Urinary Metabolomics Study on the Protective Role of Cocoa in Zucker Diabetic Rats via 1H-NMR-Based Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets, Animals, and Experimental Design
2.2. Biochemical Determinations
2.3. Preparation of Urine Samples and NMR Analysis
2.4. Statistical and Multivariate Analysis
3. Results
3.1. Effect of Cocoa Supplementation on Physiological Parameters
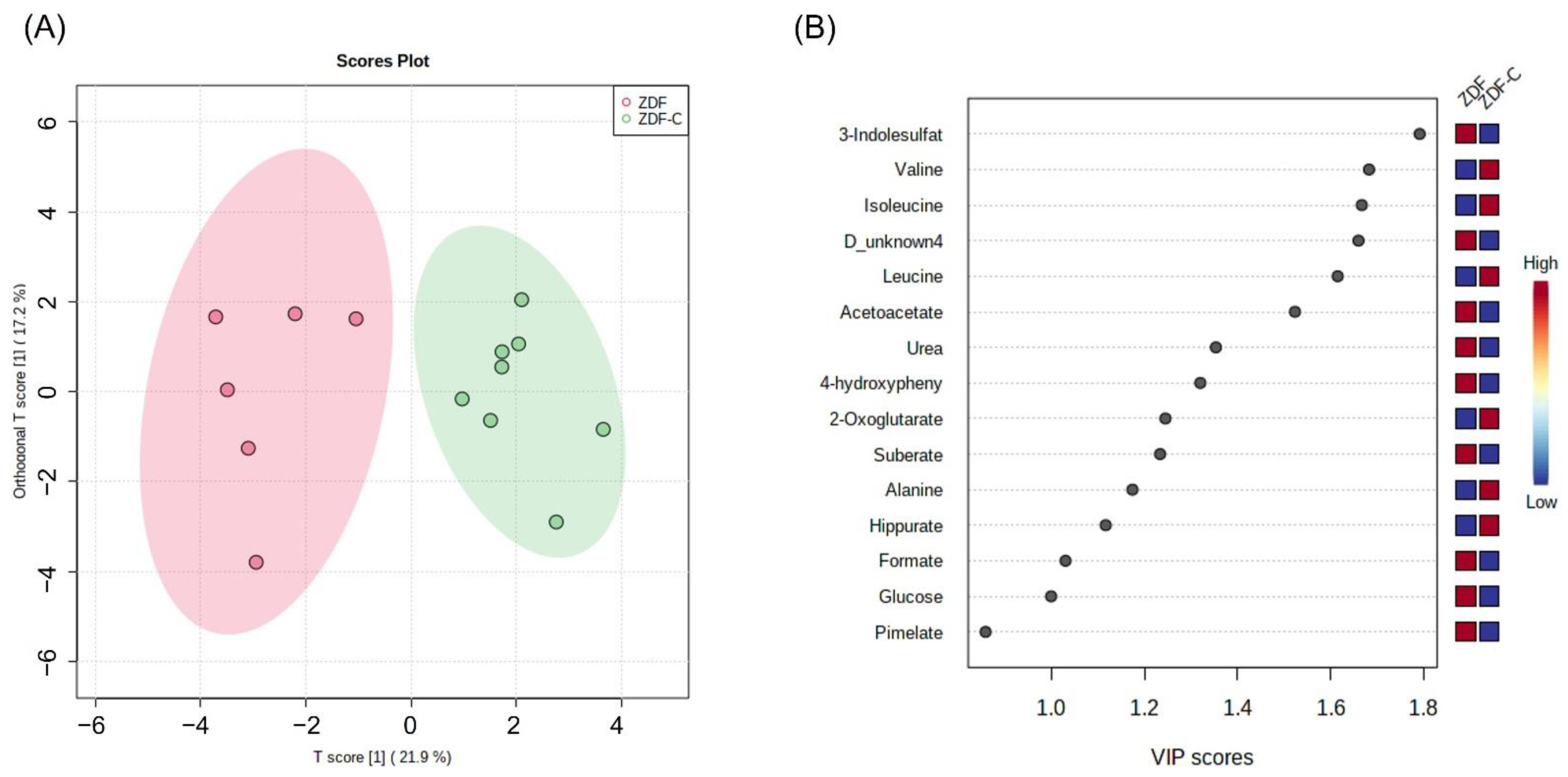
