The Relationship between Gastrointestinal Health, Micronutrient Concentrations, and Autoimmunity: A Focus on the Thyroid
Abstract
:1. Introduction
2. Overview of Association between Thyroid Dysfunction, GI Dysfunction, and Nutrient Insufficiency
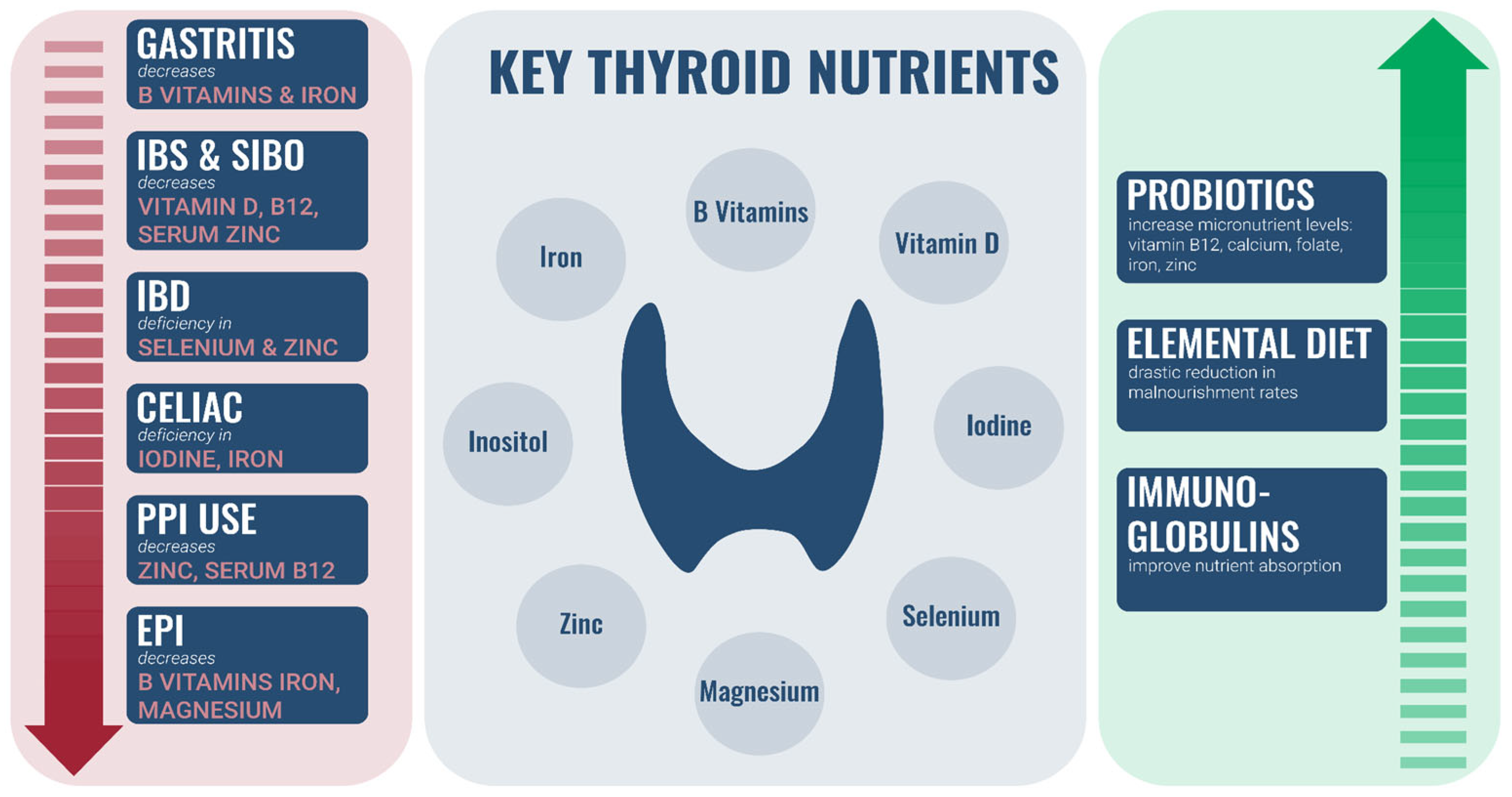
2.1. Important Nutrients Required for Thyroid Health
2.2. Iodine
2.3. Selenium
2.4. Inositol
2.5. Vitamin D
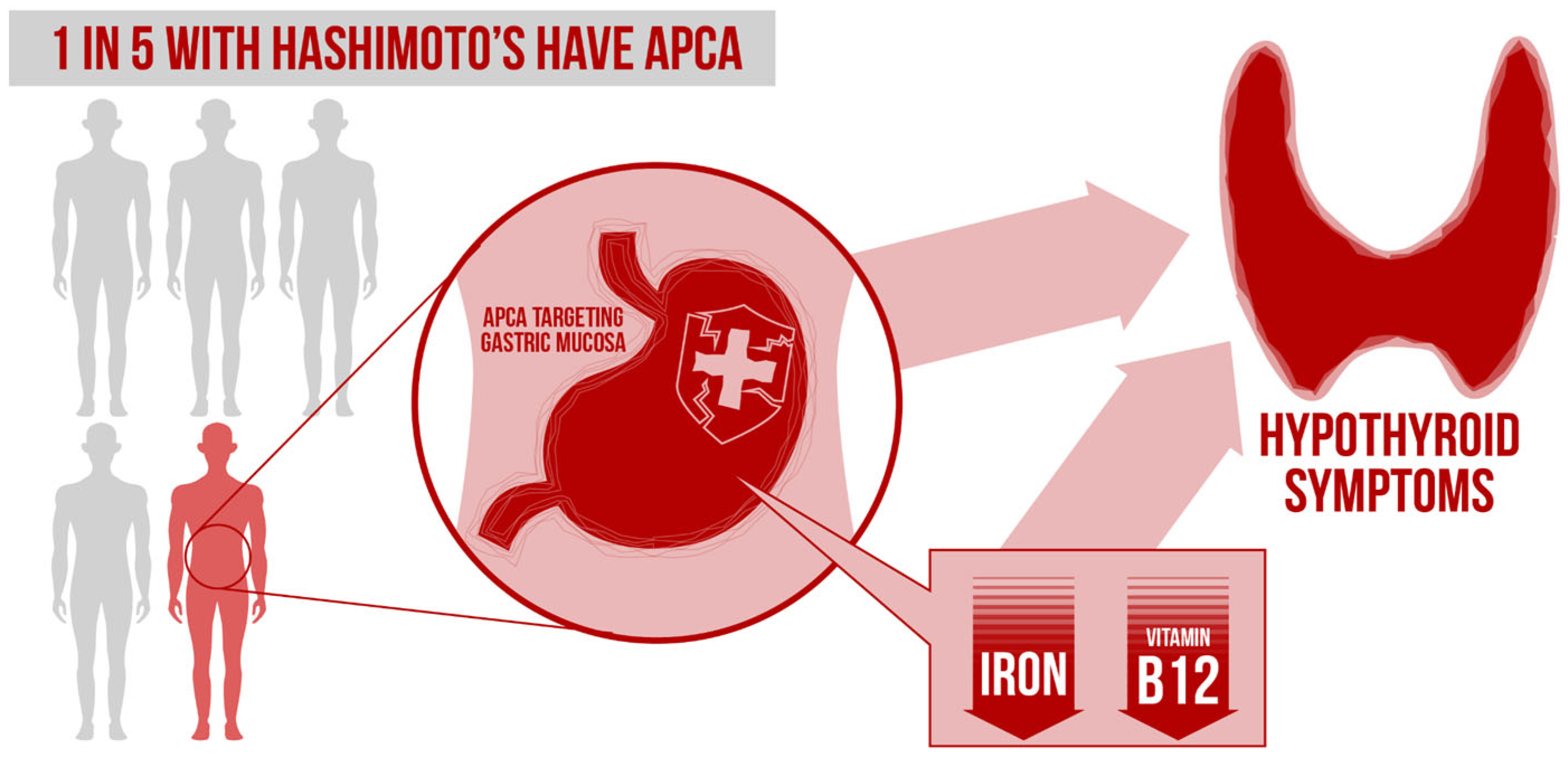
2.6. Iron
2.7. Vitamin B12
2.8. Zinc
2.9. Magnesium
3. Impact of GI Conditions on Status of Micronutrients Important for Thyroid Health
3.1. Gastritis
3.2. Irritable Bowel Syndrome (IBS) and SIBO
3.3. Inflammatory Bowel Disease (IBD)
3.4. Celiac Disease
3.5. Proton Pump Inhibitor (PPI) Use
3.6. Exocrine Pancreatic Insufficiency (EPI)
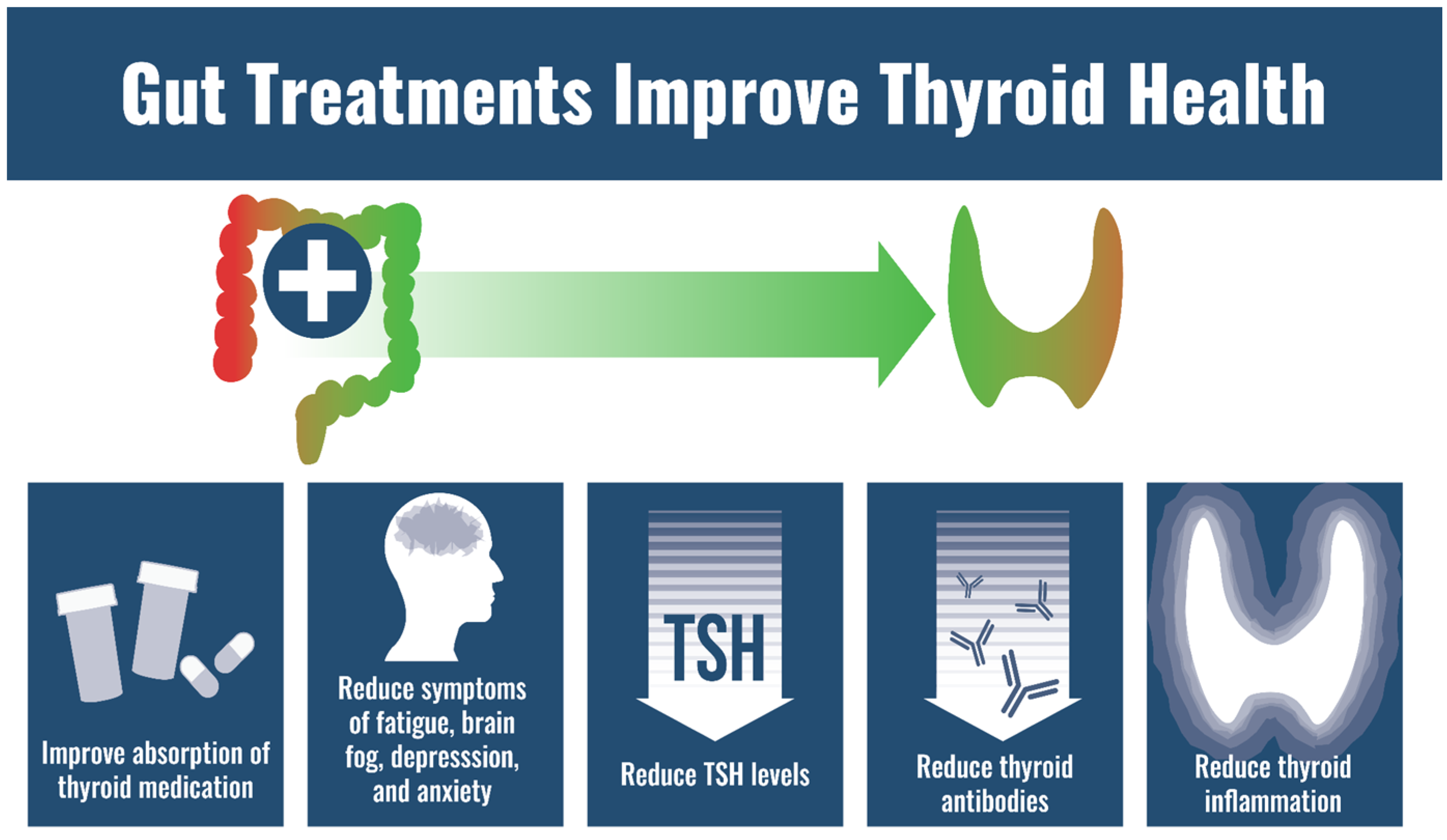
4. GI Therapies Can Improve Nutrient Absorption
4.1. Probiotics
4.2. Elemental Diet
4.3. Immunoglobulins
5. Are Symptoms Emanating from the GI Tract or Thyroid?
6. GI Care Can Resolve Symptoms Thought to Be from Thyroid Dysfunction
7. GI Dysfunction Is at Least 45 Times More Common Than Hypothyroidism
8. Hypothyroidism Is Incorrectly Diagnosed and Overdiagnosed
9. GI Therapies Improve Exogenous Thyroid Hormone Absorption
10. Impact of GI System on Autoimmunity and Thyroid Autoimmunity
11. Conclusions
- -
- GI conditions can lower thyroid-specific nutrients.
- -
- GI care can improve status of thyroid-specific nutrients.
- -
- GI conditions are much more common than hypothyroidism.
- -
- GI care can resolve symptoms thought to be from “thyroid dysfunction”.
- -
- GI health can affect thyroid autoimmunity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saravanan, P.; Chau, W.F.; Roberts, N.; Vedhara, K.; Greenwood, R.; Dayan, C.M. Psychological well-being in patients on “adequate” doses of l-thyroxine: Results of a large, controlled community-based questionnaire study. Clin. Endocrinol. 2002, 57, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J. Persistent hypothyroid symptoms in a patient with a normal thyroid stimulating hormone level. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.J.; Cappola, A.R.; Castro, M.R.; Dayan, C.M.; Farwell, A.P.; Hennessey, J.V.; Kopp, P.A.; Ross, D.S.; Samuels, M.H.; Sawka, A.M.; et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid 2018, 28, 707–721. [Google Scholar] [CrossRef]
- Wouters, H.J.C.M.; Slagter, S.N.; Muller Kobold, A.C.; van der Klauw, M.M.; Wolffenbuttel, B.H.R. Epidemiology of thyroid disorders in the Lifelines Cohort Study (The Netherlands). PLoS ONE 2020, 15, e0242795. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Wert, Y.; Cheriyath, P.; Joshi, R. Effects of Long-Term Combination LT4 and LT3 Therapy for Improving Hypothyroidism and Overall Quality of Life. South Med. J. 2018, 111, 363–369. [Google Scholar] [CrossRef]
- Brechmann, T.; Sperlbaum, A.; Schmiegel, W. Levothyroxine therapy and impaired clearance are the strongest contributors to small intestinal bacterial overgrowth: Results of a retrospective cohort study. World J. Gastroenterol. 2017, 23, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Konrad, P.; Chojnacki, J.; Kaczka, A.; Pawłowicz, M.; Rudnicki, C.; Chojnacki, C. Thyroid dysfunction in patients with small intestinal bacterial overgrowth. Pol. Merkur. Lek. 2018, 44, 15–18. [Google Scholar]
- Talebi, S.; Karimifar, M.; Heidari, Z.; Mohammadi, H.; Askari, G. The effects of synbiotic supplementation on thyroid function and inflammation in hypothyroid patients: A randomized, double-blind, placebo-controlled trial. Complementary Ther. Med. 2020, 48, 102234. [Google Scholar] [CrossRef]
- Lowe, J.R.; Briggs, A.M.; Whittle, S.; Stephenson, M.D. A systematic review of the effects of probiotic administration in inflammatory arthritis. Complement Ther. Clin. Pract. 2020, 40, 101207. [Google Scholar] [CrossRef]
