Zinc Fortification: Current Trends and Strategies
Abstract
1. Introduction
2. Dietary Factors Influencing Zinc Absorption and Retention
3. Techniques for Estimating the Impact of Zinc Fortification on Zinc Absorption
4. Zinc Utilization and Trends in Zinc Biomarkers for Monitoring Fortification
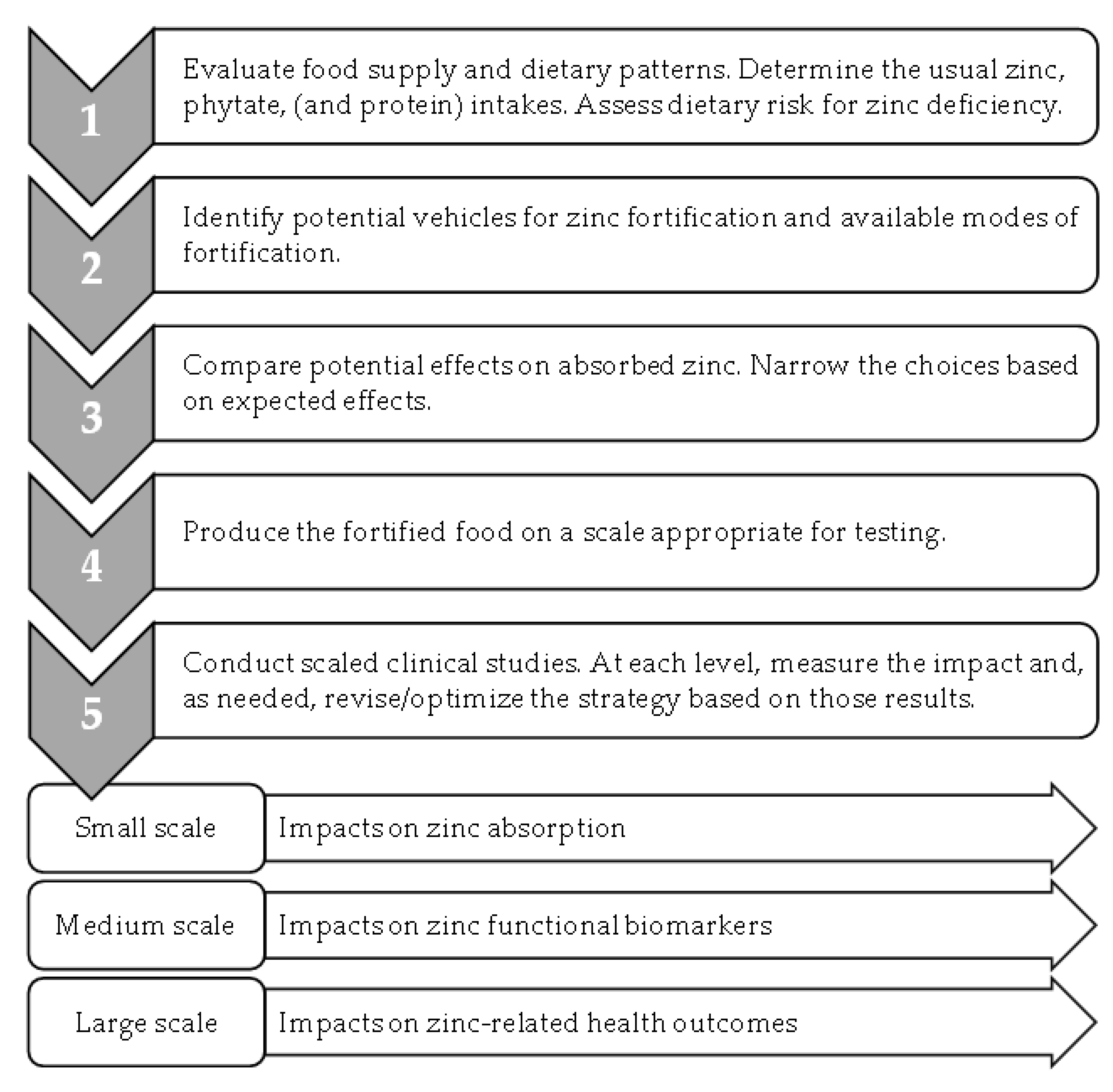
5. Trends in Zinc Fortification Strategies
6. Building on Current Trends for Future Progress in Zinc Fortification Efforts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc deficiency in low- and middle-income countries: Prevalence and approaches for mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Southon, S. Bioavailability of Nutrients. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; Volume 1, pp. 478–484. [Google Scholar] [CrossRef]
- Brown, K.H.; Hambidge, K.M.; Ranum, P.; Zinc Fortification Working Group. Zinc fortification of cereal flours: Current recommendations and research needs. Food Nutr. Bull. 2010, 31, S62–S74. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H.; Wessells, K.R.; Hess, S.Y. Zinc bioavailability from zinc-fortified foods. Int. J. Vitam. Nutr. Res. 2007, 77, 174–181. [Google Scholar] [CrossRef] [PubMed]
- WHO Guideline: Fortification of Maize Flour and Corn Meal with Vitamins and Minerals; World Health Organization: Geneva, Switzerland, 2016.
