Thyroidal and Extrathyroidal Requirements for Iodine and Selenium: A Combined Evolutionary and (Patho)Physiological Approach
Abstract
1. Introduction
2. Physical and (Bio)Chemical Properties of Iodine and Selenium
3. World Distribution and Food Chains
4. Evolutionary Background
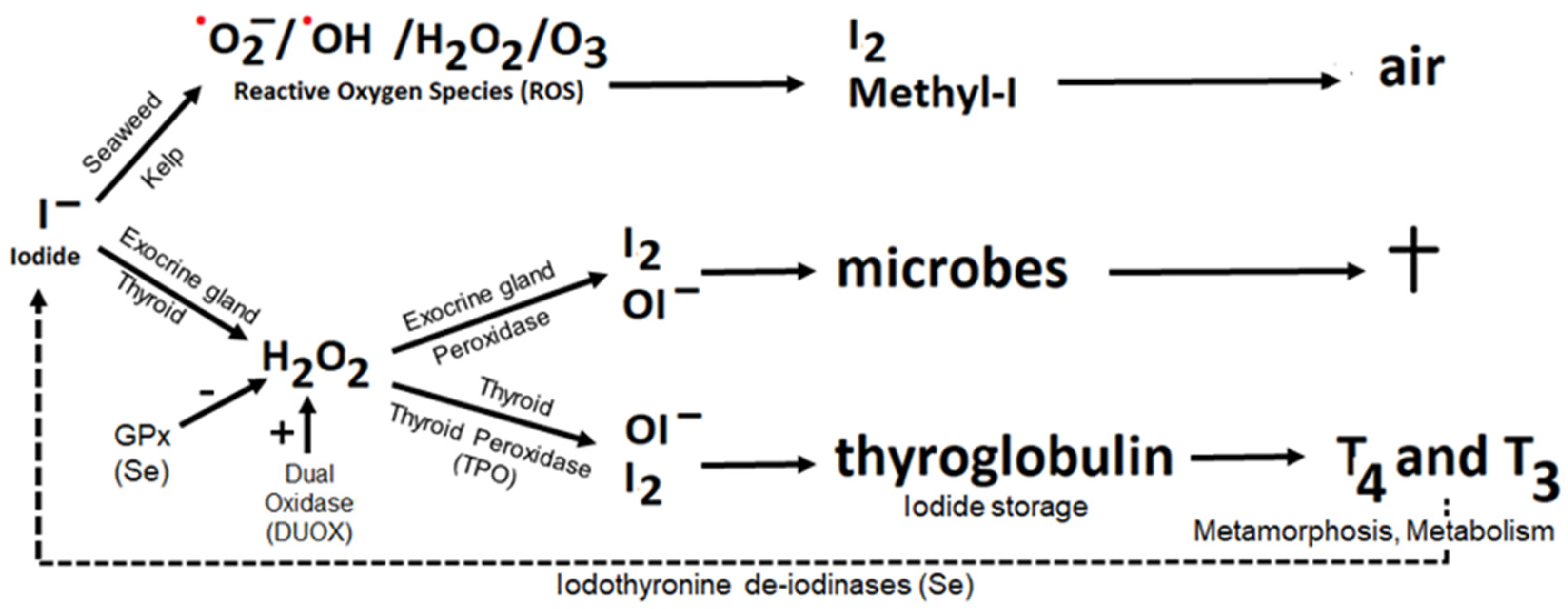
4.1. Iodide as Antioxidant
4.2. Iodine as Oxidant, the Peroxidase Partner System
4.3. Evolution Explains the Present
5. Substantiation of Current Recommendations
6. Considerations Pertaining to Iodine and Selenium Dietary Reference Intakes
6.1. Iodine and Selenium Regulation
6.2. Our Diet Has Changed
6.3. Ancient Diet and Brain Selective Nutrients
6.4. Iodine Intake from Seafood Is Unconstrained, but Selenium Intake Is Constrained
6.5. Iodine and Selenium Interact in Thyroid Function
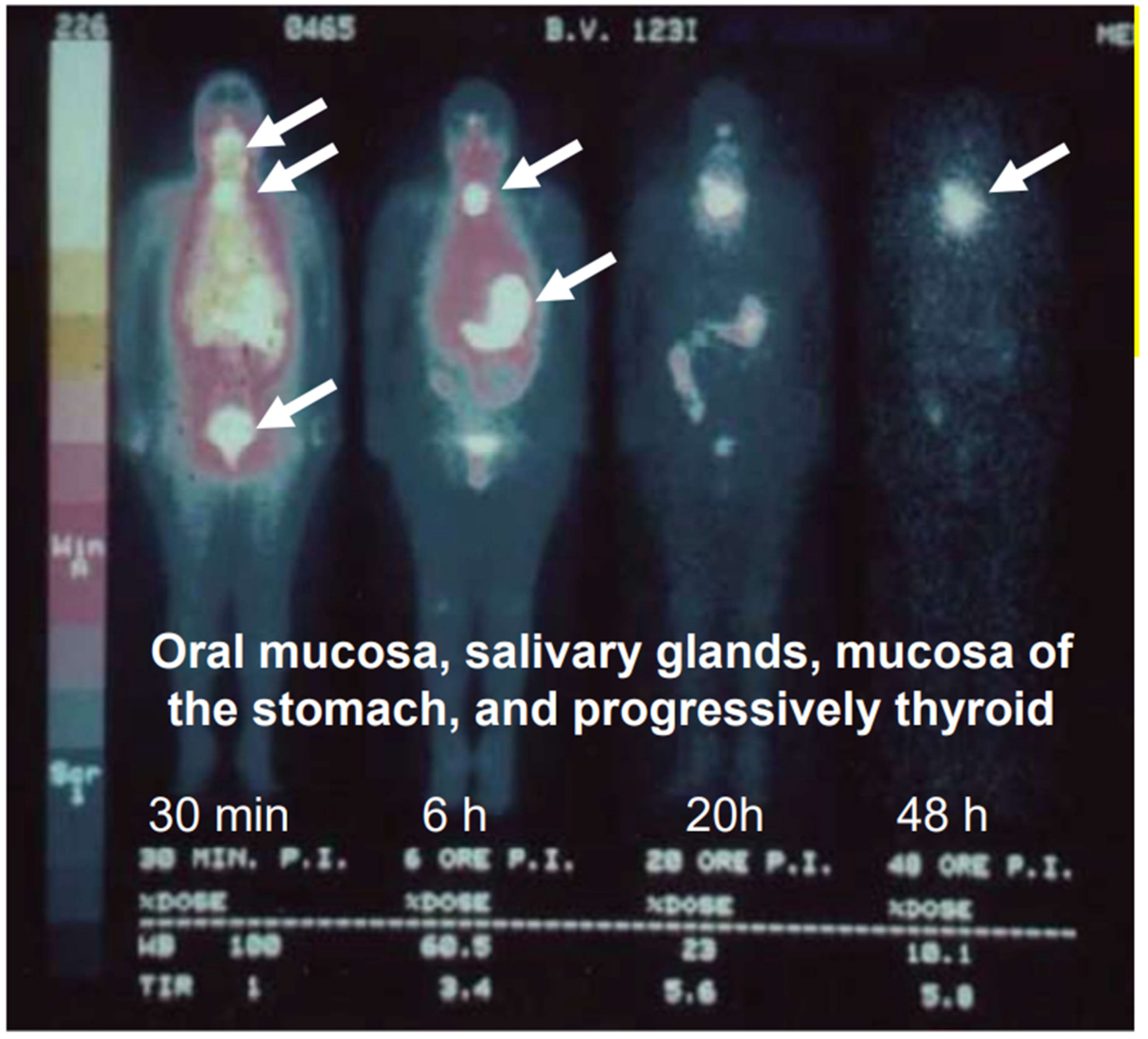
6.6. Iodine and Selenium Interact in Exocrine Glands
7. Towards Optimal Intakes and Upper Limits
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Ding, W. Isotopes of iodine in thyroid and urine: Source, application, level, and determination. In Comprehensive Handbook of Iodine; Preedy, V.R., Burrow, G.N., Watson, R.R., Eds.; Academic Press: London, UK, 2009; pp. 437–448. ISBN 978-0-12-374135-6. [Google Scholar]
- Oh, J.-R.; Ahn, B.-C. False-positive uptake on radioiodine whole-body scintigraphy: Physiologic and pathologic variants unrelated to thyroid cancer. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 362–385. [Google Scholar] [PubMed]
- Venturi, S. Evolutionary Significance of Iodine. Curr. Chem. Biol. 2011, 5, 155–162. [Google Scholar] [CrossRef]
- De la Vieja, A.; Santisteban, P. Role of iodide metabolism in physiology and cancer. Endocrin. Relat. Cancer 2018, 25, R225–R245. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, T. Iodine and cancer a summary of the evidence to date. Nat. Med. J. 2014, 6, 1–14. [Google Scholar]
- Aceves, C.; Anguiano, B.; Delgado, G. The extrathyronine actions of iodine as antioxidant, apoptotic, and differentiation factor in various tissues. Thyroid 2013, 23, 938–946. [Google Scholar] [CrossRef]
- Miller, D.W. Extrathyroidal benefits of iodine. J. Am. Physicians Surg. 2006, 11, 106–110. [Google Scholar]
- Venturi, S.; Donati, F.M.; Venturi, A.; Venturi, M. Environmental iodine deficiency: A challenge to the evolution of terrestrial life? Thyroid 2000, 10, 727–729. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology; Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., Smedley, P., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. ISBN 978-94-007-4374-8. [Google Scholar]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and its supplementation in cardiovascular disease—What do we know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a bioactive micronutrient in the human diet and its cancer chemopreventive activity. Nutrients 2021, 13, 1049. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. The Role and Mechanism of Essential Selenoproteins for Homeostasis. Antioxidants 2022, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochem. 2022, 87, S168–S177. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef]
- Talbert, L.E.; Julian, R.R. Methionine and Selenomethionine as Energy Transfer Acceptors for. J. Am. Soc. Mass Spectrom. 2019, 30, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Goldsztejn, G.; Mundlapati, V.R.; Brenner, V.; Gloaguen, E.; Mons, M. Selenium in Proteins: Conformational Changes Induced by Se Substitution on Methionine, as Studied in Isolated Model Peptides by Optical Spectroscopy and Quantum Chemistry. Molecules 2022, 27, 3163. [Google Scholar] [CrossRef] [PubMed]
- Nasim, M.J.; Zuraik, M.M.; Abdin, A.Y.; Ney, Y.; Jacob, C. Selenomethionine: A pink trojan redox horse with implications in aging and various age-related diseases. Antioxidants 2021, 10, 882. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Venturi, S.; Begin, M.E. Thyroid hormone, iodine, and human brain evolution. In Human Brain Evolution: The Influence of Freshwater and Marine Food Resources; Cunnane, S.C., Stewart, K.M., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 105–124. ISBN 978-0-470-45268-4. [Google Scholar]
- Kendrick, M.A. Halogens in Seawater, Marine Sediments and the Altered Oceanic Lithosphere. In The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes; Harlov, D.E., Aranovich, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 591–648. ISBN 978-3-319-61667-4. [Google Scholar]
- Nancharaiah, Y.V.; Lens, P.N.L. Ecology and Biotechnology of Selenium-Respiring Bacteria. Microbiol. Mol. Biol. Rev. 2015, 79, 61–80. [Google Scholar] [CrossRef]
- Floor, G.H.; Román-Ross, G. Selenium in volcanic environments: A review. Appl. Geochem. 2012, 27, 517–531. [Google Scholar] [CrossRef]
- Fuge, R.; Johnson, C.C. Iodine and human health, the role of environmental geochemistry and diet, a review. Appl. Geochem. 2015, 63, 282–302. [Google Scholar] [CrossRef]
- Šeda, M.; Švehla, J.; Trávníček, J.; Kroupová, V.; Konečný, R.; Fiala, K.; Svozilová, M.; Krhovjaková, J. The effect of volcanic activity of the Eyjafjallajökul volcano on iodine concentration in precipitation in the Czech Republic. Geochemistry 2012, 72, 279–281. [Google Scholar] [CrossRef]
- Fuge, R. Anthropogenic Sources. In Essentials of Medical Geology; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 59–74. ISBN 978-94-007-4374-8. [Google Scholar]
- Nielsen, F.H. Evolutionary events culminating in specific minerals becoming essential for life. Eur. J. Nutr. 2000, 39, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Lyons, G. Biofortification of cereals with foliar selenium and iodine could reduce hypothyroidism. Front. Plant Sci. 2018, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Fuge, R. Soils and Iodine Deficiency. In Essentials of medical geology; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 417–432. ISBN 978-94-007-4374-8. [Google Scholar]
- Eastman, C.J.; Zimmermann, M.B. The Iodine Deficiency Disorders. In Endotext. Comprehensice Free Online Endocrinology Book; Feingold, K.R., Ed.; The National Center for Biotechnology: Bethesda, MD, USA, 2018. [Google Scholar]
