Effect of Green Tea Extract Ingestion on Fat Oxidation during Exercise in the Menstrual Cycle: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Menstrual Cycle Monitoring and Phase Determination
2.3. Study Design
2.4. Preliminary Testing
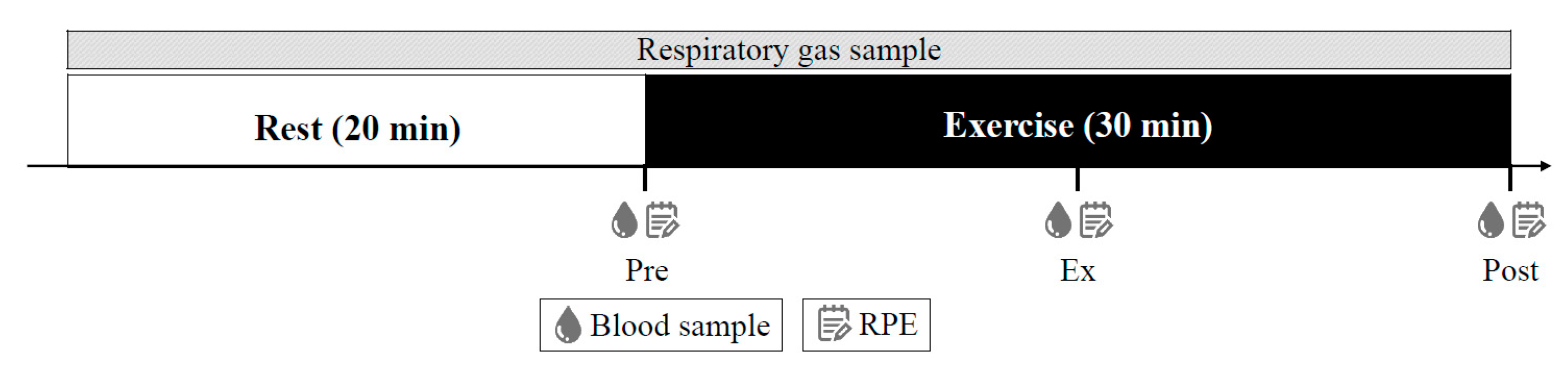
2.5. Experimental Protocols
2.6. Diet
2.7. Supplements
2.8. Blood Sample Analysis
2.9. Substrate Oxidation
2.10. Statistical Analysis
3. Results
3.1. Menstrual Cycle and Ovarian Hormones
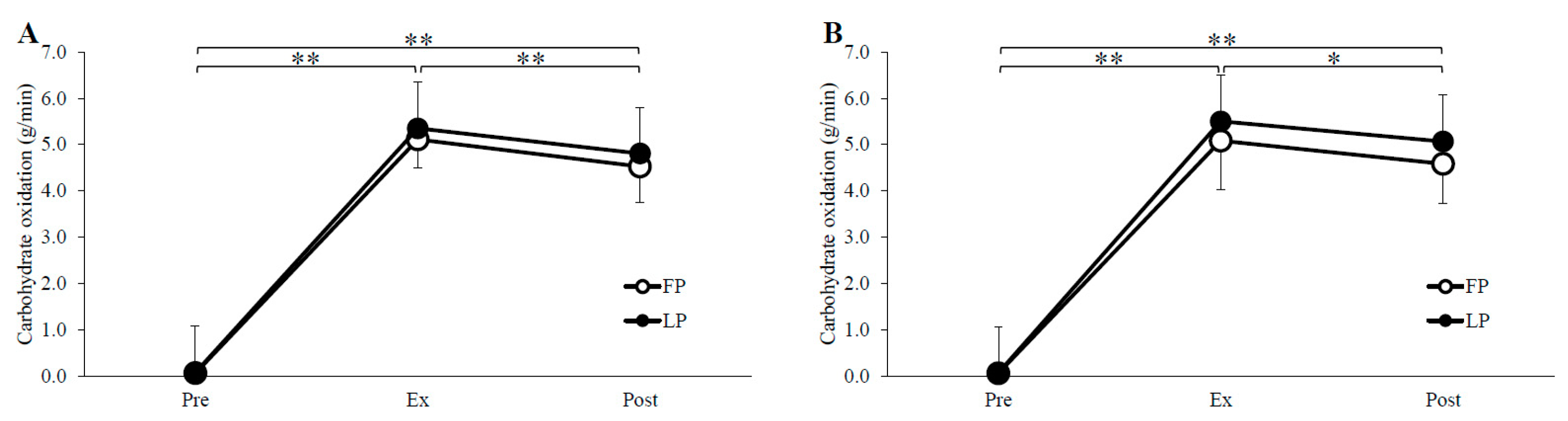
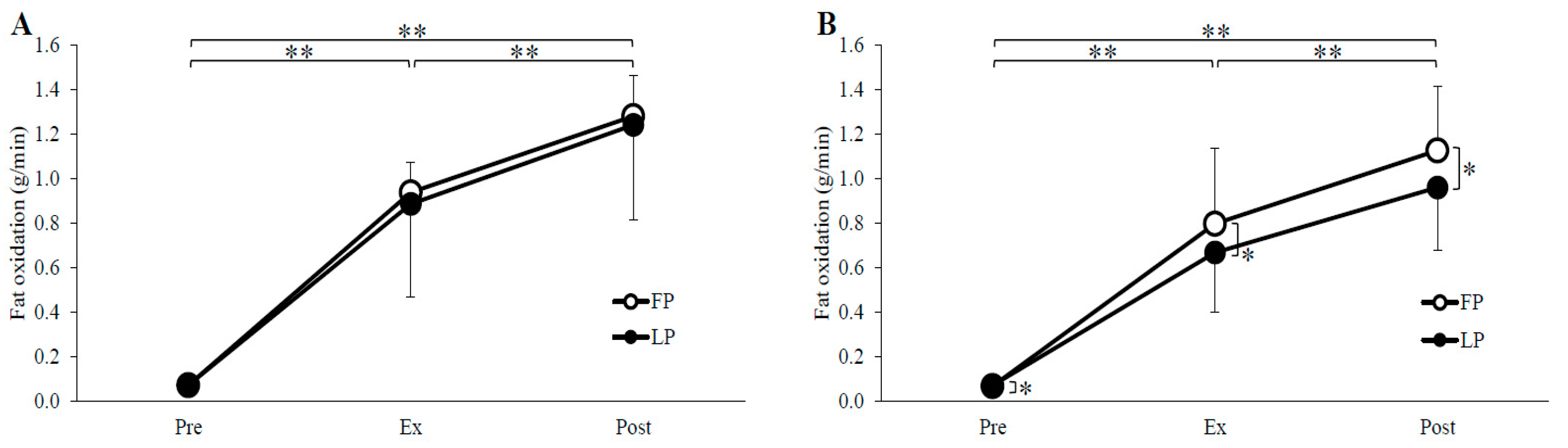
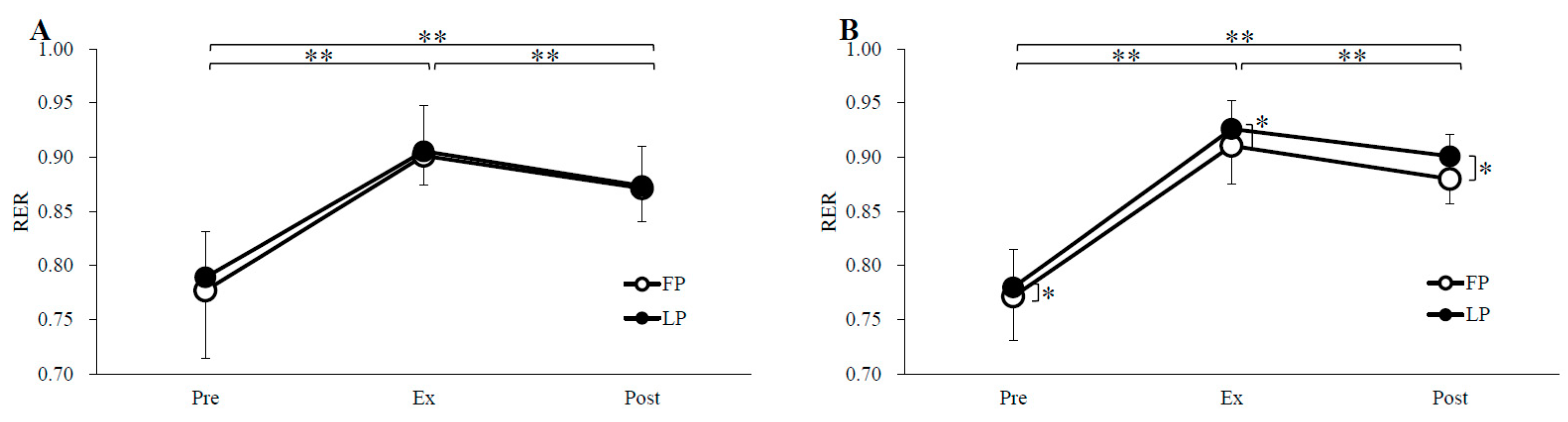
3.2. Substrate Oxidation
3.3. Metabolites and Hormones
3.4. RPE
3.5. Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, D. Obesity in Women: A Life Cycle of Medical Risk. Int. J. Obes. 2007, 31, S3–S7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, X.; Xie, Y.J.; Liu, Y.T.; Long, S.L.; Mo, Z.C. Polycystic Ovarian Syndrome: Correlation between Hyperandrogenism, Insulin Resistance and Obesity. Clin. Chim. Acta 2020, 502, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Sabolović Rudman, S. Pregnancy Outcome in Gestational Diabetes Compared to Body Mass Index. Acta Clin. Croat. 2019, 58, 37–41. [Google Scholar] [CrossRef]
- Butwick, A.J.; Bentley, J.; Leonard, S.A.; Carmichael, S.L.; El-Sayed, Y.Y.; Stephansson, O.; Guo, N. Prepregnancy Maternal Body Mass Index and Venous Thromboembolism: A Population-Based Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 581–588. [Google Scholar] [CrossRef]
- Ghimire, P.R.; Akombi-Inyang, B.J.; Tannous, C.; Agho, K.E. Association between Obesity and Miscarriage among Women of Reproductive Age in Nepal. PLoS ONE 2020, 15, e0236435. [Google Scholar]
- Rich-Edwards, J.W.; Goldman, M.B.; Willett, W.C.; Hunter, D.J.; Stampfer, M.J.; Colditz, G.A.; Manson, J.E. Adolescent Body Mass Index and Infertility Caused by Ovulatory Disorder. Am. J. Obstet. Gynecol. 1994, 171, 171–177. [Google Scholar] [CrossRef]