- Miller, L.V.; Krebs, N.F.; Hambidge, K.M. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J. Nutr. 2007, 137, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.V.; Krebs, N.F.; Hambidge, K.M. Mathematical model of zinc absorption: Effects of dietary calcium, protein and iron on zinc absorption. Br. J. Nutr. 2013, 109, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Cheryan, M. Phytic acid interactions in food systems. Crit. Rev. Food Sci. Nutr. 1980, 13, 297–335. [Google Scholar] [CrossRef]
- FAO/IZiNCG. FAO/INFOODS/IZiNCG Global Food Composition Database for Phytate Version 1.0—PhyFoodComp 1.0. 2018. Available online: https://www.fao.org/3/i8542en/I8542EN.pdf (accessed on 16 August 2022).
- Matseshe, J.W.; Phillips, S.F.; Malagelada, J.R.; McCall, J.T. Recovery of dietary iron and zinc from the proximal intestine of healthy man: Studies of different meals and supplements. Am. J. Clin. Nutr. 1980, 33, 1946–1953. [Google Scholar] [CrossRef]
- Neve, J.; Hanocq, M.; Peretz, A.; Abi Khalil, F.; Pelen, F.; Famaey, J.P.; Fontaine, J. Pharmacokinetic study of orally administered zinc in humans: Evidence for an enteral recirculation. Eur. J. Drug Metab. Pharmacokinet. 1991, 16, 315–323. [Google Scholar] [CrossRef]
- Neve, J.; Hanocq, M.; Peretz, A.; Khalil, F.A.; Pelen, F. Absorption and metabolism of oral zinc gluconate in humans in fasting state, during, and after a meal. Biol. Trace Elem. Res. 1992, 32, 201–212. [Google Scholar] [CrossRef]
- Gibson, R.S.; King, J.C.; Lowe, N. A Review of Dietary Zinc Recommendations. Food Nutr. Bull. 2016, 37, 443–460. [Google Scholar] [CrossRef]
- Rothman, S.; Liebow, C.; Isenman, L. Conservation of digestive enzymes. Physiol. Rev. 2002, 82, 1–18. [Google Scholar] [CrossRef]
- Sauer, A.K.; Pfaender, S.; Hagmeyer, S.; Tarana, L.; Mattes, A.K.; Briel, F.; Kury, S.; Boeckers, T.M.; Grabrucker, A.M. Characterization of zinc amino acid complexes for zinc delivery in vitro using Caco-2 cells and enterocytes from hiPSC. Biometals 2017, 30, 643–661. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Sheng, X.; Krebs, N.F. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 2010, 91, 1478S–1483S. [Google Scholar] [CrossRef]
- Shames, D.M.; Woodhouse, L.R.; Lowe, N.M.; King, J.C. Accuracy of simple techniques for estimating fractional zinc absorption in humans. J. Nutr. 2001, 131, 1854–1861. [Google Scholar] [CrossRef]
- Rosado, J.L.; Díaz, M.; Muñoz, E.; Westcott, J.L.; González, K.E.; Krebs, N.F.; Caamaño, M.C.; Hambidge, M. Bioavailability of zinc oxide added to corn tortilla is similar to that of zinc sulfate and is not affected by simultaneous addition of iron. Food Nutr. Bull. 2012, 33, 261–266. [Google Scholar] [CrossRef]
- Brnic, M.; Wegmuller, R.; Zeder, C.; Senti, G.; Hurrell, R.F. Influence of phytase, EDTA, and polyphenols on zinc absorption in adults from porridges fortified with zinc sulfate or zinc oxide. J. Nutr. 2014, 144, 1467–1473. [Google Scholar] [CrossRef]
- Brnic, M.; Wegmuller, R.; Melse-Boonstra, A.; Stomph, T.; Zeder, C.; Tay, F.M.; Hurrell, R.F. Zinc Absorption by Adults Is Similar from Intrinsically Labeled Zinc-Biofortified Rice and from Rice Fortified with Labeled Zinc Sulfate. J. Nutr. 2016, 146, 76–80. [Google Scholar] [CrossRef]
- Signorell, C.; Zimmermann, M.B.; Cakmak, I.; Wegmuller, R.; Zeder, C.; Hurrell, R.; Aciksoz, S.B.; Boy, E.; Tay, F.; Frossard, E.; et al. Zinc Absorption from Agronomically Biofortified Wheat Is Similar to Post-Harvest Fortified Wheat and Is a Substantial Source of Bioavailable Zinc in Humans. J. Nutr. 2019, 149, 840–846. [Google Scholar] [CrossRef]
- Galetti, V.; Kujinga, P.; Mitchikpe, C.E.; Zeder, C.; Tay, F.; Tossou, F.; Hounhouigan, J.D.; Zimmermann, M.B.; Moretti, D. Efficacy of highly bioavailable zinc from fortified water: A randomized controlled trial in rural Beninese children. Am. J. Clin. Nutr. 2015, 102, 1238–1248. [Google Scholar] [CrossRef]