- Chung, S.; Chan, A.; Xiao, Y.; Lin, V.; Ho, Y.Y. Iodine content in commonly consumed food in Hong Kong and its changes due to cooking. Food Addit. Contam. Part B Surveill. 2013, 6, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Van der Reijden, O.L.; Zimmermann, M.B.; Galetti, V. Iodine in dairy milk: Sources, concentrations and importance to human health. Best Pr. Res. Clin. Endocrinol. Metab. 2017, 31, 385–395. [Google Scholar] [CrossRef]
- Van de Kamp, M.E.; Saridakis, I.; Verkaik-Kloosterman, J. Iodine content of semi-skimmed milk available in the Netherlands depending on farming (organic versus conventional) and heat treatment (pasteurized versus UHT) and implications for the consumer. J. Trace Elem. Med. Biol. 2019, 56, 178–183. [Google Scholar] [CrossRef]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Harthill, M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Sun, G.X.; Meharg, A.A.; Li, G.; Chen, Z.; Yang, L.; Chen, S.C.; Zhu, Y.G. Distribution of soil selenium in China is potentially controlled by deposition and volatilization? Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Fraile, J.G. Get Enough Selenium to Prevent a Viral Catastrophe!! Available online: https://medium.com/@Nutrisphere/get-enough-selenium-to-prevent-a-viral-catastrophe-be4c03a36d6d (accessed on 3 August 2022).
- Nordic Council of Ministers. Nordic Nutrition Recommendations, 5th ed.; Molander, E., Virtanen, S., Thorgeirsdottir, H., Aarum, A.K.O., Mattisson, I., Eds.; Narayana Press: Copenhagen, Denmark, 2012; ISBN 978-92-893-2670-4. [Google Scholar]
- Silva Junior, E.C.; Wadt, L.H.O.; Silva, K.E.; Lima, R.M.B.; Batista, K.D.; Guedes, M.C.; Carvalho, G.S.; Carvalho, T.S.; Reis, A.R.; Lopes, G.; et al. Natural variation of selenium in Brazil nuts and soils from the Amazon region. Chemosphere 2017, 188, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Varo, P.; Alfthan, G.; Ekholm, P.; Aro, A.; Koivistoinen, P. Selenium intake and serum selenium in Finland: Effects of soil fertilization with selenium. Am. J. Clin. Nutr. 1988, 48, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Pang, K.; Fu, T.; Phillips, C.J.C.; Gao, T. Nano-selenium Supplementation Increases Selenoprotein (Sel) Gene Expression Profiles and Milk Selenium Concentration in Lactating Dairy Cows. Biol. Trace Elem. Res. 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Tinggi, U.; Patterson, C.; Reilly, C. Selenium levels in cow’s milk from different regions of Australia. Int. J. Food Sci. Nutr. 2001, 52, 43–51. [Google Scholar] [CrossRef]
- Van Hulzen, K.J.E.; Sprong, R.C.; van der Meer, R.; van Arendonk, J.A.M. Genetic and nongenetic variation in concentration of selenium, calcium, potassium, zinc, magnesium, and phosphorus in milk of Dutch Holstein-Friesian cows. J. Dairy Sci. 2009, 92, 5754–5759. [Google Scholar] [CrossRef] [PubMed]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedüs, L. Selenium in thyroid disorders–essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 1–67. [CrossRef]
- Schomburg, L. Selenium deficiency due to diet, pregnancy, severe illness, or covid-19—A preventable trigger for autoimmune disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (2000); Food and Nutrition board, Ed.; National Academies Press (US): Washington, DC, USA, 2000; ISBN 978-0-309-06949-6. [Google Scholar]
- Zachara, B.A.; Pawluk, H.; Bloch-Boguslawska, E.; Sliwka, K.M.; Korenkiewicz, J.; Skok, Z.; Ryć, K. Tissue level, distribution, and total body selenium content in healthy and diseased humans in Poland. Arch. Environ. Health 2001, 56, 461–466. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; McFiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954–6958. [Google Scholar] [CrossRef]
- Teas, J.; Pino, S.; Critchley, A.; Braverman, L.E. Variability of iodine content in common commercially available edible seaweeds. Thyroid 2004, 14, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Zava, T.T.; Zava, D.T. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Harbige, L.S.; Crawford, M.A. The importance of energy and nutrient supply in human brain evolution. Nutr. Health 1993, 9, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.; Stewart, K. (Eds.) Human Brain Evolution: The Influence of Freshwater and Marine Food Resources, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; ISBN 978-0-470-45268-4. [Google Scholar]
- Broadhurst, C.L.; Cunnane, S.C.; Crawford, M.A. Rift Valley lake fish and shellfish provided brain-specific nutrition for early Homo. Br. J. Nutr. 1998, 79, 3–21. [Google Scholar] [CrossRef]
- Kyriacou, K.; Blackhurst, D.M.; Parkington, J.E.; Marais, A.D. Marine and terrestrial foods as a source of brain-selective nutrients for early modern humans in the southwestern Cape, South Africa. J. Hum. Evol. 2016, 97, 86–96. [Google Scholar] [CrossRef]
- Cunnane, S.C. (Ed.) . Survival of the Fattest: The Key to Human Brain Evolution; World Scientific Publishing: Singapore, 2005; ISBN 981-256-191-9. [Google Scholar]
- Muskiet, F.A.J.; Kuipers, R.S. Lessons from Shore based Hunter-Gatherer Diets Iin East Africa. In Human Brain Evolution: The Influence of Freshwater and Marine Food Resources; Cunnane, S.C., Stewart, K., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 74–104. ISBN 978-0-470-45268-4. [Google Scholar]
- Cunnane, S.C.; Crawford, M.A. Survival of the fattest: Fat babies were the key to evolution of the large human brain. Comp Biochem. Physiol. A Mol. Integr. Physiol. 2003, 136, 17–26. [Google Scholar] [CrossRef]
- Zimmermann, M.; Adou, P.; Torresani, T.; Zeder, C.; Hurrell, R. Iron supplementation in goitrous, iron-deficient children improves their response to oral iodized oil. Eur. J. Endocrinol. 2000, 142, 217–223. [Google Scholar] [CrossRef][Green Version]
- Zimmermann, M.; Adou, P.; Torresani, T.; Zeder, C.; Hurrell, R. Persistence of goiter despite oral iodine supplementation in goitrous children with iron deficiency anemia in Cote d’Ivoire. Am. J. Clin. Nutr. 2000, 71, 88–93. [Google Scholar] [CrossRef]