3.2. Metabolic Analysis of Urine Samples
3.3. Identification of Potential Biomarkers Associated with the Cocoa Ingestion in ZDF Rats
3.4. Correlation Analysis between Urine Metabolites and Biomarkers Associated with Cocoa Intake
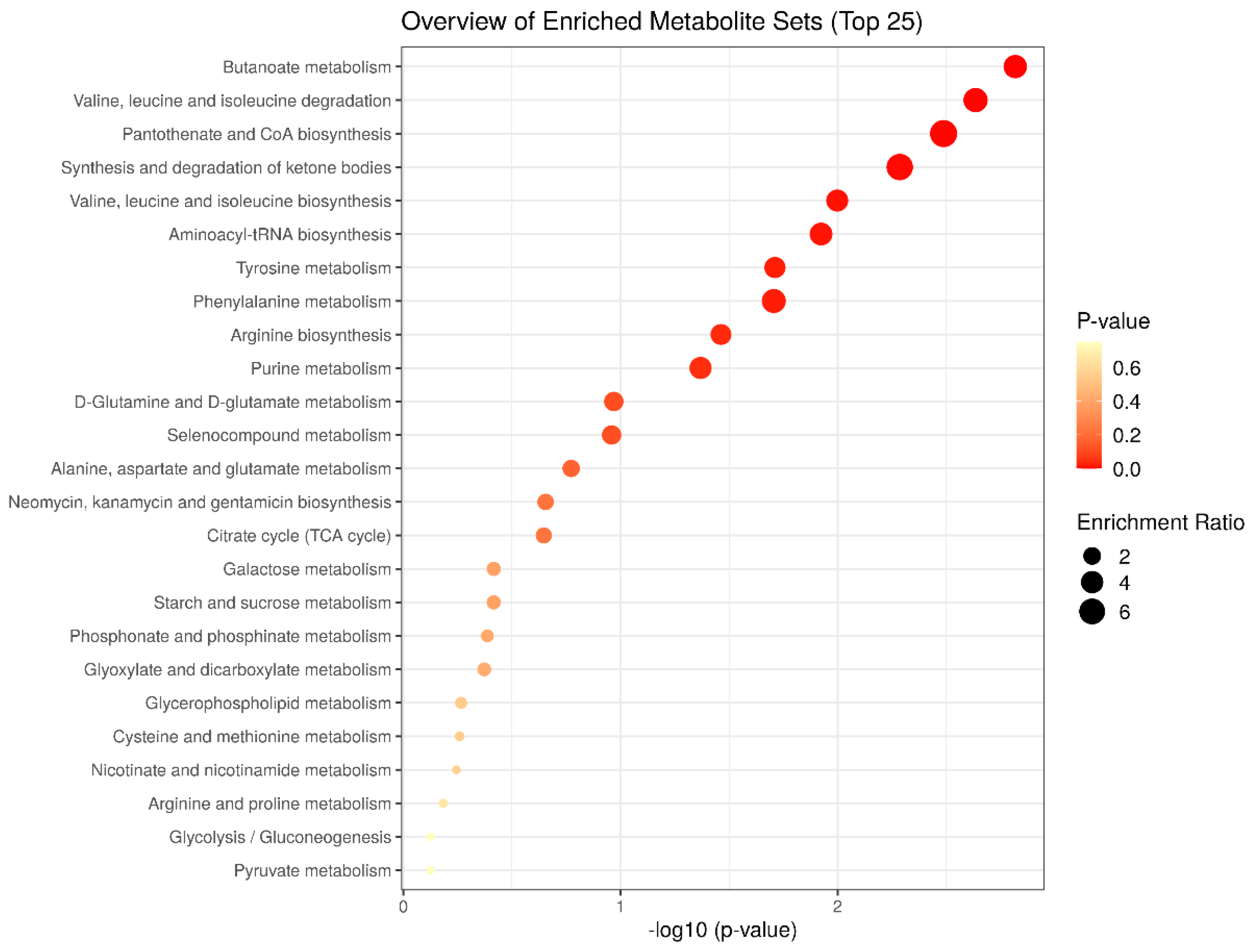
3.5. Analysis of Biomarker Networks and Reconstruction of Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 21 March 2020).