- Kodkany, B.S.; Bellad, R.M.; Mahantshetti, N.S.; Westcott, J.E.; Krebs, N.F.; Kemp, J.F.; Hambidge, K.M. Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorption of these minerals above physiologic requirements in young children. J. Nutr. 2013, 143, 1489–1493. [Google Scholar] [CrossRef]
- Ariff, S.; Krebs, N.F.; Soofi, S.; Westcott, J.; Bhatti, Z.; Tabassum, F.; Bhutta, Z.A. Absorbed zinc and exchangeable zinc pool size are greater in Pakistani infants receiving traditional complementary foods with zinc-fortified micronutrient powder. J. Nutr. 2014, 144, 20–26. [Google Scholar] [CrossRef]
- Chomba, E.; Westcott, C.M.; Westcott, J.E.; Mpabalwani, E.M.; Krebs, N.F.; Patinkin, Z.W.; Palacios, N.; Hambidge, K.M. Zinc absorption from biofortified maize meets the requirements of young rural Zambian children. J. Nutr. 2015, 145, 514–519. [Google Scholar] [CrossRef]
- Mendez, R.O.; Hambidge, M.; Baker, M.; Salgado, S.A.; Ruiz, J.; Garcia, H.S.; Calderon de la Barca, A.M. Zinc absorption from fortified milk powder in adolescent girls. Biol. Trace Elem. Res. 2015, 168, 61–66. [Google Scholar] [CrossRef]
- Brnic, M.; Hurrell, R.F.; Songre-Ouattara, L.T.; Diawara, B.; Kalmogho-Zan, A.; Tapsoba, C.; Zeder, C.; Wegmuller, R. Effect of phytase on zinc absorption from a millet-based porridge fed to young Burkinabe children. Eur. J. Clin. Nutr. 2017, 71, 137–141. [Google Scholar] [CrossRef]
- Zyba, S.J.; Wegmuller, R.; Woodhouse, L.R.; Ceesay, K.; Prentice, A.M.; Brown, K.H.; Wessells, K.R. Effect of exogenous phytase added to small-quantity lipid-based nutrient supplements (SQ-LNS) on the fractional and total absorption of zinc from a millet-based porridge consumed with SQ-LNS in young Gambian children: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1465–1475. [Google Scholar] [CrossRef]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lonnerdal, B.; Ruel, M.T.; Sandstrom, B.; Wasantwisut, E.; Hotz, C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar]
- Miller, L.V.; Hambidge, K.M.; Krebs, N.F. Zinc absorption is not related to dietary phytate intake in infants and young children based on modeling combined data from multiple studies. J. Nutr. 2015, 145, 1763–1769. [Google Scholar] [CrossRef]
- Manary, M.J.; Hotz, C.; Krebs, N.F.; Gibson, R.S.; Westcott, J.E.; Arnold, T.; Broadhead, R.L.; Hambidge, K.M. Dietary phytate reduction improves zinc absorption in Malawian children recovering from tuberculosis but not in well children. J. Nutr. 2000, 130, 2959–2964. [Google Scholar] [CrossRef]
- Krebs, N.F.; Westcott, J.E.; Culbertson, D.L.; Sian, L.; Miller, L.V.; Hambidge, K.M. Comparison of complementary feeding strategies to meet zinc requirements of older breastfed infants. Am. J. Clin. Nutr. 2012, 96, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Maqsood, M.A.; Miller, L.V. Bioavailable Zinc in Grains of Bread Wheat Varieties of Pakistan. Cereal Res. Commun. 2012, 40, 62–73. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Zhang, W.; Chen, X.; Zou, C. Agronomic Approach of Zinc Biofortification Can Increase Zinc Bioavailability in Wheat Flour and thereby Reduce Zinc Deficiency in Humans. Nutrients 2017, 9, 465. [Google Scholar] [CrossRef]
- Yu, B.G.; Liu, Y.M.; Chen, X.X.; Cao, W.Q.; Ding, T.B.; Zou, C.Q. Foliar Zinc Application to Wheat May Lessen the Zinc Deficiency Burden in Rural Quzhou, China. Front. Nutr. 2021, 8, 697817. [Google Scholar] [CrossRef] [PubMed]