- Hess, S.Y.; Zimmermann, M.B.; Adou, P.; Torresani, T.; Hurrell, R.F. Treatment of iron deficiency in goitrous children improves the efficacy of iodized salt in Côte d’Ivoire. Am. J. Clin. Nutr. 2002, 75, 743–748. [Google Scholar] [CrossRef][Green Version]
- Silva Morais, J.B.; Soares Severo, J.; Rocha Dos Santos, L.; Rodrigues de Sousa Melo, S.; de Oliveira Santos, R.; Soares de Oliveira, A.R.; Clímaco Cruz, K.J.; do Nascimento Marreiro, D. Role of Magnesium in Oxidative Stress in Individuals with Obesity. Biol. Trace Elem. Res. 2017, 176, 20–26. [Google Scholar] [CrossRef]
- Saranac, L.; Zivanovic, S.; Bjelakovic, B.; Stamenkovic, H.; Novak, M.; Kamenov, B. Why is the thyroid so prone to autoimmune disease? Horm. Res. Paediatr. 2011, 75, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Teng, X.; Zheng, H.; Shan, Z.; Li, J.; Jin, T.; Xiong, C.; Zhang, H.; Fan, C.; Teng, W. Iron deficiency without anemia causes maternal hypothyroxinemia in pregnant rats. Nutr. Res. 2014, 34, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Khatiwada, S.; Gelal, B.; Baral, N.; Lamsal, M. Association between iron status and thyroid function in Nepalese children. Thyroid Res. 2016, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific opinion on the substantiation of a health claim related to vitamin C and increasing non-haem iron absorption pursuant to Article 14 regulation (EC) no 1924/2006. EFSA J. 2014, 12, 3514.
- Cavalieri, R.R. Iodine metabolism and thyroid physiology: Current concepts. Thyroid 1997, 7, 177–181. [Google Scholar] [CrossRef]
- Food Standards. Iodine in Food and Iodine Requirements. Available online: https://www.foodstandards.gov.au/consumer/nutrition/iodinefood/Pages/default.aspx (accessed on 4 August 2022).
- Wright, A.C.; Fan, Y.; Baker, G.L. Nutritional Value and Food Safety of Bivalve Molluscan Shellfish. J. Shellfish Res. 2018, 37, 695–708. [Google Scholar] [CrossRef]
- Suzuki, H.; Higuchi, T.; Sawa, K.; Ohtaki, S.; Horiuchi, Y. Endemic coast goitre in Hokkaido, Japan. Eur. J. Endocrinol. 1965, 50, 161–176. [Google Scholar] [CrossRef]
- Fuse, Y.; Ito, Y.; Shishiba, Y.; Irie, M. Current Iodine Status in Japan: A Cross-sectional Nationwide Survey of Schoolchildren, 2014-2019. J. Clin. Endocrinol. Metab. 2022, 107, e2065–e2079. [Google Scholar] [CrossRef]
- Zhou, B.F.; Stamler, J.; Dennis, B.; Moag-Stahlberg, A.; Okuda, N.; Robertson, C.; Zhao, L.; Chan, Q.; Elliott, P. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: The INTERMAP Study. J. Hum. Hypertens. 2003, 17, 623–630. [Google Scholar] [CrossRef]
- Sakurai, H.; Tsuchya, K. A tentative recommendation for the maximum daily intake of selenium. Environ. Physiol. Biochem. 1975, 5, 107–118. [Google Scholar]
- Oster, O.; Schmiedel, G.; Prellwitz, W. The organ distribution of selenium in German adults. Biol. Trace Elem. Res. 1988, 15, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Bjorn, L.O.; Widell, S.; Wang, T. Evolution of UV-B regulation and protection in plants. Adv. Sp. Res. 2002, 30, 1557–1562. [Google Scholar] [CrossRef]
- Gonzales, J.; Tymon, T.; Küpper, F.C.; Edwards, M.S.; Carrano, C.J. Correction: The potential role of kelp forests on iodine speciation in coastal seawater. PLoS ONE 2017, 12, e0189559. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Venturi, S.; Venturi, M. Iodine in evolution of salivary glands and in oral health. Nutr. Health 2009, 20, 119–134. [Google Scholar] [CrossRef]
- Venturi, S.; Donati, F.M.; Venturi, M.; Venturi, A.; Grossi, L.; Guidi, A. Role of iodine in evolution and carcinogenesis of thyroid, breast and stomach. Adv. Clin. Path. 2000, 4, 11–17. [Google Scholar]
- Dohán, O.; De La Vieja, A.; Paroder, V.; Riedel, C.; Artani, M.; Reed, M.; Ginter, C.S.; Carrasco, N. The sodium/iodide symporter (NIS): Characterization, regulation, and medical significance. Endocr. Rev. 2003, 24, 48–77. [Google Scholar] [CrossRef]
- Ravera, S.; Reyna-Neyra1, A.; Ferrandino, G.; Mario Amzel, L.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef]
- Patel, U.; Gingerich, A.; Widman, L.; Sarr, D.; Tripp, R.A.; Rada, B. Susceptibility of influenza viruses to hypothiocyanite and hypoiodite produced by lactoperoxidase in a cell-free system. PLoS ONE 2018, 13, e0199167. [Google Scholar] [CrossRef]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The significance of lactoperoxidase system in oral health: Application and efficacy in oral hygiene products. Int. J. Mol. Sci. 2019, 20, 1443. [Google Scholar] [CrossRef]
- Szanto, I.; Pusztaszeri, M.; Mavromati, M. H2O2 metabolism in normal thyroid cells and in thyroid tumorigenesis: Focus on NADPH oxidases. Antioxidants 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Furtmüller, P.G.; Jantschko, W.; Regelsberger, G.; Jakopitsch, C.; Arnhold, J.; Obinger, C. Reaction of lactoperoxidase compound I with halides and thiocyanate. Biochemistry 2002, 41, 11895–11900. [Google Scholar] [CrossRef] [PubMed]
- Bafort, F.; Parisi, O.; Perraudin, J.P.; Jijakli, M.H. Mode of action of lactoperoxidase as related to its antimicrobial activity: A review. Enzyme Res. 2014, 2014, 517164. [Google Scholar] [CrossRef]
- Bosch, E.H.; van Doorne, H.; de Vries, S. The lactoperoxidase system: The influence of iodide and the chemical and antimicrobial stability over the period of about 18 months. J. Appl. Microbiol. 2000, 89, 215–224. [Google Scholar] [CrossRef]
- Derscheid, R.J.; Van Geelen, A.; Berkebile, A.R.; Gallup, J.M.; Hostetter, S.J.; Banfi, B.; McCray, P.B.; Ackermann, M.R. Increased concentration of iodide in airway secretions is associated with reduced respiratory syncytial virus disease severity. Am. J. Respir. Cell Mol. Biol. 2014, 50, 389–397. [Google Scholar] [CrossRef]
- Mazumdar, A.; Adak, S.; Chatterjee, R.; Banerjee, R.K. Mechanism-based inactivation of lacrimal-gland peroxidase by phenylhydrazine: A suicidal substrate to probe the active site. Biochem. J. 1997, 324, 713–719. [Google Scholar] [CrossRef]
- Dunford, H.B. Peroxidase-catalyzed halide ion oxidation. Redox. Rep. 2000, 5, 169–171. [Google Scholar] [CrossRef]