- Mohammed, A.T.; Khattab, M.; Ahmed, A.M.; Turk, T.; Sakr, N.; MKhalil, A.; Abdelhalim, M.; Sawaf, B.; Hirayama, K.; Huy, N.T. The therapeutic effect of probiotics on rheumatoid arthritis: A systematic review and meta-analysis of randomized control trials. Clin. Rheumatol. 2017, 36, 2697–2707. [Google Scholar] [CrossRef]
- García-Collinot, G.; Madrigal-Santillán, E.O.; Martínez-Bencomo, M.A.; Carranza-Muleiro, R.A.; Jara, L.J.; Vera-Lastra, O.; Cruz-Domínguez, M.P. Effectiveness of Saccharomyces boulardii and Metronidazole for Small Intestinal Bacterial Overgrowth in Systemic Sclerosis. Dig. Dis. Sci. 2020, 65, 1134–1143. [Google Scholar] [CrossRef]
- Leventogiannis, K.; Gkolfakis, P.; Spithakis, G.; Tsatali, A.; Pistiki, A.; Sioulas, A.; Triantafyllou, K. Effect of a Preparation of Four Probiotics on Symptoms of Patients with Irritable Bowel Syndrome: Association with Intestinal Bacterial Overgrowth. Probiotics Antimicrob. Proteins 2019, 11, 627–634. [Google Scholar] [CrossRef]
- Küçükemre Aydın, B.; Yıldız, M.; Akgün, A.; Topal, N.; Adal, E.; Önal, H. Children with Hashimoto’s Thyroiditis Have Increased Intestinal Permeability: Results of a Pilot Study. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef]
- Bjarnason, I.; So, A.; Levi, A.J.; Peters, T.; Williams, P.; Zanelli, G.; Ansell, B. Intestinal permeability and inflammation in rheumatoid arthritis: Effects of non-steroidal anti-inflammatory drugs. Lancet 1984, 2, 1171–1174. [Google Scholar] [CrossRef]
- Goebel, A.; Buhner, S.; Schedel, R.; Lochs, H.; Sprotte, G. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology 2008, 47, 1223–1227. [Google Scholar] [CrossRef]
- Zheng, D.; Liao, H.; Chen, S.; Liu, X.; Mao, C.; Zhang, C.; Chen, Y. Elevated levels of circulating biomarkers related to leaky gut syndrome and bacterial translocation are associated with graves’ disease. Front. Endocrinol. 2021, 12, 796212. [Google Scholar] [CrossRef]
- Youssefi, M.; Tafaghodi, M.; Farsiani, H.; Ghazvini, K.; Keikha, M. Helicobacter pylori infection and autoimmune diseases; Is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. J. Microbiol. Immunol. Infect. 2021, 54, 359–369. [Google Scholar] [CrossRef]
- Boutzios, G.; Koukoulioti, E.; Goules, A.V.; Kalliakmanis, I.; Giovannopoulos, I.; Vlachoyiannopoulos, P.; Tzioufas, A.G. Hashimoto Thyroiditis, Anti-Parietal Cell Antibodies: Associations With Autoimmune Diseases and Malignancies. Front. Endocrinol. 2022, 13, 860880. [Google Scholar] [CrossRef]
- Jabbar, A.; Yawar, A.; Waseem, S.; Islam, N.; Ul Haque, N.; Zuberi, L.; Akhter, J. Vitamin B12 deficiency common in primary hypothyroidism. J. Pak. Med. Assoc. 2008, 58, 258–261. [Google Scholar] [PubMed]
- Foley, T.P. The relationship between autoimmune thyroid disease and iodine intake: A review. Endokrynol. Pol. 1992, 43 (Suppl. S1), 53–69. [Google Scholar] [PubMed]
- Zhang, Y.; Sun, Y.; He, Z.; Xu, S.; Liu, C.; Li, Y.; Teng, W. Age-specific thyrotropin references decrease over-diagnosis of hypothyroidism in elderly patients in iodine-excessive areas. Clin. Endocrinol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Yuan, X.; Kobayashi, S.; Sasaki, S. Effect of excess iodine intake on thyroid diseases in different populations: A systematic review and meta-analyses including observational studies. PLoS ONE 2017, 12, e0173722. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, Q.; Wang, N.; Zhang, W.; Zhu, C.; Wang, Y.; Lu, Y. Are ethnic differences, urinary iodine status, lead and cadmium exposure associated with thyroid autoimmunity and hypothyroid status? A cross-sectional study. BMJ Open 2022, 12, e056909. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Choi, S.R.; Kim, D.M.; Kim, J.U.; Kim, K.W.; Ahn, C.W.; Huh, K.B. The effect of iodine restriction on thyroid function in patients with hypothyroidism due to Hashimoto’s thyroiditis. Yonsei Med. J. 2003, 44, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, J.; Higashi, K.; Morita, M.; Umeda, T.; Sato, T. Studies of hypothyroidism in patients with high iodine intake. J. Clin. Endocrinol. Metab. 1986, 63, 412–417. [Google Scholar] [CrossRef]