- Joy, E.J.; Ander, E.L.; Young, S.D.; Black, C.R.; Watts, M.J.; Chilimba, A.D.; Chilima, B.; Siyame, E.W.; Kalimbira, A.A.; Hurst, R.; et al. Dietary mineral supplies in Africa. Physiol. Plant. 2014, 151, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Nankap, M.; Ndjebayi, A.O.; Allen, L.H.; Shahab-Ferdows, S.; Hampel, D.; Killilea, D.W.; Gimou, M.M.; Houghton, L.A.; Friedman, A.; et al. Iron, Zinc, Folate, and Vitamin B-12 Status Increased among Women and Children in Yaoundé and Douala, Cameroon, 1 Year after Introducing Fortified Wheat Flour. J. Nutr. 2017, 147, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Haile, D.; Luo, H.; Vosti, S.A.; Dodd, K.W.; Arnold, C.D.; Engle-Stone, R. Micronutrient Fortification of Commercially Available Biscuits Is Predicted to Have Minimal Impact on Prevalence of Inadequate Micronutrient Intakes: Modeling of National Dietary Data from Cameroon. Curr. Dev. Nutr. 2020, 4, nzaa132. [Google Scholar] [CrossRef]
- Jou, M.Y.; Du, X.; Hotz, C.; Lonnerdal, B. Biofortification of rice with zinc: Assessment of the relative bioavailability of zinc in a Caco-2 cell model and suckling rat pups. J. Agric. Food Chem. 2012, 60, 3650–3657. [Google Scholar] [CrossRef]
- Kruger, J.; Taylor, J.R.; Du, X.; De Moura, F.F.; Lonnerdal, B.; Oelofse, A. Effect of phytate reduction of sorghum, through genetic modification, on iron and zinc availability as assessed by an in vitro dialysability bioaccessibility assay, Caco-2 cell uptake assay, and suckling rat pup absorption model. Food Chem. 2013, 141, 1019–1025. [Google Scholar] [CrossRef]
- Vaz-Tostes, M.D.; Verediano, T.A.; de Mejia, E.G.; Brunoro Costa, N.M. Evaluation of iron and zinc bioavailability of beans targeted for biofortification using in vitro and in vivo models and their effect on the nutritional status of preschool children. J. Sci. Food Agric. 2016, 96, 1326–1332. [Google Scholar] [CrossRef]
- Kruger, J. Potential of food-to-food fortification with cowpea leaves and orange-fleshed sweet potato, in combination with conventional fortification, to improve the cellular uptake of iron and zinc from ready-to-eat maize porridges. Food Sci. Nutr. 2020, 8, 3190–3199. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Y.; Ma, Y.; Liu, L.; Wu, D.; Shu, X.; Pan, L.; Lai, Q. Combination of High Zn Density and Low Phytic Acid for Improving Zn Bioavailability in Rice (Oryza stavia L.) Grain. Rice 2021, 14, 23. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Goodall, M.J.; Stall, C.; Pritts, J. Post-prandial and daily changes in plasma zinc. J. Trace Elem. Electrolytes Health Dis. 1989, 3, 55–57. [Google Scholar]
- Wallock, L.M.; King, J.C.; Hambidge, K.M.; English-Westcott, J.E.; Pritts, J. Meal-induced changes in plasma, erythrocyte, and urinary zinc concentrations in adult women. Am. J. Clin. Nutr. 1993, 58, 695–701. [Google Scholar] [CrossRef]
- Wessells, K.R.; Jorgensen, J.M.; Hess, S.Y.; Woodhouse, L.R.; Peerson, J.M.; Brown, K.H. Plasma zinc concentration responds rapidly to the initiation and discontinuation of short-term zinc supplementation in healthy men. J. Nutr. 2010, 140, 2128–2133. [Google Scholar] [CrossRef]
- Massih, Y.N.; Hall, A.G.; Suh, J.; King, J.C. Zinc Supplements Taken with Food Increase Essential Fatty Acid Desaturation Indices in Adult Men Compared with Zinc Taken in the Fasted State. J. Nutr. 2021, 151, 2583–2589. [Google Scholar] [CrossRef]
- Lo, N.B.; Aaron, G.J.; Hess, S.Y.; Dossou, N.I.; Guiro, A.T.; Wade, S.; Brown, K.H. Plasma zinc concentration responds to short-term zinc supplementation, but not zinc fortification, in young children in Senegal1,2. Am. J. Clin. Nutr. 2011, 93, 1348–1355. [Google Scholar] [CrossRef]
- Baer, M.T.; King, J.C. Tissue zinc levels and zinc excretion during experimental zinc depletion in young men. Am. J. Clin. Nutr. 1984, 39, 556–570. [Google Scholar] [CrossRef]
- Hennigar, S.R.; Lieberman, H.R.; Fulgoni, V.L., 3rd; McClung, J.P. Serum Zinc Concentrations in the US Population Are Related to Sex, Age, and Time of Blood Draw but Not Dietary or Supplemental Zinc. J. Nutr. 2018, 148, 1341–1351. [Google Scholar] [CrossRef]