- Kwakkel, J.; Fliers, E.; Boelen, A. Illness-induced changes in thyroid hormone metabolism: Focus on the tissue level. Neth. J. Med. 2011, 69, 224–228. [Google Scholar]
- Day, B.J.; Bratcher, P.E.; Chandler, J.D.; Kilgore, M.B.; Min, E.; LiPuma, J.J.; Hondal, R.J.; Nichols, D.P. The thiocyanate analog selenocyanate is a more potent antimicrobial pro-drug that also is selectively detoxified by the host. Free Radic. Biol. Med. 2020, 146, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Saad, R.; Taylor, E.W.; Rayman, M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020, 37, 101715. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infection. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Beck, M.A.; Levander, O.A.; Handy, J. Oxidative Stress Mediated by Trace Elements Selenium Deficiency and Viral Infection. J. Nutr. 2003, 133, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Hiffler, L.; Rakotoambinina, B. Selenium and RNA virus interactions: Potential implications for SARS-CoV-2 infection (COVID-19). Front. Nutr. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Venturi, S.; Venturi, M. Iodide, thyroid and stomach carcinogenesis: Evolutionary story of a primitive antioxidant? Eur. J. Endocrinol. 1999, 140, 371–372. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat. Rev. Endocrinol. 2012, 8, 160–171. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rhee, C.M.; Leung, A.M.; Braverman, L.E.; Brent, G.A.; Pearce, E.N. A review: Radiographic iodinated contrast media-induced thyroid dysfunction. J. Clin. Endocrinol. Metab. 2015, 100, 376–383. [Google Scholar] [CrossRef]
- Wilcox, C. Evolution: Out of The Sea. Available online: https://blogs.scientificamerican.com/science-sushi/evolution-out-of-the-sea/ (accessed on 3 August 2022).
- Ames, B.N. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrient by triage. Proc. Natl. Acad. Sci. USA 2006, 103, 17589–17594. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: Why modest selenium deficiency may increase risk of diseases of aging. FASEB J. 2011, 25, 1793–1814. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Ames, B.N. Vitamin K, an example of triage theory: Is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009, 90, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Hisada, A.; Takatani, R.; Yamamoto, M.; Nakaoka, H.; Sakurai, K.; Mori, C. Maternal Iodine Intake and Neurodevelopment of Offspring: The Japan Environment and Children’s Study. Nutrients 2022, 14, 1826. [Google Scholar] [CrossRef]
- Ames, B.N. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat. Res. 2001, 475, 7–20. [Google Scholar] [CrossRef]
- Clark, L.C.; Dalkin, B.; Krongrad, A.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Witherington, R.; Herlong, J.H.; Janosko, E.; Carpenter, D.; et al. Decreased incidence of prostate cancer with selenium supplementation: Results of a double-blind cancer prevention trial. Br. J. Urol. 1998, 81, 730–734. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; White, D.A.; Schneider, C.J. Cancer mortality correlation studies--IV: Associations with dietary intakes and blood levels of certain trace elements, notably Se-antagonists. Bioinorg. Chem. 1977, 7, 35–56. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; White, D.A.; Schneider, C.J. Cancer mortality correlation studies-III: Statistical associations with dietary selenium intakes. Bioinorg. Chemorg. 1977, 7, 23–34. [Google Scholar] [CrossRef]
- Shimizu, H.; Ross, R.K.; Bernstein, L.; Henderson, B.E.; Mack, T.M.; Yatani, R. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles county. Br. J. Cancer 1991, 63, 963–966. [Google Scholar] [CrossRef]
- Cann, S.A.; van Netten, J.P.; van Netten, C. Hypothesis: Iodine, selenium and the development of breast cancer. Cancer Causes Control 2000, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Smyth, P.P.A. Iodine, Seaweed, and the Thyroid. Eur. Thyroid. J. 2021, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Karita, K.; Hamada, G.S.; Tsugane, S. Comparison of selenium status between Japanese living in Tokyo and Japanese Brazilians in São Paulo, Brazil. Asia Pac. J. Clin. Nutr. 2002, 10, 197–199. [Google Scholar] [CrossRef]
- Manson, J.E. Multivitamins slow cognitive aging in older adults. Medscape 2021.
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Völzke, H.; Erlund, I.; Hubalewska-Dydejczyk, A.; Ittermann, T.; Peeters, R.P.; Rayman, M.; Buchberger, M.; Siebert, U.; Thuesen, B.H.; Zimmermann, M.B.; et al. How do we improve the impact of iodine deficiency disorders prevention in Europe and beyond? Eur. Thyroid. J. 2018, 7, 193–200. [Google Scholar] [CrossRef]
- Institute of Medicine Iodine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academic Press: Washington, DC, USA, 2001; pp. 258–289. [Google Scholar]
- Scientific Committee on Food (Ed.) Tolerable Upper Intake Levels for Vitamins and Minerals; European Food Safety Authority: Luxembourg, 2006; ISBN 9291990140.
- FAO/WHO. Human Vitamin and Mineral Requirements; FAO Food and Nutrition Division: Rome, Italy, 2001. [Google Scholar]
- Bürgi, H. Iodine excess. Best Pr. Res. Clin. Endocrinol. Metab. 2010, 24, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhou, R. Further observations on the human maximum safe dietary selenium intake in a seleniferous area of China. J. Trace Elem. Electrol. Health Dis. 1994, 8, 159–165. [Google Scholar]
- Longnecker, M.P.; Taylor, P.R.; Levander, O.A.; Howe, M.; Veillon, C.; McAdam, P.A.; Patterson, K.Y.; Holden, J.M.; Stampfer, M.J.; Morris, J.S.; et al. Selenium in diet, blood, and toenails in relation to human health in a seleniferous area. Am. J. Clin. Nutr. 1991, 53, 1288–1294. [Google Scholar] [CrossRef]
- Shearer, T.R.; Hadjimarkos, D.M. Geographic distribution of selenium in human milk. Arch. Environ. Health 1975, 30, 230–233. [Google Scholar] [CrossRef]
- Aburto, N.J.; Abudou, M.; Candeias, V.; Wu, T. Effect and Safety of Salt Iodization to Prevent Iodine Deficiency Disorders: A Systematic Review with Meta-Analyses; WHO: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/148175 (accessed on 23 July 2022).