- American Diabetes Association; Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care 2021, 44, S15–S33.
- Palanisamy, S.; Yien, E.L.H.; Shi, L.W.; Si, L.Y.; Qi, S.H.; Ling, L.S.C.; Lun, T.W.; Chen, Y.N. Systematic review of efficacy and safety of newer antidiabetic drugs approved from 2013 to 2017 in controlling HbA1c in diabetes patients. Pharmacy 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Márquez Campos, E.; Jakobs, L.; Simon, M.-C. Antidiabetic Effects of Flavan-3-ols and Their Microbial Metabolites. Nutrients 2020, 12, 1592. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Roy, A.; Dinda, S. Dietary plant flavonoids in prevention of obesity and diabetes. Adv. Protein Chem. Struct. Biol. 2020, 120, 159–235. [Google Scholar]
- Ramos, S.; Martín, M.A.; Goya, L. Effects of Cocoa Antioxidants in Type 2 Diabetes Mellitus. Antioxidants 2017, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Millán, E.; Cordero-Herrera, I.; Ramos, S.; Escrivá, F.; Alvarez, C.; Goya, L.; Martín, M.A. Cocoa-rich diet attenuates beta cell mass loss and function in young Zucker diabetic fatty rats by preventing oxidative stress and beta cell apoptosis. Mol. Nutr. Food Res. 2015, 59, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cilleros, D.; López-Oliva, M.E.; Morales-Cano, D.; Barreira, B.; Pérez-Vizcaíno, F.; Goya, L.; Ramos, S.; Martín, M.A. Dietary cocoa prevents aortic remodeling and vascular oxidative stress in diabetic rats. Mol. Nutr. Food Res. 2019, 30, e1900044. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cilleros, D.; López-Oliva, E.; Goya, L.; Martín, M.A.; Ramos, S. Cocoa intake attenuates renal injury in Zucker Diabetic fatty rats by improving glucose homeostasis. Food Chem. Toxicol. 2019, 127, 101–109. [Google Scholar] [CrossRef]
- Martín, M.A.; Goya, L.; Ramos, S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016, 60, 1756–1769. [Google Scholar] [CrossRef]
- Álvarez-Cilleros, D.; Ramos, S.; López-Oliva, M.E.; Escrivá, F.; Álvarez, C.; Fernández-Millán, E.; Martín, M.A. Cocoa diet modulates gut microbiota composition and improves intestinal health in Zucker diabetic rats. Food Res. Int. 2020, 132, 109058. [Google Scholar] [CrossRef]
- Arneth, B.; Arneth, R.; Shams, M. Metabolomics of Type 1 and Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 2467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Recent and potential developments of biofluid analyses in metabolomics. J. Proteome 2012, 75, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Klassen, A.; Faccio, A.T.; Canuto, G.A.; da Cruz, P.L.; Ribeiro, H.C.; Tavares, M.F.; Sussulini, A. Metabolomics: Definitions and significance in systems biology. Adv. Exp. Med. Biol. 2017, 965, 3–17. [Google Scholar] [PubMed]
- Hasanpour, M.; Iranshahy, M.; Iranshahi, M. The application of metabolomics in investigating anti-diabetic activity of medicinal plants. Biomed. Pharmacother. 2020, 128, 110263. [Google Scholar] [CrossRef] [PubMed]