- Consolo, L.Z.; Melnikov, P.; Consolo, F.Z.; Nascimento, V.A.; Pontes, J.C. Zinc supplementation in children and adolescents with acute leukemia. Eur. J. Clin. Nutr. 2013, 67, 1056–1059. [Google Scholar] [CrossRef]
- Zyba, S.J.; Shenvi, S.V.; Killilea, D.W.; Holland, T.C.; Kim, E.; Moy, A.; Sutherland, B.; Gildengorin, V.; Shigenaga, M.K.; King, J.C. A moderate increase in dietary zinc reduces DNA strand breaks in leukocytes and alters plasma proteins without changing plasma zinc concentrations. Am. J. Clin. Nutr. 2017, 105, 343–351. [Google Scholar] [CrossRef]
- Radhakrishna, K.V.; Hemalatha, R.; Geddam, J.J.; Kumar, P.A.; Balakrishna, N.; Shatrugna, V. Effectiveness of zinc supplementation to full term normal infants: A community based double blind, randomized, controlled, clinical trial. PLoS ONE 2013, 8, e61486. [Google Scholar] [CrossRef]
- Joray, M.L.; Yu, T.W.; Ho, E.; Clarke, S.L.; Stanga, Z.; Gebreegziabher, T.; Hambidge, K.M.; Stoecker, B.J. Zinc supplementation reduced DNA breaks in Ethiopian women. Nutr. Res. 2015, 35, 49–55. [Google Scholar] [CrossRef]
- Shah, D.; Sachdev, H.S.; Gera, T.; De-Regil, L.M.; Pena-Rosas, J.P. Fortification of staple foods with zinc for improving zinc status and other health outcomes in the general population. Cochrane Database Syst. Rev. 2016, CD010697. [Google Scholar] [CrossRef]
- Tsang, B.L.; Holsted, E.; McDonald, C.M.; Brown, K.H.; Black, R.; Mbuya, M.N.N.; Grant, F.; Rowe, L.A.; Manger, M.S. Effects of Foods Fortified with Zinc, Alone or Cofortified with Multiple Micronutrients, on Health and Functional Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1821–1837. [Google Scholar] [CrossRef]
- Chimhashu, T.; Malan, L.; Baumgartner, J.; van Jaarsveld, P.J.; Galetti, V.; Moretti, D.; Smuts, C.M.; Zimmermann, M.B. Sensitivity of fatty acid desaturation and elongation to plasma zinc concentration: A randomised controlled trial in Beninese children. Br. J. Nutr. 2018, 119, 610–619. [Google Scholar] [CrossRef]
- Jongstra, R.; Hossain, M.M.; Galetti, V.; Hall, A.G.; Holt, R.R.; Cercamondi, C.I.; Rashid, S.F.; Zimmermann, M.B.; Mridha, M.K.; Wegmueller, R. The effect of zinc-biofortified rice on zinc status of Bangladeshi pre-school children: A randomized, double-masked, household-based controlled trial. Am. J. Clin. Nutr. 2022, 115, 724–737. [Google Scholar] [CrossRef]
- Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef]
- Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; DeMartiis, M.; Giannandrea, E.; Renieri, C.; Busco, F.; Galeazzi, R.; et al. Effects of zinc-fortified drinking skim milk (as functional food) on cytokine release and thymic hormone activity in very old persons: A pilot study. Age 2014, 36, 9656. [Google Scholar] [CrossRef]
- Jou, M.Y.; Hall, A.G.; Philipps, A.F.; Kelleher, S.L.; Lonnerdal, B. Tissue-specific alterations in zinc transporter expression in intestine and liver reflect a threshold for homeostatic compensation during dietary zinc deficiency in weanling rats. J. Nutr. 2009, 139, 835–841. [Google Scholar] [CrossRef]
- Mendez, R.O.; Santiago, A.; Yepiz-Plascencia, G.; Peregrino-Uriarte, A.B.; de la Barca, A.M.; Garcia, H.S. Zinc fortification decreases ZIP1 gene expression of some adolescent females with appropriate plasma zinc levels. Nutrients 2014, 6, 2229–2239. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Chavez, M.N.; Brown, R.M.; Walravens, P.A. Zinc nutritional status of young middle-income children and effects of consuming zinc-fortified breakfast cereals. Am. J. Clin. Nutr. 1979, 32, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Zinc. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Donangelo, C.M.; Woodhouse, L.R.; King, S.M.; Viteri, F.E.; King, J.C. Supplemental zinc lowers measures of iron status in young women with low iron reserves. J. Nutr. 2002, 132, 1860–1864. [Google Scholar] [CrossRef] [PubMed]
