.2 ± 1.3 | 99.2 ± 1.6 | 0.848 | 0.001 | 0.948 |
| Rate of change | 0.6 ± 0.5 | 0.6 ± 0. 9 | 0.6 ± 0. 4 | 1.1 ± 0.9 | 0.534 | 0.616 | 0.538 |
| Arm circumference (cm) | |||||||
| Initial | 28.5 ± 0.6 | 28.3 ± 0.6 | 30.5 ± 0.6 | 30.4 ± 0.6 | 0.848 | 0.001 | 0.948 |
| Final | 28.3 ± 0.6 | 28.6 ± 0.7 | 30.3 ± 0.6 | 30.5 ± 0.6 | 0.945 | 0.002 | 0.920 |
| Rate of change | −0.5 ± 0. 5 | 0.1 ± 0. 4 | −0.7 ± 0. 5 | 0.2 ± 0. 4 | 0.130 | 0.962 | 0.559 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rámila, S.; Sarriá, B.; Seguido, M.Á.; García-Cordero, J.; Bravo-Clemente, L.; Mateos, R. Effect of Olive Pomace Oil on Cardiovascular Health and Associated Pathologies. Nutrients 2022, 14, 3927. https://doi.org/10.3390/nu14193927
González-Rámila S, Sarriá B, Seguido MÁ, García-Cordero J, Bravo-Clemente L, Mateos R. Effect of Olive Pomace Oil on Cardiovascular Health and Associated Pathologies. Nutrients. 2022; 14(19):3927. https://doi.org/10.3390/nu14193927
Chicago/Turabian StyleGonzález-Rámila, Susana, Beatriz Sarriá, Miguel Ángel Seguido, Joaquín García-Cordero, Laura Bravo-Clemente, and Raquel Mateos. 2022. "Effect of Olive Pomace Oil on Cardiovascular Health and Associated Pathologies" Nutrients 14, no. 19: 3927. https://doi.org/10.3390/nu14193927
APA StyleGonzález-Rámila, S., Sarriá, B., Seguido, M. Á., García-Cordero, J., Bravo-Clemente, L., & Mateos, R. (2022). Effect of Olive Pomace Oil on Cardiovascular Health and Associated Pathologies. Nutrients, 14(19), 3927. https://doi.org/10.3390/nu14193927