- Sajak, A.A.B.; Mediani, A.; Dom, N.S.M.; Machap, C.; Hamid, M.; Ismail, A.; Khatib, A.; Abas, F. Effect of Ipomoea aquatica ethanolic extract in streptozotocin (STZ) induced diabetic rats via 1H NMR-based metabolomics approach. Phytomedicine 2017, 36, 201–209. [Google Scholar] [CrossRef]
- Azam, A.A.; Pariyani, R.; Ismail, I.S.; Ismail, A.; Khatib, A.; Abas, F.; Shaari, K. Urinary metabolomics study on the protective role of Orthosiphon stamineus in Streptozotocin induced diabetes mellitus in rats via 1H NMR spectroscopy. BMC Complement. Altern. Med. 2017, 17, 278. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Maulidiani, M.; Khatib, A.; Tan, C.P.; Ismail, I.S.; Shaari, K.; Ismail, A.; Lajis, N. Metabolic and biochemical changes in streptozotocin induced obese-diabetic rats treated with Phyllanthus niruri extract. J. Pharm. Biomed. Anal. 2016, 128, 302–312. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Zhang, J.; Pariyani, R.; Jokioja, J.; Kortesniemi, M.; Linderborg, K.M.; Heinonen, J.; Sainio, T.; Zhang, Y.; et al. Effects of Anthocyanin Extracts from Bilberry (Vaccinium myrtillus L.) and Purple Potato (Solanum tuberosum L. Var. ‘Synkeä Sakari’) on the Plasma Metabolomic Profile of Zucker Diabetic Fatty Rats. J. Agric. Food Chem. 2020, 68, 9436–9450. [Google Scholar] [CrossRef]
- Singh, A.K.; Raj, V.; Keshari, A.K.; Rai, A.; Kumar, P.; Rawat, A.; Maity, B.; Kumar, D.; Prakash, A.; De, A.; et al. Isolated mangiferin and naringenin exert antidiabetic effect via PPARγ/GLUT4 dual agonistic action with strong metabolic regulation. Chem. Biol. Interact. 2018, 280, 33–44. [Google Scholar] [CrossRef]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Serkova, N.; Fuller, T.F.; Klawitter, J.; Freise, C.E.; Niemann, C.U. 1H-NMR–based metabolic signatures of mild and severe ischemia/reperfusion injury in rat kidney transplants. Kidney Int. 2005, 67, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Mayneris-Perxachs, J.; Costabile, A.; Swann, J.R.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Association between urinary metabolic profile and the intestinal effects of cocoa in rats. Br. J. Nutr. 2017, 117, 623–634. [Google Scholar] [CrossRef][Green Version]
- Tabone, M.; García-Merino, J.A.; Bressa, C.; Guzman, N.E.R.; Rocha, K.H.; Chu Van, E.; Castelli, F.A.; Fenaille, F.; Larrosa, M. Chronic Consumption of Cocoa Rich in Procyanidins Has a Marginal Impact on Gut Microbiota and on Serum and Fecal Metabolomes in Male Endurance Athletes. J. Agric. Food Chem. 2022, 70, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.P.; Rezzi, S.; Peré-Trepat, E.; Kamlage, B.; Collino, S.; Leibold, E.; Kastler, J.; Rein, D.; Fay, L.B.; Kochhar, S. Metabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjects. J. Proteome Res. 2009, 8, 5568–5579. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef]