- Manousou, S.; Stål, M.; Larsson, C.; Mellberg, C.; Lindahl, B.; Eggertsen, R.; Nyström, H.F. A Paleolithic-type diet results in iodine deficiency: A 2-year randomized trial in postmenopausal obese women. Eur. J. Clin. Nutr. 2018, 72, 124–129. [Google Scholar] [CrossRef]
- Eveleigh, E.R.; Coneyworth, L.J.; Avery, A.; Welham, S.J.M. Vegans, vegetarians, and omnivores: How does dietary choice influence iodine intake? A systematic review. Nutrients 2020, 12, 1606. [Google Scholar] [CrossRef]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhu, M.; Li, L.; Fan, H.; Lv, F.; Zhu, D. Clinical Observation of Levothyroxine Sodium Combined with Selenium in the Treatment of Patients with Chronic Lymphocytic Thyroiditis and Hypothyroidism and the Effects on Thyroid Function, Mood, and Inflammatory Factors. Evid.-Based Complementary Altern. Med. 2021, 2021, 5471281. [Google Scholar] [CrossRef] [PubMed]
- Kryczyk-Kozioł, J.; Zagrodzki, P.; Prochownik, E.; Błażewska-Gruszczyk, A.; Słowiaczek, M.; Sun, Q.; Bartyzel, M. Positive effects of selenium supplementation in women with newly diagnosed Hashimoto’s thyroiditis in an area with low selenium status. Int. J. Clin. Pract. 2021, 75, e14484. [Google Scholar] [CrossRef]
- Qiu, Y.; Xing, Z.; Xiang, Q.; Yang, Q.; Zhu, J.; Su, A. Insufficient evidence to support the clinical efficacy of selenium supplementation for patients with chronic autoimmune thyroiditis. Endocrine 2021, 73, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Is selenium supplementation in autoimmune thyroid diseases justified? Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 348–355. [Google Scholar] [CrossRef]
- Duntas, L.H. The evolving role of selenium in the treatment of graves’ disease and ophthalmopathy. J. Thyroid Res. 2012, 2012, 736161. [Google Scholar] [CrossRef]
- Wertenbruch, T.; Willenberg, H.S.; Sagert, C.; Nguyen TB, T.; Bahlo, M.; Feldkamp, J.; Schott, M. Serum selenium levels in patients with remission and relapse of graves’ disease. Med. Chem. 2007, 3, 281–284. [Google Scholar] [CrossRef]
- Filipowicz, D.; Majewska, K.; Kalantarova, A.; Szczepanek-Parulska, E.; Ruchała, M. The rationale for selenium supplementation in patients with autoimmune thyroiditis, according to the current state of knowledge. Endokrynol. Pol. 2021, 72, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Pirola, I.; Rotondi, M.; Cristiano, A.; Maffezzoni, F.; Pasquali, D.; Marini, F.; Cappelli, C. Selenium supplementation in patients with subclinical hypothyroidism affected by autoimmune thyroiditis: Results of the SETI study. Endocrinol. Diabetes Nutr. 2020, 67, 28–35. [Google Scholar] [CrossRef]
- Pace, C.; Tumino, D.; Russo, M.; Le Moli, R.; Naselli, A.; Borzì, G.; Frasca, F. Role of selenium and myo-inositol supplementation on autoimmune thyroiditis progression. Endocr. J. 2020, 67, 1093–1098. [Google Scholar] [CrossRef]
- Nordio, M.; Basciani, S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto’s patients with subclinical hypothyroidism. Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (Suppl. S2), 51–59. [Google Scholar]
- Nordio, M.; Pajalich, R. Combined treatment with Myo-inositol and selenium ensures euthyroidism in subclinical hypothyroidism patients with autoimmune thyroiditis. J. Thyroid Res. 2013, 2013, 424163. [Google Scholar] [CrossRef]
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Saxena, A.; Tonse, R.; Veledar, E.; McGranaghan, P. Association between vitamin D deficiency and hypothyroidism: Results from the National Health and Nutrition Examination Survey (NHANES) 2007–2012. BMC Endocr. Disord. 2021, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, N.C.; Karbek, B.; Ucan, B.; Sahin, M.; Cakal, E.; Ozbek, M.; Delibasi, T. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr. Pract. 2013, 19, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211060675. [Google Scholar] [CrossRef] [PubMed]