- Wegmuller, R.; Tay, F.; Zeder, C.; Brnic, M.; Hurrell, R.F. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef]
- De Grande, A.; Leleu, S.; Delezie, E.; Rapp, C.; De Smet, S.; Goossens, E.; Haesebrouck, F.; Van Immerseel, F.; Ducatelle, R. Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poult. Sci. 2020, 99, 441–453. [Google Scholar] [CrossRef]
- Pieper, R.; Dadi, T.H.; Pieper, L.; Vahjen, W.; Franke, A.; Reinert, K.; Zentek, J. Concentration and chemical form of dietary zinc shape the porcine colon microbiome, its functional capacity and antibiotic resistance gene repertoire. ISME J. 2020, 14, 2783–2793. [Google Scholar] [CrossRef]
- Sreenivasulu, K.; Raghu, P.; Ravinder, P.; Nair, K.M. Effect of dietary ligands and food matrices on zinc uptake in Caco-2 cells: Implications in assessing zinc bioavailability. J. Agric. Food Chem. 2008, 56, 10967–10972. [Google Scholar] [CrossRef]
- Miquel, E.; Farré, R. Effects and future trends of casein phosphopeptides on zinc bioavailability. Trends Food Sci. Technol. 2007, 18, 139–143. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef]
- Scholmerich, J.; Freudemann, A.; Kottgen, E.; Wietholtz, H.; Steiert, B.; Lohle, E.; Haussinger, D.; Gerok, W. Bioavailability of zinc from zinc-histidine complexes. I. Comparison with zinc sulfate in healthy men. Am. J. Clin. Nutr. 1987, 45, 1480–1486. [Google Scholar] [CrossRef]
- Gandia, P.; Bour, D.; Maurette, J.M.; Donazzolo, Y.; Duchene, P.; Bejot, M.; Houin, G. A bioavailability study comparing two oral formulations containing zinc (Zn bis-glycinate vs. Zn gluconate) after a single administration to twelve healthy female volunteers. Int. J. Vitam. Nutr. Res. 2007, 77, 243–248. [Google Scholar] [CrossRef]
- Hotz, C. The potential to improve zinc status through biofortification of staple food crops with zinc. Food Nutr. Bull. 2009, 30, S172–S178. [Google Scholar] [CrossRef]
- Praharaj, S.; Skalicky, M.; Maitra, S.; Bhadra, P.; Shankar, T.; Brestic, M.; Hejnak, V.; Vachova, P.; Hossain, A. Zinc Biofortification in Food Crops Could Alleviate the Zinc Malnutrition in Human Health. Molecules 2021, 26, 3509. [Google Scholar] [CrossRef]
- Mahboob, U.; Ceballos-Rasgado, M.; Moran, V.H.; Joy, E.J.M.; Ohly, H.; Zaman, M.; Lowe, N.M. Community Perceptions of Zinc Biofortified Flour during an Intervention Study in Pakistan. Nutrients 2022, 14, 817. [Google Scholar] [CrossRef]
- Sazawal, S.; Dhingra, U.; Dhingra, P.; Dutta, A.; Deb, S.; Kumar, J.; Devi, P.; Prakash, A. Efficacy of high zinc biofortified wheat in improvement of micronutrient status, and prevention of morbidity among preschool children and women—A double masked, randomized, controlled trial. Nutr. J. 2018, 17, 86. [Google Scholar] [CrossRef]
- Trijatmiko, K.R.; Duenas, C.; Tsakirpaloglou, N.; Torrizo, L.; Arines, F.M.; Adeva, C.; Balindong, J.; Oliva, N.; Sapasap, M.V.; Borrero, J.; et al. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 2016, 6, 19792. [Google Scholar] [CrossRef]
- Woods, B.J.; Gallego-Castillo, S.; Talsma, E.F.; Alvarez, D. The acceptance of zinc biofortified rice in Latin America: A consumer sensory study and grain quality characterization. PLoS ONE 2020, 15, e0242202. [Google Scholar] [CrossRef]
- Gallego-Castillo, S.; Taleon, V.; Talsma, E.F.; Rosales-Nolasco, A.; Palacios-Rojas, N. Effect of maize processing methods on the retention of minerals, phytic acid and amino acids when using high kernel-zinc maize. Curr. Res. Food Sci. 2021, 4, 279–286. [Google Scholar] [CrossRef]
- Mehta, S.; Finkelstein, J.L.; Venkatramanan, S.; Huey, S.L.; Udipi, S.A.; Ghugre, P.; Ruth, C.; Canfield, R.L.; Kurpad, A.V.; Potdar, R.D.; et al. Effect of iron and zinc-biofortified pearl millet consumption on growth and immune competence in children aged 12–18 months in India: Study protocol for a randomised controlled trial. BMJ Open 2017, 7, e017631. [Google Scholar] [CrossRef]