- Rodriguez-Diaz, E.; Pearce, E.N. Iodine status and supplementation before, during, and after pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101430. [Google Scholar] [CrossRef]
- Hubalewska-Dydejczyk, A.; Duntas, L.; Gilis-Januszewska, A. Pregnancy, thyroid, and the potential use of selenium. Hormones 2020, 19, 47–53. [Google Scholar] [CrossRef]
- Ames, B.N.; McCann, J.C.; Stampfer, M.J.; Willett, W.C. Evidence-based decision making on micronutrients and chronic disease: Long-term randomized controlled trials are not enough. Am. J. Clin. 2007, 86, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.L.; Rosenberg, C. The need for evidence-based medicine. J. R. Soc. Med. 1995, 88, 620–624. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Urinary Iodine Concentrations for Determining Iodine Status Deficiency in Populations. Vitamin and Mineral Nutrition Information System; WHO Report: Geneva, Switzerland, 2013. [Google Scholar]
- Zimmermann, M.B.; Boelaert, K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E.; He, X.; Heeren, T.; Pearce, E.N. Breastmilk iodine concentrations following acute dietary iodine intake. Thyroid 2012, 22, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Iodine in human milk: A systematic review. Adv. Nutr. 2018, 9, 347S–357S. [Google Scholar] [CrossRef]
- Oster, O.; Prellwitz, W. The renal excretion of selenium. Biol. Trace Elem. Res. 1990, 24, 119–146. [Google Scholar] [CrossRef]
- Thomson, C.D. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef]
- Sziklai-László, I.; Majchrzak, D.; Elmadfa, I.; Cser, M.Á. Selenium and vitamin e concentrations in human milk and formula milk from Hungary. J. Radioanal. Nucl. Chem. 2009, 279, 585–590. [Google Scholar] [CrossRef]
- Lonnerdal, B. Regulation of mineral and trace elements in human milk: Exogenous and endogenous factors. Nutr. Rev. 2000, 58, 223–229. [Google Scholar] [CrossRef]
- Huber, M.; Knottnerus, A.; Green, L.; van der Horst, H.; Jadad, A.R.; Kromhout, D.; Leonard, B.; Lorig, K.; Loureiro, M.I.; van der Meer, J.W.M.; et al. How should we define health? Br. Med. J. 2011, 343, d4163. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.P.; Schulting, R.J.; Hedges, R.E.M. Archaeology: Sharp shift in diet at onset of Neolithic. Nature 2003, 425, 366. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Essential fatty acids in health and chronic diseases. Am. J. Clin. Nutr. 1999, 70, 560s–569s. [Google Scholar] [CrossRef] [PubMed]
- Muskiet, F.A.J.; Fokkema, M.R.; Schaafsma, A.; Boersma, E.R.; Crawford, M.A. Is Docosahexaenoic Acid (DHA) Essential? Lessons from DHA Status Regulation, Our Ancient Diet, Epidemiology and Randomized Controlled Trials. J. Nutr. 2004, 134, 183–186. [Google Scholar] [CrossRef]
- Koletzko, B.; Boey, C.C.M.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current information and asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Systematic review and practice recommendations from an early nutrition academy workshop. Ann. Nutr. Metab. 2014, 65, 49–80. [Google Scholar] [CrossRef]
- Rappaport, J. Changes in dietary iodine explains increasing incidence of breast cancer with distant involvement in young women. J. Cancer 2017, 8, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Health Council of the Netherlands Towards Maintaining an Optimal Iodine intake Report; Health Council of The Netherlands: The Hague, The Netherlands, 2008.
- Dinnissen, C.S.; de Jong, M.H.; Verkaik-Kloosterman, J.; Hendriksen, M. Jodiuminname Van Volwassenen in Noord Nederland in 2020–2021 en Trend Sinds 2006-2007. Resultaten van Voedingsstatusonderzoek in Het Lifelines Cohort; RIVM: Bilthoven, The Netherlands, 2022.
- Geurts, M.; Verkaik-Kloosterman, J. De Jodiuminname Van de Nederlands Bevolking na Verdere Zoutverlaging in Brood; RIVM: Bilthoven, The Netherlands, 2014.
- Stoutjesdijk, E.; Schaafsma, A.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Iodine status during pregnancy and lactation: A pilot study in the Netherlands. Neth. J. Med. 2018, 76, 210–217. [Google Scholar]
- Mayunga, K.C.; Lim, A.; Po, M.; Lubberts, J.; Stoutjesdijk, E.; Touw, D.J.; Muskiet, F.A.J.; Dijck-Brouwer, D.A.J. Pregnant Dutch women have inadequate iodine status and selenium intake. Nutrients 2022, 11, 69. [Google Scholar]
- Zimmermann, M.B.; Andersson, M. Global perspectives in endocrinology: Coverage of iodized salt programs and iodine status in 2020. Eur. J. Endocrinol. 2021, 185, R13–R21. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J.; Lazarus, J.H.; Smyth, P.P.; Laurberg, P.; Holder, R.L.; Boelaert, K.; Franklyn, J.A. British Thyroid Association UK Iodine Survey Group Iodine status of UK schoolgirls: A cross-sectional survey. Lancet 2011, 377, 2007–2012. [Google Scholar] [CrossRef]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Hynes, K.L.; Otahal, P.; Hay, I.; Burgess, J.R. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J. Clin. Endocrinol. Metab. 2013, 98, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.C.; Rose, M.C.; Skeaff, S.A.; Gray, A.R.; Morgan, K.M.D.; Ruffman, T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am. J. Clin. Nutr. 2009, 90, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Chandrakant, S.P. Statement South Asia Iodine Glob. Netw.; 73rd Session of the WHO Regional Committee for South-East Asia, Statement letter; WHO: Nonthaburi, Thailand, 2020. [Google Scholar]
- Cordain, L.; Boyd-Eaton, S.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J.; Cordain, L. Origins and evolution of the Western diet. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Kuipers, R.S.; Luxwolda, M.F.; Dijck-brouwer, D.A.J.; Eaton, S.B.; Crawford, M.A.; Cordain, L.; Muskiet, F.A.J. Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. Br. J. Nutr. 2010, 104, 1666–1687. [Google Scholar] [CrossRef]
- Kuipers, R.S.; Luxwolda, M.F.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Fatty acid compositions of preterm and term colostrum, transitional and mature milks in a sub-Saharan population with high fish intakes. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 201–207. [Google Scholar] [CrossRef]
- Crawford, M.A.; Bloom, M.; Cunnane, S.; Holmsen, H.; Ghebremeskel, K.; Parkington, J.; Schmidt, W.; Sinclair, A.J.; Broadhurst, C.L. Docosahexaenoic acid and cerebral evolution. World Rev. Nutr. Diet Nutr. Diet 2001, 88, 6–17. [Google Scholar] [CrossRef]
- Verhaegen, M. Aquatic versus Savanna: Comparative and paleo-environmental evidence. Nutr. Health 1993, 9, 165–191. [Google Scholar] [CrossRef]
- Benderc, R.; Tobias, P.V.; Bender, N. The Savannah hypotheses: Origin, reception and impact on paleoanthropology. Hist. Philos. Life Sci. 2012, 34, 147–184. [Google Scholar]
- Byelashov, O.A.; Sinclair, A.J.; Kaur, G. Dietary souces, current intakes, and nutritional role of omega-3 docosapentaenoic acid. Lipid Technol. 2015, 27, 79–82. [Google Scholar] [CrossRef]
- Crawford, M.A.; Bloom, M.; Broadhurst, C.L.; Schmidt, W.F.; Cunnane, S.C.; Galli, C.; Gehbremeskel, K.; Linseisen, F.; Lloyd-Smith, J.; Parkington, J. Evidence for the unique function of docosahexaenoic acid during the evolution of the modern hominid brain. Lipids 1999, 34, s39–s47. [Google Scholar] [CrossRef]
- Burdge, C.G. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Fokkema, M.R.; van Rieke, H.M.; Bauermann, O.J.; Smit, E.N.; Muskiet, F.A.J. Short-term carnitine supplementation does not augment LCPomega3 status of vegans and lacto-ovo-vegetarians. J. Am. Coll. Nutr. 2005, 24, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, C.L.; Wang, Y.; Crawford, M.A.; Cunnane, S.C.; Parkington, J.E.; Schmidt, W.F. Brain-specific lipids from marine, lacustrine, or terrestrial food resources: Potential impact on early African Homo sapiens. Comp. Biochem. Physiol. Part B 2002, 131, 653–673. [Google Scholar] [CrossRef]
- Gibbons, A. In search of the first hominids. Science 2002, 295, 1214–1219. [Google Scholar] [CrossRef]
- Gibbons, A. Ardipithecus ramidus. The view from Afar. Science 2009, 326, 41–43. [Google Scholar] [CrossRef]
- WoldeGabriel, G.; Ambrose, S.H.; Barboni, D.; Bonnefille, R.; Bremond, L.; Currie, B.; DeGusta, D.; Hart, W.K.; Murray, A.M.; Renne, P.R.; et al. The geological, isotopic, botanical, invertebrate, and lower vertebrate surroundings of Ardipithecus ramidus. Science 2009, 326, 65.e1–e5. [Google Scholar] [CrossRef]
- Biggs, J.; Ayele, A.; Fischer, T.P.; Fontijn, K.; Hutchison, W.; Kazimoto, E.; Whaler, K.; Wright, T.J. Volcanic activity and hazard in the East African Rift Zone. Nat. Commun. 2021, 12, 6881. [Google Scholar] [CrossRef]