- Martorell, R.; Ad, S.; Dg, S. Early Nutrition and Later Adiposity. Symposium: Obesity in Developing Countries: Biological and Ecological Factors. J. Nutr. 2001, 131, 874S–880S. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, A.E. Optimizing Fat Oxidation through Exercise and Diet. Nutrition 2004, 20, 716–727. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Randell, R. Fat Burners: Nutrition Supplements That Increase Fat Metabolism. Obes. Rev. 2011, 12, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.P.; Sugita, M.; Fukuzawa, Y.; Okubo, T. Physiological Effects of Epigallocatechin-3-Gallate (EGCG) on Energy Expenditure for Prospective Fat Oxidation in Humans: A Systematic Review and Meta-Analysis. J. Nutr. Biochem. 2017, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kurogi, M.; Miyashita, M.; Emoto, Y.; Kubo, Y.; Saitoh, O. Green Tea Polyphenol Epigallocatechin Gallate Activates Trpa1 in an Intestinal Enteroendocrine Cell Line, STC-1. Chem. Senses 2012, 37, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Kurogi, M.; Kawai, Y.; Nagatomo, K.; Tateyama, M.; Kubo, Y.; Saitoh, O. Auto-Oxidation Products of Epigallocatechin Gallate Activate TRPA1 and TRPV1 in Sensory Neurons. Chem. Senses. 2015, 40, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222–234. [Google Scholar] [CrossRef]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green Tea Extract Ingestion, Fat Oxidation, and Glucose Tolerance in Healthy Humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Şahin, M.A.; Cook, M.D. Matcha Green Tea Drinks Enhance Fat Oxidation during Brisk Walking in Females. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.; Nomura, S.; Someya, Y.; Akimoto, S.; Tachiyashiki, K.; Imaizumi, K. Effect of Endurance Training Supplemented with Green Tea Extract on Substrate Metabolism during Exercise in Humans. Scand. J. Med. Sci. Sport 2011, 21, 598–605. [Google Scholar] [CrossRef]
- Ota, N.; Soga, S.; Shimotoyodome, A.; Haramizu, S.; Inaba, M.; Murase, T.; Tokimitsu, I. Effects of Combination of Regular Exercise and Tea Catechins Intake on Energy Expenditure in Humans. J. Health Sci. 2005, 51, 233–236. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. Oestrogen’s Regulation of Fat Metabolism during Exercise and Gender Specific Effects. Curr. Opin. Pharmacol. 2012, 12, 363–371. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Sharoff, C.; Chipkin, S.R.; Grow, D.; Ruby, B.C.; Braun, B. Regulation of Exercise Carbohydrate Metabolism by Estrogen and Progesterone in Women. Am. J. Physiol.-Endocrinol. Metab. 2002, 283, 1046–1055. [Google Scholar] [CrossRef]