- Menni, C.; Fauman, E.; Erte, I.; Perry, J.R.; Kastenmuller, G.; Shin, S.Y.; Petersen, A.K.; Hyde, C.; Psatha, M.; Ward, K.J.; et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 2013, 62, 4270–4276. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Bloomgarden, Z.J. Diabetes and branched-chain amino acids: What is the link? Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Arriola Apelo, S.I.; et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2018, 596, 623–645. [Google Scholar] [CrossRef]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R.D.; et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol. Genom. 2007, 29, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, M.; Kemme, M.; Ouwens, M.; van Hoogdalem, E.J.; Jones, R.; Romijn, H.; de Kam, M.; Schoemaker, R.; Burggraaf, K.; Cohen, A. Evaluation of proinflammatory cytokines and inflammation markers as biomarkers for the action of thiazolidinediones in Type 2 diabetes mellitus patients and healthy volunteers. Br. J. Clin. Pharmacol. 2006, 62, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Benetti, E.; Liberto, E.; Bressanello, D.; Bordano, V.; Rosa, A.C.; Miglio, G.; Haxhi, J.; Pugliese, G.; Balducci, S.; Cordero, C. Sedentariness and Urinary Metabolite Profile in Type 2 Diabetic Patients, a Cross-Sectional Study. Metabolites 2020, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Herrera, I.; Martín, M.A.; Escrivá, F.; Álvarez, C.; Goya, L.; Ramos, S. Cocoa-rich diet ameliorates hepatic insulin resistance by modulating insulin signaling and glucose homeostasis in Zucker diabetic fatty rats. J. Nutr. Biochem. 2015, 26, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Wohl, P.; Krušinová, E.; Klementová, M.; Wohl, P.; Kratochvílová, S.; Pelikánová, T. Urinary urea nitrogen excretion during the hyperinsulinemic euglycemic clamp in type 1 diabetic patients and healthy subjects. Physiol. Res. 2008, 57, 247–252. [Google Scholar] [CrossRef]
- Clarke, E.D.; Collins, C.E.; Rollo, M.E.; Kroon, P.A.; Philo, M.; Haslam, R.L. The relationship between urinary polyphenol metabolites and dietary polyphenol intakes in young adults. Br. J. Nutr. 2022, 127, 589–598. [Google Scholar] [CrossRef]
- Clifford, M.N.; Copeland, E.L.; Bloxsidge, J.P.; Mitchell, L.A. Hippuric acid as a major excretion product associated with black tea consumption. Xenobiotica 2000, 30, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Olmo, M.; Oneca, M.; Torre, P.; Sainz, N.; Moreno-Aliaga, M.J.; Guruceaga, E.; Díaz, J.V.; Encio, I.J.; Barajas, M.; Araña, M. A Fermented Food Product Containing Lactic Acid Bacteria Protects ZDF Rats from the Development of Type 2 Diabetes. Nutrients 2019, 11, 2530. [Google Scholar] [CrossRef]
- Calvani, R.; Miccheli, A.; Capuani, G.; Tomassini Miccheli, A.; Puccetti, C.; Delfini, M.; Iaconelli, A.; Nanni, G.; Mingrone, G. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype. Int. J. Obes. 2010, 34, 1095–1098. [Google Scholar] [CrossRef]
- Wang, Y.; Lawler, D.; Larson, B.; Ramadan, Z.; Kochhar, S.; Holmes, E.; Nicholson, J.K. Metabonomic investigations of aging and caloric restriction in a life-long dog study. J. Proteome Res. 2007, 6, 1846–1854. [Google Scholar] [CrossRef]