- Iron Deficiency May Explain Persistent Hypothyroidism Symptoms|MDedge Endocrinology. Available online: https://www.mdedge.com/endocrinology/article/104350/pituitary-thyroid-adrenal-disorders/iron-deficiency-may-explain (accessed on 1 July 2021).
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, X.; Yuan, L.; Guo, L. Iron Deficiency, a Risk Factor of Thyroid Disorders in Reproductive-Age and Pregnant Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 629831. [Google Scholar] [CrossRef]
- Aktaş, H.Ş. Vitamin B12 and Vitamin D Levels in Patients with Autoimmune Hypothyroidism and Their Correlation with Anti-Thyroid Peroxidase Antibodies. Med. Princ. Pract. 2020, 29, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Kacharava, T.; Giorgadze, E.; Janjgava, S.; Lomtadze, N.; Iamze, T. Correlation between Vitamin B12 Deficiency and Autoimmune Thyroid Diseases. Endocr. Metab. Immune Disord. Drug Targets 2022. [Google Scholar] [CrossRef]
- Talebi, S.; Ghaedi, E.; Sadeghi, E.; Mohammadi, H.; Hadi, A.; Clark, C.C.; Askari, G. Trace Element Status and Hypothyroidism: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2020, 197, 1–14. [Google Scholar] [CrossRef]
- Ertek, S.; Cicero, A.F.; Caglar, O.; Erdogan, G. Relationship between serum zinc levels, thyroid hormones and thyroid volume following successful iodine supplementation. Hormones 2010, 9, 263–268. [Google Scholar] [CrossRef]
- Szczepanik, J.; Podgórski, T.; Domaszewska, K. The Level of Zinc, Copper and Antioxidant Status in the Blood Serum of Women with Hashimoto’s Thyroiditis. Int. J. Environ. Res. Public Health 2021, 18, 7805. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Banach, M. Effect of magnesium supplements on serum C-reactive protein: A systematic review and meta-analysis. Arch. Med. Sci. 2018, 14, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wei, H.; Zhang, W.; Li, Z.; Ding, L.; Yu, T.; Zhu, M. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study. Sci. Rep. 2018, 8, 9904. [Google Scholar] [CrossRef] [PubMed]
- Cox, I.M.; Campbell, M.J.; Dowson, D. Red blood cell magnesium and chronic fatigue syndrome. Lancet 1991, 337, 757–760. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Brenner, D.M. Efficacy and Safety of Over-the-Counter Therapies for Chronic Constipation: An Updated Systematic Review. Am. J. Gastroenterol. 2021, 116, 1156–1181. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Veasey, R.C.; Watson, A.W.; Dodd, F.L.; Jones, E.K.; Tiplady, B.; Haskell, C.F. Vitamins and psychological functioning: A mobile phone assessment of the effects of a B vitamin complex, vitamin C and minerals on cognitive performance and subjective mood and energy. Hum. Psychopharmacol. 2011, 26, 338–347. [Google Scholar] [CrossRef]
- Moncayo, R.; Moncayo, H. Proof of concept of the WOMED model of benign thyroid disease: Restitution of thyroid morphology after correction of physical and psychological stressors and magnesium supplementation. BBA Clin. 2015, 3, 113–122. [Google Scholar] [CrossRef]
- Carabotti, M.; Lahner, E.; Esposito, G.; Sacchi, M.C.; Severi, C.; Annibale, B. Upper gastrointestinal symptoms in autoimmune gastritis: A cross-sectional study. Medicine 2017, 96, e5784. [Google Scholar] [CrossRef]
- Sipponen, P.; Maaroos, H.-I. Chronic gastritis. Scand. J. Gastroenterol. 2015, 50, 657–667. [Google Scholar] [CrossRef]
- Khayyat, Y.; Attar, S. Vitamin D Deficiency in Patients with Irritable Bowel Syndrome: Does it Exist? Oman Med. J. 2015, 30, 115–118. [Google Scholar] [CrossRef]
- Nwosu, B.U.; Maranda, L.; Candela, N. Vitamin D status in pediatric irritable bowel syndrome. PLoS ONE 2017, 12, e0172183. [Google Scholar] [CrossRef]