- Hackney, A.C. Influence of Oestrogen on Muscle Glycogen Utilization during Exercise. Acta Physiol. Scand. 1999, 167, 273–274. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Hackney, A.C.; Sharp, R.L. The Menstrual Cycle and Exercise: Performance, Muscle Glycogen, and Substrate Responses. Int. J. Sports Med. 1989, 10, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Zderic, T.W.; Coggan, A.R.; Ruby, B.C. Glucose Kinetics and Substrate Oxidation during Exercise in the Follicular and Luteal Phases. J. Appl. Physiol. 2001, 90, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Janse DE Jonge, X.; Thompson, B.; Han, A. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Chiazze, L.; Brayer, F.T.; Macisco, J.J.; Parker, M.P.; Duffy, B.J. The Length and Variability of the Human Menstrual Cycle. JAMA J. Am. Med. Assoc. 1968, 203, 377–380. [Google Scholar] [CrossRef]
- Borg, G.A.V. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Smekal, G.; Von Duvillard, S.P.; Frigo, P.; Tegelhofer, T.; Pokan, R.; Hofmann, P.; Tschan, H.; Baron, R.; Wonisch, M.; Renezeder, K.; et al. Menstrual Cycle: No Effect on Exercise Cardiorespiratory Variables or Blood Lactate Concentration. Med. Sci. Sports Exerc. 2007, 39, 1098–1106. [Google Scholar] [CrossRef]
- Redman, L.M.; Scroop, G.C.; Norman, R.J. Impact of Menstrual Cycle Phase on the Exercise Status of Young, Sedentary Women. Eur J. Appl. Physiol. 2003, 90, 505–513. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best Practice Methods to Apply to Measurement of Resting Metabolic Rate in Adults: A Systematic Review. J. Am. Diet Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Narumi, K.; Sonoda, J.I.; Shiotani, K.; Shigeru, M.; Shibata, M.; Kawachi, A.; Tomishige, E.; Sato, K.; Motoya, T. Simultaneous Detection of Green Tea Catechins and Gallic Acid in Human Serum after Ingestion of Green Tea Tablets Using Ion-Pair High-Performance Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 945–946, 147–153. [Google Scholar] [CrossRef]