- Jungers, W.L. These feet were made for walking. Elife 2016, 5, e19568. [Google Scholar] [CrossRef]
- Will, M.; Kandel, A.W.; Conard, N.J. Midden or molehill: The role of coastal adaptations for human evolution and dispersal. J. World Prehistory 2019, 32, 33–72. [Google Scholar] [CrossRef]
- Marean, C.W.; Bar-Matthews, M.; Bernatchez, J.; Fisher, E.; Goldberg, P.; Herries, A.I.R.; Jacobs, Z.; Jerardino, A.; Karkanas, P.; Minichillo, T.; et al. Early human use of marine resources and pigment in South Africa during the Middle Pleistocene. Nature 2007, 449, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Stringer, C.B.; Finlayson, J.C.; Barton, R.N.E.; Fernández-Jalvo, Y.; Cáceres, I.; Sabin, R.C.; Rhodes, E.J.; Currant, A.P.; Rodríguez-Vidal, J.; Giles-Pacheco, F.; et al. Neanderthal exploitation of marine mammals in Gibraltar. Proc. Natl. Acad. Sci. USA 2008, 105, 14319–14324. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.R.; Harris, J.W.K.; Levin, N.E.; Mccoy, J.T.; Herries, A.I.R.; Bamford, M.K.; Bishop, L.C.; Richmond, B.G.; Kibunjia, M. Early hominin diet included diverse terrestrial and aquatic animals 1.95 Ma in East Turkana, Kenya. Proc. Natl. Acad. Sci. USA 2010, 107, 10002–10007. [Google Scholar] [CrossRef]
- O’Connor, S.; Ono, R.; Clarkson, C. Pelagic fishing at 42,000 years before the present and the maritime skills of modern humans. Science 2011, 334, 1117–1121. [Google Scholar] [CrossRef]
- Joordens, J.C.A.; D’Errico, F.; Wesselingh, F.P.; Munro, S.; de Vos, J.; Wallinga, J.; Ankjærgaard, C.; Reimann, T.; Wijbrans, J.R.; Kuiper, K.F.; et al. Homo erectus at Trinil on Java used shells for tool production and engraving. Nature 2015, 518, 228–231. [Google Scholar] [CrossRef]
- Cortés-Sánchez, M.; Morales-Muñiz, A.; Simón-Vallejo, M.D.; Lozano-Francisco, M.C.; Vera-Peláez, J.L.; Finlayson, C.; Rodríguez-Vidal, J.; Delgado-Huertas, A.; Jiménez-Espejo, F.J.; Martínez-Ruiz, F.; et al. Earliest known use of marine resources by neanderthals. PLoS ONE 2011, 6, e24026. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shang, H.; Tong, H.; Nehlicha, O.; Liuc, W.; Zhao, C.; Yu, J.; Wang, C.; Trinkaus, E.; Richards, M.P. Stable isotope dietary analysis of the Tianyuan 1 early modern human. Proc. Natl. Acad. Sci. USA 2009, 106, 10971–10974. [Google Scholar] [CrossRef]
- Craig, O.E.; Saul, H.; Lucquin, A.; Nishida, Y.; Taché, K.; Clarke, L.; Thompson, A.; Altoft, D.T.; Uchiyama, J.; Ajimoto, M.; et al. Earliest evidence for the use of pottery. Nature 2013, 496, 351–354. [Google Scholar] [CrossRef]
- Kummu, M.; De Moel, H.; Ward, P.J.; Varis, O. How Close Do We Live to Water? A Global Analysis of Population Distance to Freshwater Bodies. PLoS ONE 2011, 6, e20578. [Google Scholar] [CrossRef]
- Edmonds, D.; Caldwell, R.; Baumgardner, S.; Paola, C.; Roy, S.; Nelson, A.; Nienhuis, J. A global analysis of human habitation on river deltas. Geophys. Res. Abstr. 2017, 19, 10832. [Google Scholar]
- Smith, E.I.; Jacobs, Z.; Johnsen, R.; Ren, M.; Fisher, E.C.; Oestmo, S.; Wilkins, J.; Harris, J.A.; Karkanas, P.; Fitch, S.; et al. Humans thrived in South Africa through the Toba eruption about 74,000 years ago. Nature 2018, 555, 511–515. [Google Scholar] [CrossRef]
- Marean, C. Humanity Thrived in Africa after the Eruption of a Supervolcano 74,000 Years Ago that Plunged Parts of Earth into a Decade-Long Winter. Available online: https://www.dailymail.co.uk/sciencetech/article-5483389/How-humans-survived-eruption-apocalyptic-SUPERVOLCANO.html (accessed on 23 July 2022).
- Henn, B.M.; Gignoux, C.R.; Jobin, M.; Granka, J.M.; Macpherson, J.M.; Kidd, J.M.; Feldman, M.W. Hunter-gatherer genomic diversity suggests a southern African origin for modern humans. Proc. Natl. Acad. Sci. USA 2011, 108, 5154–5162. [Google Scholar] [CrossRef]
- Lawson Handley, L.J.; Manica, A.; Goudet, J.; Balloux, F. Going the distance: Human population genetics in a clinal world. Trends Genet. 2007, 23, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Stringer, C. Palaeoanthropology. Coasting out of Africa. Nature 2000, 405, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lewis, C.M.L., Jr.; Jakobsson, M.; Ramachandran, S.; Ray, N.; Bedoya, G.; Rojas, W.; Parra, M.V.; Molina, J.A.; Gallo, C.; et al. Genetic Variation and Population Structure in Native Americans. PLoS Genet. 2007, 3, e30185. [Google Scholar] [CrossRef]
- Kuipers, R.S.; Joordens, J.C.A.; Muskiet, F.A.J. A multidisciplinary reconstruction of Palaeolithic nutrition that holds promise for the prevention and treatment of diseases of civilisation. Nutr. Res. Rev. 2012, 25, 96–129. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthale, M.U.; Swinkels, D.W. 4 Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Kumar, S.N.; Arnipalli, S.R.; Mehta, P.; Carrau, S.; Ziouzenkova, O. Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies. Nutrients 2022, 14, 2976. [Google Scholar] [CrossRef]
- Triggiani, V.; Tafaro, E.; Giagulli, V.A.; Sabbà, C.; Resta, F.; Licchelli, B.; Guastamacchia, E. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr. Metab. Immune. Disord. Drug Targets 2009, 9, 277–294. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Shreenath, A.P.; Ameer, M.A.; Dooley, J. Selenium Deficiency; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B 12 deficiency. Nat. Rev. Dis. Prim. 2017, 29, 17040. [Google Scholar] [CrossRef] [PubMed]
- Aakre, I.; Solli, D.D.; Markhus, M.W.; Mæhre, H.K.; Henjum, S.; Alexander, J.; Korneliussen, P.; Madsen, L.; Kjellevold, M. Commercially available kelp and seaweed products–valuable iodine source or risk of excess intake? Food Nutr. Res. 2021, 65, 7584. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academic Press: Washington, DC, USA, 2001. [Google Scholar]
- Katagiri, R.; Yuan, X.; Kobayashi, S.; Sasaki, S. Effect of excess iodine intake on thyroid diseases in different populations: A systematic review and meta-analyses including observational studies. PLoS ONE 2017, 12, e0173722. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef]
- Thomson, J.D.; Campbell, J.M.; Miller, J.; Skeaff, S.A. Minimal impact of excess iodate intake on thyroid hormones and selenium status in older New Zealanders. Eu. J. Endocrinol. 2011, 165, 745–752. [Google Scholar] [CrossRef]
- Alfthan, G.; Aro, A.; Arvilommi, H.; Huttunen, J.K. Selenium metabolism and platelet glutathione peroxidase activity in healthy Finnish men: Effects of selenium yeast, selenite, and selenate. Am. J. Clin. Nutr. 1991, 53, 120–125. [Google Scholar] [CrossRef]
- Noahsen, P.; Kleist, I.; Larsen, H.M.; Andersen, S. Intake of seaweed as part of a single sushi meal, iodine excretion and thyroid function in euthyroid subjects: A randomized dinner study. J. Endocrinol. Investig. 2020, 43, 431–438. [Google Scholar] [CrossRef]
- Aakre, I.; Evensen, L.T.; Kjellevold, M.; Dahl, L.; Henjum, S.; Alexander, J.; Madsen, L.; Markhus, M.W. Iodine status and thyroid function in a group of seaweed consumers in Norway. Nutrients 2020, 12, 3483. [Google Scholar] [CrossRef]
- Leung, A.; Pearce, E.N.; Braverman, L.E. Role of iodine in thyroid physiology. Expert Rev. Endocrinol. Metab. 2010, 5, 593–602. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Ponton, D.E.; Graves, S.D.; Fortin, C.; Janz, D.; Amyot, M.; Schiavon, M. Selenium interactions with algea: Chemical processes at biological uptake sites, bioaculmulation, and intracellular metabolism. Plants 2020, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C.; Raymond, L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Noda, K.; Danbara, H. Selenium intake based on representative diets in Japan, 1957 to 1989. Nutr. Res. 1996, 16, 1471–1477. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yamashita, M.; Iida, H. Selenium Content in Seafood in Japan. Nutrients 2013, 5, 388–395. [Google Scholar] [CrossRef]
- Sakata, M.; Marumoto, K.; Narukawa, M.; Asakura, K. Mass balance and sources of mercury in Tokyo Bay. J. Oceanogr. 2006, 62, 767–775. [Google Scholar] [CrossRef]
- Olmedo, P.; Hernández, A.F.; Pla, A.; Femia, P.; Navas-Acien, A.; Gil, F. Determination of essential elements (copper, manganese, selenium and zinc) in fish and shellfish samples. Risk and nutritional assessment and mercury-selenium balance. Food Chem. Toxicol. 2013, 62, 299–307. [Google Scholar] [CrossRef]
- Diet & Fitness Today Selenium Rich Fish and Shellfish. Available online: http://www.dietandfitnesstoday.com/shellfish-high-in-selenium.php (accessed on 31 July 2022).