- Losurdo, G.; Salvatore D’Abramo, F.; Indellicati, G.; Lillo, C.; Ierardi, E.; Di Leo, A. The Influence of Small Intestinal Bacterial Overgrowth in Digestive and Extra-Intestinal Disorders. Int. J. Mol. Sci. 2020, 21, 3531. [Google Scholar] [CrossRef] [PubMed]
- Rezazadegan, M.; Soheilipour, M.; Tarrahi, M.J.; Amani, R. Correlation Between Zinc Nutritional Status with Serum Zonulin and Gastrointestinal Symptoms in Diarrhea-Predominant Irritable Bowel Syndrome: A Case-Control Study. Dig. Dis. Sci. 2022, 67, 3632–3638. [Google Scholar] [CrossRef] [PubMed]
- Melchior, C.; Algera, J.; Colomier, E.; Törnblom, H.; Simrén, M.; Störsrud, S. Food avoidance and restriction in irritable bowel syndrome: Relevance for symptoms, quality of life and nutrient intake. Clin. Gastroenterol. Hepatol. 2022, 20, 1290–1298.e4. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Bischoff, S.C. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Pappas, D.; Miglioretto, C.; Javadpour, A.; Reveley, H.; Frank, L.; Grimm, M.C.; Samocha-Bonet, D.; Hold, G.L. Systematic review with meta-analysis: Dietary intake in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021, 54, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, J.; Arai, K.; Kudo, T.; Nambu, R.; Tajiri, H.; Aomatsu, T.; Mizuochi, T. Serum Zinc and Selenium in Children with Inflammatory Bowel Disease: A Multicenter Study in Japan. Dig. Dis. Sci. 2022, 67, 2485–2491. [Google Scholar] [CrossRef]
- Delvecchio, M.; Bizzoco, F.; Lapolla, R.; Gentile, A.; Carrozza, C.; Barone, M.; Francavilla, R. Iodine absorption in celiac children: A longitudinal pilot study. Nutrients 2021, 13, 808. [Google Scholar] [CrossRef]
- Hutchinson, C.; Geissler, C.A.; Powell, J.J.; Bomford, A. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut 2007, 56, 1291–1295. [Google Scholar] [CrossRef]
- Ozutemiz, A.O.; Aydin, H.H.; Isler, M.; Celik, H.A.; Batur, Y. Effect of omeprazole on plasma zinc levels after oral zinc administration. Indian J. Gastroenterol. 2002, 21, 216–218. [Google Scholar]
- Allen, L.H. Vitamin B-12. Adv. Nutr. 2012, 3, 54–55. [Google Scholar] [CrossRef]
- Jalal, M.; Campbell, J.A.; Tesfaye, S.; Al-Mukhtar, A.; Hopper, A.D. Yield of testing for micronutrient deficiencies associated with pancreatic exocrine insufficiency in a clinical setting: An observational study. World J. Clin. Cases 2021, 9, 11320–11329. [Google Scholar] [CrossRef]
- Phillips, M.E.; Hopper, A.D.; Leeds, J.S.; Roberts, K.J.; McGeeney, L.; Duggan, S.N.; Kumar, R. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol. 2021, 8, e000643. [Google Scholar] [CrossRef]
- Barkhidarian, B.; Roldos, L.; Iskandar, M.M.; Saedisomeolia, A.; Kubow, S. Probiotic supplementation and micronutrient status in healthy subjects: A systematic review of clinical trials. Nutrients 2021, 13, 3001. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Molloy, A.; Scott, J.; Hussein, L. Plasma cobalamin and folate and their metabolic markers methylmalonic acid and total homocysteine among Egyptian children before and after nutritional supplementation with the probiotic bacteria Lactobacillus acidophilus in yoghurt matrix. Int. J. Food Sci. Nutr. 2006, 57, 470–480. [Google Scholar] [CrossRef]
- Sandroni, A.; House, E.; Howard, L.; DellaValle, D.M. Synbiotic Supplementation Improves Response to Iron Supplementation in Female Athletes during Training. J. Diet Suppl. 2022, 19, 366–380. [Google Scholar] [CrossRef]
- Tremblay, A.; Xu, X.; Colee, J.; Tompkins, T.A. Efficacy of a Multi-Strain Probiotic Formulation in Pediatric Populations: A Comprehensive Review of Clinical Studies. Nutrients 2021, 13, 1908. [Google Scholar] [CrossRef]
- Axling, U.; Önning, G.; Martinsson Niskanen, T.; Larsson, N.; Hansson, S.R.; Hulthén, L. The effect of Lactiplantibacillus plantarum 299v together with a low dose of iron on iron status in healthy pregnant women: A randomized clinical trial. Acta Obstet. Gynecol. Scand. 2021, 100, 1602–1610. [Google Scholar] [CrossRef]