- Péronnet, F.; Massicotte, D. Table of Nonprotein Respiratory Quotient: An Update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar]
- Dombovy, M.L.; Bonekat, H.W.; Williams, T.J.; Staats, B.A. Exercise Performance and Ventilatory Response in the Menstrual Cycle. Med. Sci. Sports Exerc. 1987, 19, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Febbraio, M.A. Effects of Ovarian Hormones on Exercise Metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2001, 4, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Hamadeh, M.J.; Phillips, S.M.; Tarnopolsky, M.A. Menstrual Cycle Phase and Sex Influence Muscle Glycogen Utilization and Glucose Turnover during Moderate-Intensity Endurance Exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 291, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.H.; Casazza, G.A.; Horning, M.A.; Miller, B.F.; Brooks, G.A. Luteal and Follicular Glucose Fluxes during Rest and Exercise in 3-h Postabsorptive Women. J. Appl. Physiol. 2002, 93, 42–50. [Google Scholar] [CrossRef]
- Horton, T.J.; Miller, E.K.; Glueck, D.; Tench, K. No Effect of Menstrual Cycle Phase on Glucose Kinetics and Fuel Oxidation during Moderate-Intensity Exercise. Am. J. Physiol. Metab. 2002, 282, 752–762. [Google Scholar] [CrossRef]
- Jacobs, K.A.; Casazza, G.A.; Suh, S.H.; Horning, M.A.; Brooks, G.A. Fatty Acid Reesterification but Not Oxidation Is Increased by Oral Contraceptive Use in Women. J. Appl. Physiol. 2005, 98, 1720–1731. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sport Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef]
- Narasimhan, A.; Sampath, S.; Jayaraman, S.; Karundevi, B. Estradiol Favors Glucose Oxidation in Gastrocnemius Muscle through Modulation of Insulin Signaling Molecules in Adult Female Rats. Endocr. Res. 2013, 38, 251–262. [Google Scholar] [CrossRef]
- Sidossis, L.S.; Mittendorfer, B.; Chinkes, D.; Walser, E.; Wolfe, R.R. Effect of Hyperglycemia-Hyperinsulinemia on Whole Body and Regional Fatty Acid Metabolism. Am. J. Physiol.-Endocrinol. Metab. 1999, 276, 427–434. [Google Scholar] [CrossRef]
- Saddik, M.; Gamble, J.; Witters, L.A.; Lopaschuk, G.D. Acetyl-CoA Carboxylase Regulation of Fatty Acid Oxidation in the Heart. J. Biol Chem. 1993, 268, 25836–25845. [Google Scholar] [CrossRef]