- Haider, M.U.; Hussain, M.; Farooq, M.; Ul-Allah, S.; Ansari, M.J.; Alwahibi, M.S.; Farooq, S. Zinc biofortification potential of diverse mungbean [Vigna radiata (L.) Wilczek] genotypes under field conditions. PLoS ONE 2021, 16, e0253085. [Google Scholar] [CrossRef] [PubMed]
- Speranca, M.A.; Mayorquin-Guevara, J.E.; da Cruz, M.C.P.; de Almeida Teixeira, G.H.; Pereira, F.M.V. Biofortification quality in bananas monitored by energy-dispersive X-ray fluorescence and chemometrics. Food Chem. 2021, 362, 130172. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Physiological limits to zinc biofortification of edible crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Abebe, Z.; Haki, G.D.; Baye, K. Simulated effects of home fortification of complementary foods with micronutrient powders on risk of inadequate and excessive intakes in West Gojjam, Ethiopia. Matern. Child Nutr. 2018, 14. [Google Scholar] [CrossRef]
- Hayman, T.; Hickey, P.; Amann-Zalcenstein, D.; Bennett, C.; Ataide, R.; Sthity, R.A.; Khandaker, A.M.; Islam, K.M.; Stracke, K.; Yassi, N.; et al. Zinc Supplementation with or without Additional Micronutrients Does Not Affect Peripheral Blood Gene Expression or Serum Cytokine Level in Bangladeshi Children. Nutrients 2021, 13, 3516. [Google Scholar] [CrossRef]
- Esamai, F.; Liechty, E.; Ikemeri, J.; Westcott, J.; Kemp, J.; Culbertson, D.; Miller, L.V.; Hambidge, K.M.; Krebs, N.F. Zinc absorption from micronutrient powder is low but is not affected by iron in Kenyan infants. Nutrients 2014, 6, 5636–5651. [Google Scholar] [CrossRef]
- Wuehler, S.; Lopez de Romana, D.; Haile, D.; McDonald, C.M.; Brown, K.H. Reconsidering the Tolerable Upper Levels of Zinc Intake among Infants and Young Children: A Systematic Review of the Available Evidence. Nutrients 2022, 14, 1938. [Google Scholar] [CrossRef]
- Morgan, E.J.; Heath, A.L.; Szymlek-Gay, E.A.; Gibson, R.S.; Gray, A.R.; Bailey, K.B.; Ferguson, E.L. Red meat and a fortified manufactured toddler milk drink increase dietary zinc intakes without affecting zinc status of New Zealand toddlers. J. Nutr. 2010, 140, 2221–2226. [Google Scholar] [CrossRef]
- Sanchez, J.; Villada, O.A.; Rojas, M.L.; Montoya, L.; Diaz, A.; Vargas, C.; Chica, J.; Herrera, A.A. Effect of zinc amino acid chelate and zinc sulfate in the incidence of respiratory infection and diarrhea among preschool children in child daycare centers. Biomedica 2014, 34, 79–91. [Google Scholar] [CrossRef]
- Bui, V.Q.; Marcinkevage, J.; Ramakrishnan, U.; Flores-Ayala, R.C.; Ramirez-Zea, M.; Villalpando, S.; Martorell, R.; DiGirolamo, A.M.; Stein, A.D. Associations among dietary zinc intakes and biomarkers of zinc status before and after a zinc supplementation program in Guatemalan schoolchildren. Food Nutr. Bull. 2013, 34, 143–150. [Google Scholar] [CrossRef]
- Sazawal, S.; Habib, A.; Dhingra, U.; Dutta, A.; Dhingra, P.; Sarkar, A.; Deb, S.; Alam, J.; Husna, A.; Black, R.E. Impact of micronutrient fortification of yoghurt on micronutrient status markers and growth—A randomized double blind controlled trial among school children in Bangladesh. BMC Public Health 2013, 13, 514. [Google Scholar] [CrossRef]
- Talsma, E.F.; Moretti, D.; Ly, S.C.; Dekkers, R.; van den Heuvel, E.G.; Fitri, A.; Boelsma, E.; Stomph, T.J.; Zeder, C.; Melse-Boonstra, A. Zinc Absorption from Milk Is Affected by Dilution but Not by Thermal Processing, and Milk Enhances Absorption of Zinc from High-Phytate Rice in Young Dutch Women. J. Nutr. 2017, 147, 1086–1093. [Google Scholar] [CrossRef]
- Wibowo, N.; Bardosono, S.; Irwinda, R. Effects of Bifidobacterium animalis lactis HN019 (DR10TM), inulin, and micronutrient fortified milk on faecal DR10TM, immune markers, and maternal micronutrients among Indonesian pregnant women. Asia Pac. J. Clin. Nutr. 2016, 25, S102–S110. [Google Scholar] [CrossRef]