- Wang, F.; Feng, J.; Chen, P.; Liu, X.; Ma, M.; Zhou, R.; Zhao, Q. Probiotics in Helicobacter pylori eradication therapy: Systematic review and network meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 466–475. [Google Scholar] [CrossRef]
- Penumetcha, S.S.; Ahluwalia, S.; Irfan, R.; Khan, S.A.; Reddy, S.R.; Lopez ME, V.; Mohammed, L. The efficacy of probiotics in the management of helicobacter pylori: A systematic review. Cureus 2021, 13, e20483. [Google Scholar] [CrossRef]
- Ferreiro, B.; Llopis-Salinero, S.; Lardies, B.; Granados-Colomina, C.; Milà-Villarroel, R. Clinical and Nutritional Impact of a Semi-Elemental Hydrolyzed Whey Protein Diet in Patients with Active Crohn’s Disease: A Prospective Observational Study. Nutrients 2021, 13, 3623. [Google Scholar] [CrossRef] [PubMed]
- Teahon, K.; Pearson, M.; Smith, T.; Bjarnason, I. Alterations in nutritional status and disease activity during treatment of Crohn’s disease with elemental diet. Scand. J. Gastroenterol. 1995, 30, 54–60. [Google Scholar] [CrossRef]
- Wilson, D.; Evans, M.; Weaver, E.; Shaw, A.L.; Klein, G.L. Evaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndrome. Clin. Med. Insights Gastroenterol. 2013, 6, 49–60. [Google Scholar] [CrossRef]
- Petschow, B.W.; Burnett, B.; Shaw, A.L.; Weaver, E.M.; Klein, G.L. Serum-derived bovine immunoglobulin/protein isolate: Postulated mechanism of action for management of enteropathy. Clin. Exp. Gastroenterol. 2014, 7, 181–190. [Google Scholar] [CrossRef]
- Ruscio, M.; Guard, G.; Mather, J. Symptoms Originally Attributed to Thyroid Dysfunction Were Instead Caused by Suboptimal Gastrointestinal Health: A Case Series and Literature Review. Integr. Med. Clin. J. 2022, 21, 22–29. [Google Scholar]
- Carlé, A.; Pedersen, I.B.; Knudsen, N.; Perrild, H.; Ovesen, L.; Laurberg, P. Hypothyroid symptoms and the likelihood of overt thyroid failure: A population-based case-control study. Eur. J. Endocrinol. 2014, 171, 593–602. [Google Scholar] [CrossRef]
- Siegmann, E.-M.; Müller, H.H.O.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Grömer, T.W. Association of Depression and Anxiety Disorders With Autoimmune Thyroiditis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Piche, T.; Huet, P.M.; Gelsi, E.; Barjoan, E.M.; Cherick, F.; Caroli-Bosc, F.X.; Tran, A. Fatigue in irritable bowel syndrome: Characterization and putative role of leptin. Eur. J. Gastroenterol. Hepatol. 2007, 19, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Schwille-Kiuntke, J.; Ittermann, T.; Schmidt, C.O.; Grabe, H.J.; Lerch, M.M.; Völzke, H.; Schauer, B. Quality of life and sleep in individuals with irritable bowel syndrome according to different diagnostic criteria and inflammatory bowel diseases: A comparison using data from a population-based survey. Z. Für Gastroenterol. 2022, 60, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Moustafa, H.A.; Nour-Eldein, H.; Saudi, R.A. Association of anxiety-depressive disorders with irritable bowel syndrome among patients attending a rural family practice center: A comparative cross-sectional study. Gen. Psych. 2021, 34, e100553. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Kovács, F. Depressive and anxiety symptoms, coping strategies in patients with irritable bowel syndrome and inflammatory bowel disease. Psychiatr. Hung. 2007, 22, 212–221. [Google Scholar] [PubMed]
- Jerndal, P.; Ringström, G.; Agerforz, P.; Karpefors, M.; Akkermans, L.M.; Bayati, A.; Simrén, M. Gastrointestinal-specific anxiety: An important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol. Motil. 2010, 22, 646-e179. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, J.; Sjödin, I.; Dotevall, G.; Gillberg, R. Upper gastrointestinal and mental symptoms in the irritable bowel syndrome. Scand. J. Gastroenterol. 1985, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Duan, R.; Duan, L. Prevalence of sleep disorder in irritable bowel syndrome: A systematic review with meta-analysis. Saudi. J. Gastroenterol. 2018, 24, 141–150. [Google Scholar] [CrossRef]