- Landgren, B.M.; Unden, A.L.; Diczfalusy, E. Hormonal Profile of the Cycle in 68 Normally Menstruating Women. Acta Endocrinol. 1980, 94, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Puah, J.A.; Bailey, C.J. Effect of Ovarian Hormones on Glucose Metabolism in Mouse Soleus Muscle. Endocrinology 1985, 117, 1336–1340. [Google Scholar] [CrossRef]
- Kim, H.J.; Kalkhoff, R.K. Sex Steroid Influence on Triglyceride Metabolism. J. Clin. Investig. 1975, 56, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Febbraio, M.A. Effect of Ovarian Hormones on Mitochondrial Enzyme Activity in the Fat Oxidation Pathway of Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2001, 281, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Hatta, H.; Atomi, Y.; Shinohara, S.; Yamamoto, Y.; Yamada, S. The Effects of Ovarian Hormones on Glucose and Fatty Acid Oxidation during Exercise in Female Ovariectomized Rats. Horm. Metab. Res. 1988, 20, 609–611. [Google Scholar] [CrossRef]
- Lindheim, S.R.; Presser, S.C.; Ditkoff, E.C.; Vijod, M.A.; Stanczyk, F.Z.; Lobo, R.A. A Possible Bimodal Effect of Estrogen on Insulin Sensitivity in Postmenopausal Women and the Attenuating Effect of Added Progestin. Fertil. Steril. 1993, 60, 664–667. [Google Scholar] [CrossRef]
- Winder, W.W.; Arogyasami, J.; Elayan, I.M.; Cartmill, D. Time Course of Exercise-Induced Decline in Malonyl-CoA in Different Muscle Types. Am. J. Physiol.-Endocrinol. Metab. 1990, 259, E266–E271. [Google Scholar] [CrossRef]
- Kelley, D.E.; Simoneau, J.A. Impaired Free Fatty Acid Utilization by Skeletal Muscle in Non-Insulin- Dependent Diabetes Mellitus. J. Clin. Investig. 1994, 94, 2349–2356. [Google Scholar] [CrossRef]
- Kalkhoff, R.K. Metabolic Effects of Progesterone. Am. J. Obstet. Gynecol. 1982, 142, 735–738. [Google Scholar] [CrossRef]
- D’Eon, T.; Braun, B. The Roles of Estrogen and Progesterone in Regulating Carbohydrate and Fat Utilization at Rest and during Exercise. J. Womens Health Gend. Based Med. 2002, 11, 225–237. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The Effect of the Menstrual Cycle on Exercise Metabolism. Sport Med. 2010, 40, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, U.; Haller, J.; Decourt, J.P.; Girault, N.; Girault, J.; Richard-Caudron, A.S.; Pineau, B.; Weber, P. A Single Ascending Dose Study of Epigallocatechin Gallate in Healthy Volunteers. J. Int. Med. Res. 2003, 31, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Juan, C.C.; Ho, L.T.; Hsu, Y.P.; Hwang, L.S. Effect of Green Tea Supplementation on Insulin Sensitivity in Sprague-Dawley Rats. J. Agric. Food Chem. 2004, 52, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Achten, J. Fatmax: A New Concept to Optimize Fat Oxidation during Exercise? Eur. J. Sport Sci. 2001, 1, 1–5. [Google Scholar] [CrossRef]
| Trial | Phase | Pre | Ex | Post | |
|---|---|---|---|---|---|
| Estradiol (pg/mL) | GTE | FP | 32.0 ± 9.4 | 35.4 ± 9.0 | 37.6 ± 11.0 |
| LP | 119.1 ± 38.3 ** | 134.1 ± 39.4 **,†† | 147.9 ± 41.3 **,††,§§ | ||
| PLA | FP | 24.4 ± 12.8 | 30.0 ± 15.6 † | 31.0 ± 17.0 † | |
| LP | 176.3 ± 55.0 ** | 197.3 ± 56.9 **,† | 214.5 ± 51.1 **,†† | ||
| Progesterone (ng/mL) | GTE | FP | 0.4 ± 0.4 | 0.4 ± 0.4 | 0.4 ± 0.4 |
| LP | 8.3 ± 3.8 ** | 9.9 ± 4.8 **,† | 10.4 ± 5.1 **,†† | ||
| PLA | FP | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 §§ | |
| LP | 12.6 ± 3.7 ** | 15.7 ± 4.4 **,†† | 16.3 ± 4.8 **,†† | ||
| E/P ratio | GTE | LP | 14.4 | 13.5 | 14.2 |
| PLA | LP | 14.0 | 12.6 | 13.2 |
| Trial | Phase | Pre | Ex | Post | |
|---|---|---|---|---|---|
| FFA (μEq/L) | GTE | FP | 0.56 ± 0.14 | 0.56 ± 0.17 †† | 0.86 ± 0.19 ††,§§ |
| LP | 0.56 ± 0.16 | 0.56 ± 0.27 †† | 0.98 ± 0.52 ††,§§ | ||
| PLA | FP | 0.54 ± 0.19 | 0.46 ± 0.19 †† | 0.88 ± 0.37 ††,§§ | |
| LP | 0.51 ± 0.15 | 0.52 ± 0.33 †† | 0.71 ± 0.19 ††,§§ | ||
| Noradrenalin (ng/mL) | GTE | FP | 0.11 ± 0.03 | 0.55 ± 0.15 †† | 0.61 ± 0.18 †† |
| LP | 0.14 ± 0.04 | 0.63 ± 0.21 †† | 0.57 ± 0.19 †† | ||
| PLA | FP | 0.13 ± 0.05 | 0.60 ± 0.27 †† | 0.59 ± 0.32 †† | |
| LP | 0.16 ± 0.07 | 0.65 ± 0.23 †† | 0.63 ± 0.33 †† | ||
| Glucose (mg/dL) | GTE | FP | 84.9 ± 4.5 | 83.2 ± 5.8 | 86.5 ± 6.5 † |
| LP | 82.4 ± 8.4 | 86.1 ± 6.9 | 89.6 ± 7.8 § | ||
| PLA | FP | 82.2 ± 4.3 | 82.9 ± 6.3 | 88.6 ± 6.2 | |
| LP | 82.0 ± 5.8 | 82.4 ± 5.2 | 88.8 ± 6.7 | ||
| Lactate (mmol/L) | GTE | FP | 1.3 ± 0.3 | 3.3 ± 0.9 †† | 3.1 ± 1.1 †† |