- Thomson, C.D.; Chisholm, A.; Mclachlan, S.K.; Campbell, J.M. Brazil nuts: An effective way to improve selenium status. Am. J. Clin. Nutr. 2008, 87, 379–384. [Google Scholar] [CrossRef]
- Hwang, E.S.; Ki, K.N.; Chung, H.Y. Proximate composition, amino acid, mineral, and heavy metal content of dried laver. Prev. Nutr. Food Sci. 2013, 18, 139–144. [Google Scholar] [CrossRef]
- Murray-Kolb, L.E.; Acosta, A.M.; De Burga, R.R.; Chavez, C.B.; Flores, J.T.; Olotegui, M.P.; Pinedo, S.R.; Salas, M.S.; Trigoso, D.R.; Vasquez, A.O.; et al. Early childhood cognitive development is affected by interactions among illness, diet, enteropathogens and the home environment: Findings from the MAL-ED birth cohort study. BMJ Glob. Health 2018, 3, e000752. [Google Scholar] [CrossRef]
- Correia, H.; Soares, C.; Morais, S.; Pinto, E.; Marques, A.; Nunes, M.L.; Almeida, A.; Delerue-Matos, C. Seaweeds rehydration and boiling: Impact on iodine, sodium, potassium, selenium, and total arsenic contents and health benefits for consumption. Food Chem. Toxicol. 2021, 155. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.U.; Chijioke, N.O.; Adillah, N.; Heffny, B.; Bradley, D.A.; Alsubaie, A.; Sulieman, A.; Faruque, M.R.I.; Sayyed, M.I. Elevated Concentrations of Metal (loids) in Seaweed and the Concomitant Exposure to Humans. Foods 2021, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zheng, L.; Chen, H.; Lin, W.; Zhang, W. Enriched accumulation and biotransformation of selenium in the edible seaweed Laminaria japonica. J. Agric Food Chem. 2004, 52, 6460–6464. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.J. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef]
- Day, N.K.; Schmidt, T.S.; Roberts, J.J.; Osmundson, B.C.; Willacker, J.J.; Eagles-Smith, C.A. Mercury and selenium concentrations in fishes of the Upper Colorado River Basin, southwestern United States: A retrospective assessment. PLoS ONE 2020, 15, e0226824. [Google Scholar] [CrossRef]
- Rayman, M.P.; Hillert, K.; Pastor-barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef]
- Arthur, J.R.; Beckett, G.J.; Mitchell, J.H. The interactions between selenium and iodine deficiencies in man and animals. Nutr. Res. Rev. 1999, 12, 55–73. [Google Scholar] [CrossRef]
- Köhrle, J. The trace element selenium and the thyroid gland. Biochimie 1999, 81, 527–533. [Google Scholar] [CrossRef]
- Contempré, B.; Morreale de Escobar, G.; Denef, J.-F.; Dumont, J.E.; Many, M.-C. Thiocyanate induces cell necrosis and fibrosis in selenium- and iodine-deficient rat thyroids: A potential experimental model for myxedematous endemic cretinism in central Africa. Endocrinology 2004, 145, 994–1002. [Google Scholar] [CrossRef]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The role of selenium in thyroid gland pathophysiology. Endokrynol. Pol. 2017, 68, 440–465. [Google Scholar] [CrossRef]
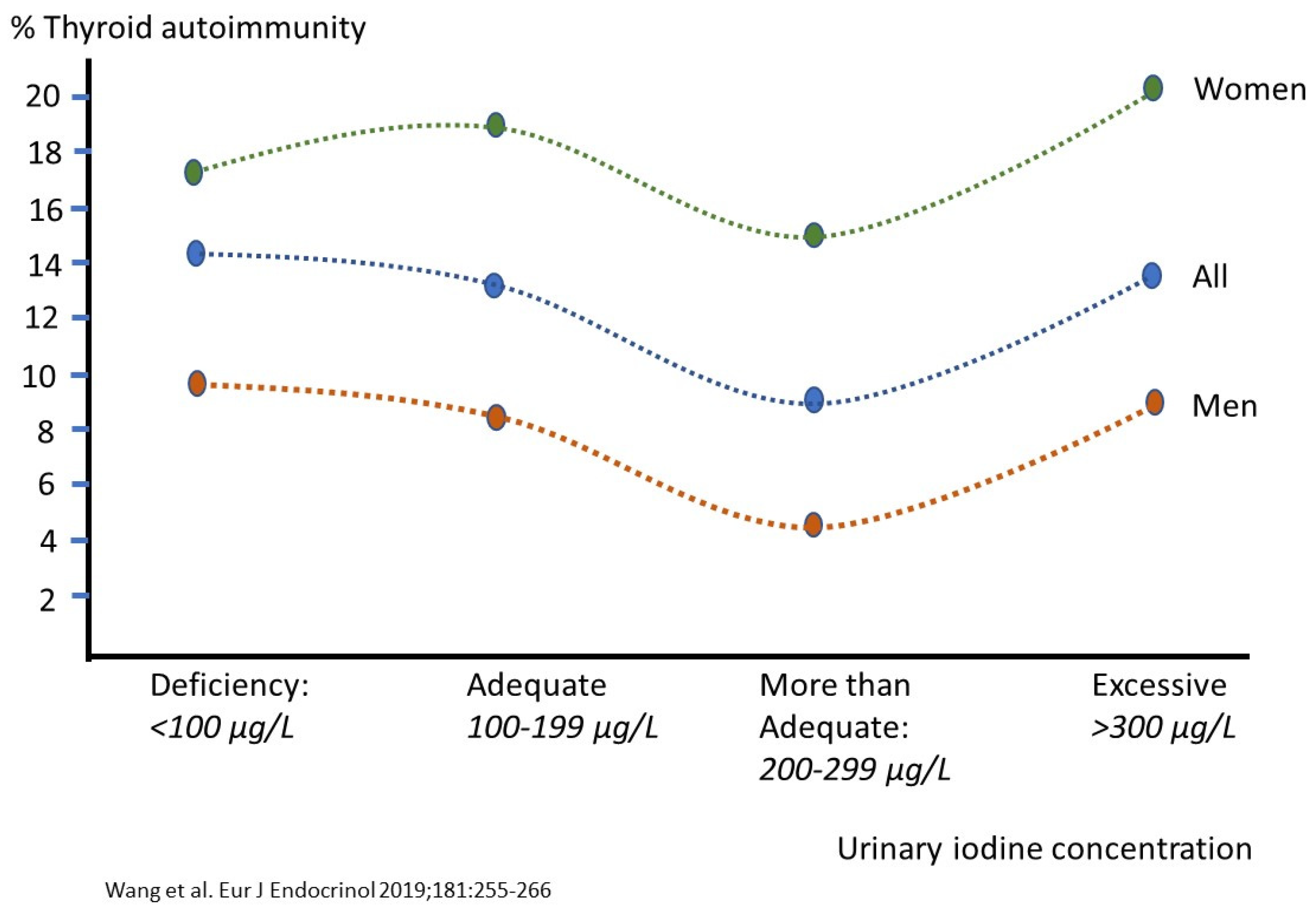
- Wang, B.; He, W.; Li, Q.; Jia, X.; Yao, Q.; Song, R.; Qin, Q.; Zhang, J.A. U-shaped relationship between iodine status and thyroid autoimmunity risk in adults. Eur. J. Endocrinol. 2019, 181, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Han, C.; Li, C.; Mao, J.; Wang, W.; Xie, X.; Li, C.; Xu, B.; Meng, T.; Du, J.; et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: A cross-sectional study of 7190 pregnant women in China. J. Clin. Endocrinol. Metab. 2015, 100, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.-C.; et al. Roles of hydrogen peroxide in thyroid physiology and disease. J. Clin. Endocrinol. Metab. 2007, 92, 3664–3773. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.-L.; Yang, X.-F.; Guo, H.-L.; Zhao, L.-N.; Sun, X.-F. Supplemental selenium alleviates the toxic effects of excessive iodine on thyroid. Biol. Trace Elem. Res. 2011, 141, 110–118. [Google Scholar] [CrossRef]
- Mooij, P.; de Wit, H.J.; Bloot, A.M.; Wilders-Truschnig, M.M.; Drexhage, H.A. Iodine deficiency induces thyroid autoimmune reactivity in Wistar rats. Endocrinol. 1993, 133, 1197–1204. [Google Scholar] [CrossRef]
- Carayanniotis, G. Molecular parameters linking thyroglobulin iodination with autoimmune thyroiditis. Hormones 2011, 10, 27–35. [Google Scholar] [CrossRef]
- Saboori, A.M.; Rose, N.R.; Bresler, H.S. Iodination of human thyroglobulin (Tg) alters its immunoreactivity I Iodination alters multiple epitopes of human Tg. Clin. Exp. Immunol. 1998, 113, 297–302. [Google Scholar] [CrossRef]
- Burek, C.L.; Taloror, M.V. Environmental triggers of autoimmune thyroiditis. J. Autoimmun. 2009, 33, 183–189. [Google Scholar] [CrossRef]
- Negro, R. Selenium and thyroid autoimmunity. Biologics 2008, 2, 265–273. [Google Scholar] [CrossRef]
- Pirola, I.; Rotondi, M.; Cristiano, A.; Maffezzoni, F.; Pasquali, D.; Marini, F.; Coperchini, F.; Paganelli, M.; Apostoli, P.; Chiovato, L.; et al. Selenium supplementation in patients with subclinical hypothyroidism affected by autoimmune thyroiditis: Results of the SETI study. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2020, 67, 28–35. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.N.A.; Mohamad Hanif, E.A.; Chin, S.F.; Neoh, H.M. Pathogens and carcinogenesis: A review. Biology 2021, 10, 533. [Google Scholar] [CrossRef]
- De Bono, J.S.; Guo, C.; Gurel, B.; De Marzo, A.M.; Sfanos, K.S.; Mani, R.S.; Gil, J.; Drake, C.G.; Alimonti, A. Prostate carcinogenesis: Inflammatory storms. Nat. Rev. Cancer 2020, 20, 455–469. [Google Scholar] [CrossRef]
- Sfanos, K.S.; De Marzo, A.M. Prostate cancer and inflammation: The evidence. Histopathology 2012, 60, 199–215. [Google Scholar] [CrossRef]