- Grover, M.; Kanazawa, M.; Palsson, O.S.; Chitkara, D.K.; Gangarosa, L.M.; Drossman, D.A.; Whitehead, W.E. Small intestinal bacterial overgrowth in irritable bowel syndrome: Association with colon motility, bowel symptoms, and psychological distress. Neurogastroenterol. Motil. 2008, 20, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, T.; Nijeboer, P.; IJssennagger, C.E.; Sanders, D.S.; Mulder, C.J.; Bouma, G. Prevalence And Characterization Of Self-Reported Gluten Sensitivity In The Netherlands. Nutrients 2016, 8, 714. [Google Scholar] [CrossRef]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R.; Study Group for Non-Celiac Gluten Sensitivity. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef]
- Losurdo, G.; Principi, M.; Iannone, A.; Amoruso, A.; Ierardi, E.; Di Leo, A.; Barone, M. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World J. Gastroenterol. 2018, 24, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Biesiekierski, J.R.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised clinical trial: Gluten may cause depression in subjects with non-coeliac gluten sensitivity—An exploratory clinical study. Aliment. Pharmacol. Ther. 2014, 39, 1104–1112. [Google Scholar] [CrossRef]
- Vergnat, M.; Suzanne, J.; Entraygues, H.; Laurent, R.; Gisselbrecht, H.; Agache, P. Cutaneous manifestations of malabsorption diseases (author’s transl). Ann. Dermatol. Venereol. 1978, 105, 1009–1016. [Google Scholar] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Prospero, L.; Riezzo, G.; Linsalata, M.; Orlando, A.; D’Attoma, B.; Russo, F. Psychological and Gastrointestinal Symptoms of Patients with Irritable Bowel Syndrome Undergoing a Low-FODMAP Diet: The Role of the Intestinal Barrier. Nutrients 2021, 13, 2469. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wei, J.; Ju, P.; Chen, J. Effects of regulating intestinal microbiota on anxiety symptoms: A systematic review. Gen Psych. 2019, 32, e100056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marum, A.P.; Moreira, C.; Saraiva, F.; Tomas-Carus, P.; Sousa-Guerreiro, C. A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients. Scand. J. Pain. 2016, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.-S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.K.; Liu, Y.-W.; Kuo, P.-H.; Chung, Y.-C.E.; Lu, M.-L.; Chen, C.-H. Effect of probiotics on depressive symptoms: A meta-analysis of human studies. Psychiatry Res. 2019, 282, 112568. [Google Scholar] [CrossRef]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.C.; Hor, Y.Y.; Yusoff NA, A.; Choi, S.B.; Yusoff, M.S.; Roslan, N.S.; Liong, M.T. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Chung, Y.C. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhou, L.; Wang, Q.; Zheng, G.; Su, S. Effect of Compound Lactic Acid Bacteria Capsules on the Small Intestinal Bacterial Overgrowth in Patients with Depression and Diabetes: A Blinded Randomized Controlled Clinical Trial. Dis. Markers 2022, 2022, 6721695. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, W.E.; Vlieg–Boerstra, B.; Warners, M.J.; Van Ampting, M.T.; van Esch, B.C.; Eussen, S.R.; Bredenoord, A.J. Effect of amino acid-based formula added to four-food elimination in adult eosinophilic esophagitis patients: A randomized clinical trial. Neurogastroenterol. Motil. 2022, 34, e14291. [Google Scholar] [CrossRef]
- McMurdy, J.M. High calorie elemental diet improves outcomes and quality of life for tube FED adolescents. J. Am. Diet Assoc. 1999, 99, A128. [Google Scholar] [CrossRef]
- Flamm, S.L.; Mullen, K.D.; Heimanson, Z.; Sanyal, A.J. Rifaximin has the potential to prevent complications of cirrhosis. Ther. Adv. Gastroenterol. 2018, 11, 1756284818800307. [Google Scholar] [CrossRef] [PubMed]