- Bitner, B.F.; Ray, J.D.; Kener, K.B.; Herring, J.A.; Tueller, J.A.; Johnson, D.K.; Tellez Freitas, C.M.; Fausnacht, D.W.; Allen, M.E.; Thomson, A.H.; et al. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in β-cells and skeletal muscle cells. J. Nutr. Biochem. 2018, 62, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Hostetter, T.H. Uremic solutes from colon microbes. Kidney Int. 2012, 81, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]




| ZL | ZDF | ZDF-C | |
|---|---|---|---|
| Body weight (g) | 329 ± 41 a | 444 ± 82 b | 400 ± 12 c |
| Glucose levels (mg/dL) | 86 ± 90 a | 238 ± 71 b | 118 ± 11 c |
| Insulin levels (ng/mL) | 0.40 ± 0.02 a | 4.36 ± 0.50 b | 1.14 ± 0.22 c |
| HbA1c (%) | 4.38 ± 0.19 a | 10.40 ± 1.58 b | 6.09 ± 0.79 c |
| AUC (mmol/L/min) | 1803 ± 96 a | 4044 ± 60 b | 3049 ± 33 c |
| HOMA IR | 2.60 ± 0.20 a | 90.96 ± 14.26 b | 12.22 ± 1.36 c |
| HOMA B | 149.81 ± 22.21 a | 169.52 ± 46.20 a | 227.83 ± 14.32 b |
| TG (mmol/L) | 0.39 ± 0.09 a | 2.74 ± 0.24 b | 2.87 ± 0.31 b |
| HDL-Cholesterol (mmol/L) | 2.27 ± 0.22 a | 2.85 ± 0.37 b | 3.08 ± 0.32 b |
| LDL-Cholesterol (mmol/L) | 0.92 ± 0.09 a | 2.28 ± 0.33 b | 2.82 ± 0.31 c |
| Top Pathways | Total Compounds | Hits | p Value | FDR * | Metabolites Identified |
|---|---|---|---|---|---|
| Butanoate metabolism | 15 | 2 | 0.0015 | 0.026 | Acetoacetate, 2-oxoglutarate |
| Valine, leucine and isoleucine degradation | 40 | 5 | 0.0023 | 0.026 | Isoleucine, Leucine, Valine, Acetoacetate, 4-Methyl-2-oxopentanoate |
| Pantothenate and CoA biosynthesis | 19 | 1 | 0.0032 | 0.026 | Valine |
| Synthesis and degradation of ketone bodies | 5 | 1 | 0.0051 | 0.031 | Acetoacetate |
| Valine, leucine and isoleucine biosynthesis | 8 | 5 | 0.01 | 0.047 | Isoleucine, Leucine, Valine, Threonine, Acetoacetate, |
| Aminoacyl-tRNA biosynthesis | 48 | 5 | 0.019 | 0.047 | Isoleucine, Leucine, Valine, Alanine, Lysine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Millán, E.; Ramos, S.; Álvarez-Cilleros, D.; Samino, S.; Amigó, N.; Correig, X.; Chagoyen, M.; Álvarez, C.; Martín, M.Á. Urinary Metabolomics Study on the Protective Role of Cocoa in Zucker Diabetic Rats via 1H-NMR-Based Approach. Nutrients 2022, 14, 4127. https://doi.org/10.3390/nu14194127
Fernández-Millán E, Ramos S, Álvarez-Cilleros D, Samino S, Amigó N, Correig X, Chagoyen M, Álvarez C, Martín MÁ. Urinary Metabolomics Study on the Protective Role of Cocoa in Zucker Diabetic Rats via 1H-NMR-Based Approach. Nutrients. 2022; 14(19):4127. https://doi.org/10.3390/nu14194127
Chicago/Turabian StyleFernández-Millán, Elisa, Sonia Ramos, David Álvarez-Cilleros, Sara Samino, Nuria Amigó, Xavier Correig, Mónica Chagoyen, Carmen Álvarez, and María Ángeles Martín. 2022. "Urinary Metabolomics Study on the Protective Role of Cocoa in Zucker Diabetic Rats via 1H-NMR-Based Approach" Nutrients 14, no. 19: 4127. https://doi.org/10.3390/nu14194127
APA StyleFernández-Millán, E., Ramos, S., Álvarez-Cilleros, D., Samino, S., Amigó, N., Correig, X., Chagoyen, M., Álvarez, C., & Martín, M. Á. (2022). Urinary Metabolomics Study on the Protective Role of Cocoa in Zucker Diabetic Rats via 1H-NMR-Based Approach. Nutrients, 14(19), 4127. https://doi.org/10.3390/nu14194127