| LP | 1.4 ± 0.2 | 3.6 ± 1.2 †† | 3.1 ± 1.0 †† | ||
| PLA | FP | 1.4 ± 0.1 | 3.6 ± 1.2 †† | 3.4 ± 1.2 †† | |
| LP | 1.3 ± 0.3 | 3.7 ± 0.7 ††,§§ | 3.1 ± 0.7 ††,§§ |
| Trial | Phase | Pre | Ex | Post | |
|---|---|---|---|---|---|
| RPE | GTE | FP | 6.6 ± 0.5 | 11.8 ± 1.9 †† | 13.7 ± 2.1 ††,§ |
| LP | 7.2 ± 1.0 | 12.2 ± 2.0 †† | 14.0 ± 1.9 ††,§ | ||
| PLA | FP | 6.8 ± 1.2 | 12.5 ± 1.7 †† | 13.6 ± 1.3 ††,§ | |
| LP | 7.1 ± 1.1 | 12.8 ± 1.1 †† | 14.8 ± 2.0 ††,§ |
| Trial | Phase | Energy (kcal) | Protein (g) | Fat (g) | Carbohydrate (g) |
|---|---|---|---|---|---|
| GTE | FP | 1281.5 ± 461.7 | 41.4 ± 15.5 | 50.9 ± 18.6 | 157.2 ± 59.1 |
| LP | 1518.8 ± 592.3 | 50.8 ± 16.7 | 61.4 ± 24.5 | 188.9 ± 84.4 | |
| PLA | FP | 1671.6 ± 633.0 | 57.1 ± 19.0 | 64.1 ± 41.0 | 217.1 ± 87.1 |
| LP | 1642.8 ± 446.8 | 63.6 ± 25.8 | 75.1 ± 36.0 | 181.5 ± 67.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, A.; Matsuda, T.; Gam, H.; Kanno, M.; Yamada, M.; Ikegami, N.; Funaki, A.; Ogata, H.; Kamemoto, K.; Ichihara, T.; et al. Effect of Green Tea Extract Ingestion on Fat Oxidation during Exercise in the Menstrual Cycle: A Pilot Study. Nutrients 2022, 14, 3896. https://doi.org/10.3390/nu14193896
Ishikawa A, Matsuda T, Gam H, Kanno M, Yamada M, Ikegami N, Funaki A, Ogata H, Kamemoto K, Ichihara T, et al. Effect of Green Tea Extract Ingestion on Fat Oxidation during Exercise in the Menstrual Cycle: A Pilot Study. Nutrients. 2022; 14(19):3896. https://doi.org/10.3390/nu14193896
Chicago/Turabian StyleIshikawa, Akira, Tomoka Matsuda, Hyunjun Gam, Moe Kanno, Mizuki Yamada, Nodoka Ikegami, Akiko Funaki, Hazuki Ogata, Kayoko Kamemoto, Takashi Ichihara, and et al. 2022. "Effect of Green Tea Extract Ingestion on Fat Oxidation during Exercise in the Menstrual Cycle: A Pilot Study" Nutrients 14, no. 19: 3896. https://doi.org/10.3390/nu14193896
APA StyleIshikawa, A., Matsuda, T., Gam, H., Kanno, M., Yamada, M., Ikegami, N., Funaki, A., Ogata, H., Kamemoto, K., Ichihara, T., & Sakamaki-Sunaga, M. (2022). Effect of Green Tea Extract Ingestion on Fat Oxidation during Exercise in the Menstrual Cycle: A Pilot Study. Nutrients, 14(19), 3896. https://doi.org/10.3390/nu14193896