- Mohan, J.; Ali, S.A.; Suvartan, R.; Kapila, S.; Sharma, R.; Tomar, S.K.; Behare, P.; Yadav, H. Bioavailability of Biotransformed Zinc Enriched Dahi in Wistar Rats. Int. J. Probiotics Prebiotics 2018, 13, 45–54. [Google Scholar]
- Shkembi, B.; Huppertz, T. Influence of Dairy Products on Bioavailability of Zinc from Other Food Products: A Review of Complementarity at a Meal Level. Nutrients 2021, 13, 4253. [Google Scholar] [CrossRef]
- Chadare, F.J.; Idohou, R.; Nago, E.; Affonfere, M.; Agossadou, J.; Fassinou, T.K.; Kenou, C.; Honfo, S.; Azokpota, P.; Linnemann, A.R.; et al. Conventional and food-to-food fortification: An appraisal of past practices and lessons learned. Food Sci. Nutr. 2019, 7, 2781–2795. [Google Scholar] [CrossRef]
- Kolapo, A.L.; Sanni, M.O. A Comparative Evaluation of the Macronutrient and Micronutrient Profiles of Soybean-Fortified Gari and Tapioca. Food Nutr. Bull. 2009, 30, 90–94. [Google Scholar] [CrossRef]
- Rosado, J.L.; Diaz, M.; Gonzalez, K.; Griffin, I.; Abrams, S.A.; Preciado, R. The addition of milk or yogurt to a plant-based diet increases zinc bioavailability but does not affect iron bioavailability in women. J. Nutr. 2005, 135, 465–468. [Google Scholar] [CrossRef]
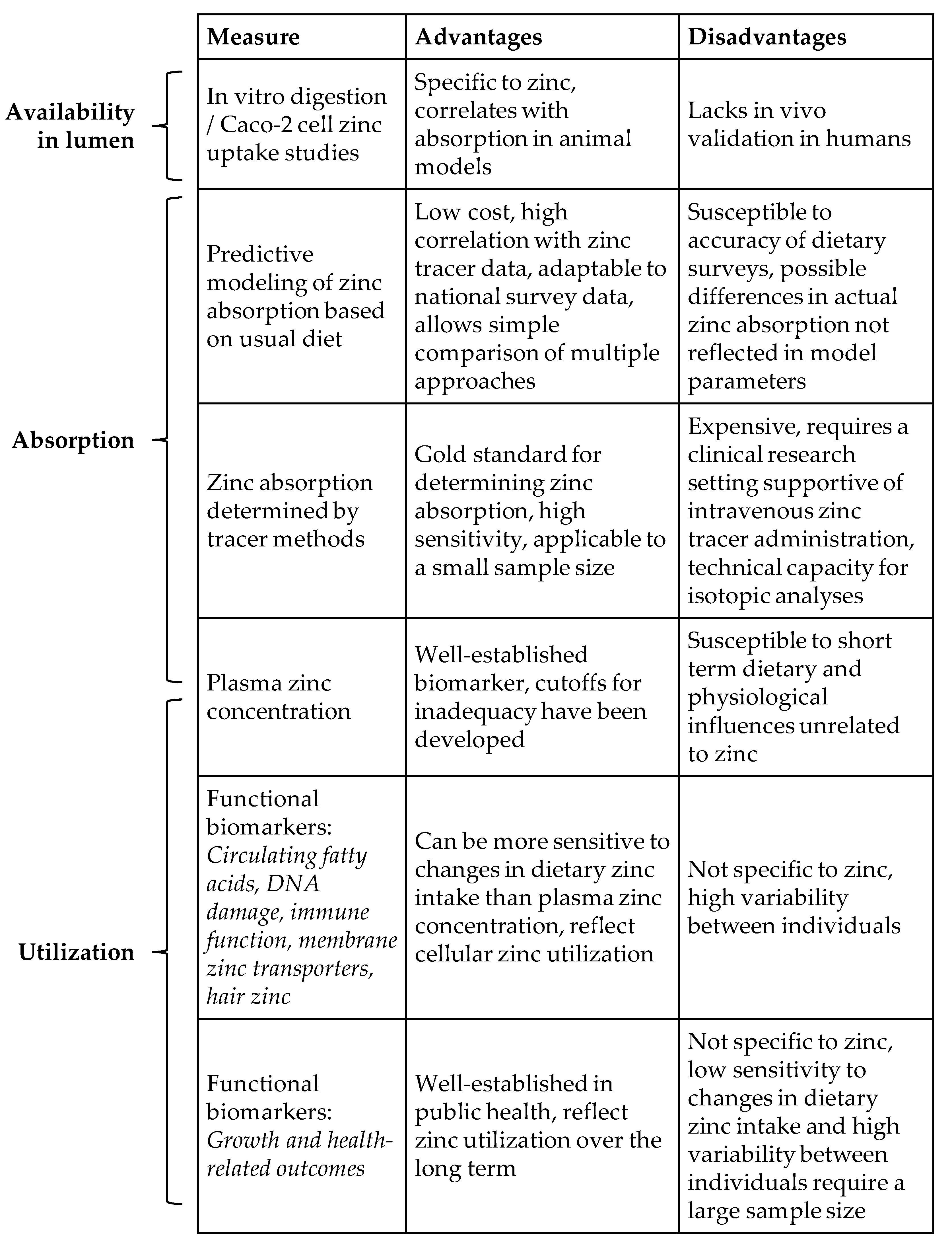
| Strategy | Advantages | Disadvantages |
|---|---|---|
| Post-harvest fortification of staples | Well-established for use in large-scale fortification of staples that are milled to flours, relatively simple to incorporate at milling | Requires capacity for centralized processing of staples, expensive to maintain coverage and sustained use within a target population, need to control zinc amount and maintain homogeneity, not practical for foods that are not milled into flours (e.g., rice, beans) |
| Biofortification | Applicable to large-scale fortification of staple crops, no need for special processing, no concern for excessive zinc, practical for crops that are not milled (e.g., rice, beans), low cost of sustained use after initial development | Time and expense of crop development, limitations to the amount of zinc that can be added |
| Fortification of manufactured food products | Well-established in fortification of population-specific products such as infant formulas or child nutrition biscuits | Production expense, challenges in coverage and sustained use within a target population, need to control zinc amount and maintain homogeneity |
| Home fortification packets | Readily produced, stored, and distributed, may improve coverage of lower income or otherwise hard to reach populations | Challenges in determining appropriate amount of zinc per packet, opportunity for overuse |
| Fortification of milk or milk products | High zinc bioavailability, may partially counter inhibitory effects of high phytate diets | Not applicable in populations that do not consume milk or milk products, need to control zinc amount and maintain homogeneity |
| Food-to-food fortification | Supports small scale implementation in low-resource settings lacking capacity for other modes of fortification | Phytate content of plant sources of zinc can be high, need to test acceptability of resulting flavor and other organoleptic properties |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, A.G.; King, J.C. Zinc Fortification: Current Trends and Strategies. Nutrients 2022, 14, 3895. https://doi.org/10.3390/nu14193895
Hall AG, King JC. Zinc Fortification: Current Trends and Strategies. Nutrients. 2022; 14(19):3895. https://doi.org/10.3390/nu14193895
Chicago/Turabian StyleHall, Andrew G., and Janet C. King. 2022. "Zinc Fortification: Current Trends and Strategies" Nutrients 14, no. 19: 3895. https://doi.org/10.3390/nu14193895
APA StyleHall, A. G., & King, J. C. (2022). Zinc Fortification: Current Trends and Strategies. Nutrients, 14(19), 3895. https://doi.org/10.3390/nu14193895