- Hoption Cann, S.A.; Qiu, Z.; van Netten, C. A prospective study of iodine status, thyroid function, and prostate cancer risk: Follow-up of the First National Health and Nutrition Examination Survey. Nutr. Cancer 2007, 58, 28–34. [Google Scholar] [CrossRef]
- Hallberg, O.; Huttunen, P.; Johansson, O. Cancer incidence vs. population average sleep duration on spring mattresses. Adv. Stud. Med. Sci. 2014, 2, 17–30. [Google Scholar] [CrossRef][Green Version]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef]
- Culp, M.B.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Katanoda, K.; Ito, Y.; Sobue, T. International comparison of trends in cancer mortality: Japan has fallen behind in screening-related cancers. Jpn. J. Clin. Oncol. 2021, 51, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Kwon, M.J.; Kim, J.H.; Kim, J.-H.; Choi, H.G. Association between Thyroid Cancer and Breast Cancer: Two Longitudinal Follow-Up Studies Using a National Health Screening Cohort. J. Pers. Med. 2022, 12, 133. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.K.; Park, H.K.; Byun, D.W.; Suh, K.; Yoo, M.H.; Min, Y.-K.; Kim, S.W.; Chung, J.H. Strong association of relatively low and extremely excessive iodine intakes with thyroid cancer in an iodine-replete area. Eur. J. Nutr. 2017, 56, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vega, A.; Vega-Riveroll, L.; Ayala, T.; Peralta, G.; Torres-Martel, J.M.; Rojas, J.; Mondragón, P.; Domínguez, A.; De Obaldía, R.; Avecilla-Guerrero, C.; et al. Adjuvant effect of molecular iodine in conventional chemotherapy for breast cancer. randomized pilot study. Nutrients 2019, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Manjer, J.; Sandsveden, M.; Borgquist, S. Serum Iodine and Breast Cancer Risk: A Prospective Nested Case-Control Study Stratified for Selenium Levels. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Rosenberg, M.; Hou, L.; Hu, M. Relationships among Environment, Climate, and Longevity in China. Int. J. Envrion. Res. Publish Health 2017, 14, 1195. [Google Scholar] [CrossRef]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef]
| Nutrient | Current RDA or (AI) * | Basis of Current RDA or AI | Basis of Current UL | Important Information Not as yet Taken into Account | Suggested Future RDA or AI | Suggested Future UL |
|---|---|---|---|---|---|---|
| Iodine (µg) | 150 UL: 600–1100 | Thyroid iodine (131I) accumulation and turnover in 292 euthyroid adults, normal urinary iodine excretion (WHO: 100–199 µg/L), TSH, serum T4 | Acute Wolff-Chaikoff effect causing mostly transient hypothyroidism (TSH increases). LOAEL = 1800. Uncertainty factor: 1.5–3 | Selenium status Iodine’s protective effect in long-term observational studies on thyroid autoimmunity, cancer and chronic diseases mg/day intakes from the traditional Japanese/Asian diet Association with iron Associations with ‘goitrogens’ that exhibit favorable effects as anti-microbials in the peroxidase partner system | mg amounts or a very safe but conservative 300 µg, as based on U-shaped relation with thyroid autoimmunity (see Figure 3) and in view of the relatively high UL. Applies only for selenium replete subjects Protection from stomach, female breast, prostate and other cancers | mg amounts, as based on the traditional Japanese/Asian diet. Applies only for selenium replete subjects |
| Selenium (µg) | 26–70 UL: 300–400 | Plateauing of plasma glutathione peroxidase-3 or plasma SEPP1. | Selenosis. Chronic high intakes by Chinese and US adults. LOAEL= 900–1000. Uncertainty factor of 2–3, using 800–850 in the calculation | Selenium form: selenomethionine may cause delayed toxicity due to its accumulation in body proteins. Selenate is better absorbed than selenite, but less retained. Protective effect of selenium in microbial infections, prevention of virus mutation, autoimmune disease, and inflammation | 105 µg, based on optimal SEPP1 at 125 ug/L (see text) May only be favorable at adequate iodine status Anti-microbial, anti-cancer and anti-thyroid autoimmunity Relation with toxic heavy metals (As, Pb, Cd, Hg) that ironically increase the selenium UL. Narrow window between RDA/AI and UL | 300–400 µg, as based on the PRECISE study (see text) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dijck-Brouwer, D.A.J.; Muskiet, F.A.J.; Verheesen, R.H.; Schaafsma, G.; Schaafsma, A.; Geurts, J.M.W. Thyroidal and Extrathyroidal Requirements for Iodine and Selenium: A Combined Evolutionary and (Patho)Physiological Approach. Nutrients 2022, 14, 3886. https://doi.org/10.3390/nu14193886
Dijck-Brouwer DAJ, Muskiet FAJ, Verheesen RH, Schaafsma G, Schaafsma A, Geurts JMW. Thyroidal and Extrathyroidal Requirements for Iodine and Selenium: A Combined Evolutionary and (Patho)Physiological Approach. Nutrients. 2022; 14(19):3886. https://doi.org/10.3390/nu14193886
Chicago/Turabian StyleDijck-Brouwer, D. A. Janneke, Frits A. J. Muskiet, Richard H. Verheesen, Gertjan Schaafsma, Anne Schaafsma, and Jan M. W. Geurts. 2022. "Thyroidal and Extrathyroidal Requirements for Iodine and Selenium: A Combined Evolutionary and (Patho)Physiological Approach" Nutrients 14, no. 19: 3886. https://doi.org/10.3390/nu14193886
APA StyleDijck-Brouwer, D. A. J., Muskiet, F. A. J., Verheesen, R. H., Schaafsma, G., Schaafsma, A., & Geurts, J. M. W. (2022). Thyroidal and Extrathyroidal Requirements for Iodine and Selenium: A Combined Evolutionary and (Patho)Physiological Approach. Nutrients, 14(19), 3886. https://doi.org/10.3390/nu14193886