- Flamm, S.L. Rifaximin treatment for reduction of risk of overt hepatic encephalopathy recurrence. Ther. Adv. Gastroenterol. 2011, 4, 199–206. [Google Scholar] [CrossRef]
- Caraceni, P.; Vargas, V.; Solà, E.; Alessandria, C.; de Wit, K.; Trebicka, J.; Watson, H. The use of rifaximin in patients with cirrhosis. Hepatology 2021, 74, 1660–1673. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Leunis, J.-C. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: Effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuroendocrinol. Lett. 2008, 29, 902–910. [Google Scholar] [PubMed]
- El-Salhy, M.; Winkel, R.; Casen, C.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Efficacy of fecal microbiota transplantation for patients with irritable bowel syndrome at three years after transplantation. Gastroenterology 2022, 6, ofz398. [Google Scholar] [CrossRef]
- Enko, D.; Meinitzer, A.; Mangge, H.; Kriegshäuser, G.; Halwachs-Baumann, G.; Reininghaus, E.Z.; Schnedl, W.J. Concomitant prevalence of low serum diamine oxidase activity and carbohydrate malabsorption. Can. J. Gastroenterol. Hepatol. 2016, 2016, 4893501. [Google Scholar] [CrossRef] [PubMed]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Aghini-Lombardi, F.; Antonangeli, L.; Martino, E.; Vitti, P.; Maccherini, D.; Leoli, F.; Pinchera, A. The spectrum of thyroid disorders in an iodine-deficient community: The Pescopagano survey. J. Clin. Endocrinol. Metab. 1999, 84, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Thyroid Profile of the Reference United States Population: Data from NHANES 2007–2012. Int. Arch. Endocrinol. Clin. Res. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef]
- Talley, N.J.; Zinsmeister, A.R.; Van Dyke, C.; Melton, L.J. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology 1991, 101, 927–934. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef]
- Thompson, W.G.; Irvine, E.J.; Pare, P.; Ferrazzi, S.; Rance, L. Functional gastrointestinal disorders in Canada: First population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig. Dis. Sci. 2002, 47, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef]
- Livadas, S.; Bothou, C.; Androulakis, I.; Boniakos, A.; Angelopoulos, N.; Duntas, L. Levothyroxine replacement therapy and overuse: A timely diagnostic approach. Thyroid 2018, 28, 1580–1586. [Google Scholar] [CrossRef]
- Ross, D.S. Treating hypothyroidism is not always easy: When to treat subclinical hypothyroidism, TSH goals in the elderly, and alternatives to levothyroxine monotherapy. J. Intern. Med. 2022, 291, 128–140. [Google Scholar] [CrossRef]
- Abu-Helalah, M.; Alshraideh, H.A.; Al-Sarayreh, S.A.; Al-Hader, A. Transient high thyroid stimulating hormone and hypothyroidism incidence during follow up of subclinical hypothyroidism. Endocr. Regul. 2021, 55, 204–214. [Google Scholar] [CrossRef]
- Meyerovitch, J.; Rotman-Pikielny, P.; Sherf, M.; Battat, E.; Levy, Y.; Surks, M.I. Serum thyrotropin measurements in the community: Five-year follow-up in a large network of primary care physicians. Arch. Intern. Med. 2007, 167, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Wang, Y.; Xiao, L.; Li, L. Effect of Levothyroxine on Older Patients With Subclinical Hypothyroidism: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 913749. [Google Scholar] [CrossRef]
- Hashimoto, K. Update on subclinical thyroid dysfunction. Endocr. J. 2022, 69, 725–738. [Google Scholar] [CrossRef]
- Lyko, C.; Blum, M.R.; Abolhassani, N.; Stuber, M.J.; Del Giovane, C.; Feller, M.; Rodondi, N. Thyroid antibodies and levothyroxine effects in subclinical hypothyroidism: A pooled analysis of two randomized controlled trials. J. Intern. Med. 2022. [Google Scholar] [CrossRef]
- Büchi, A.E.; Feller, M.; Netzer, S.; Blum, M.R.; Rodriguez, E.G.; Collet, T.H.; Aeberli, D. Bone geometry in older adults with subclinical hypothyroidism upon levothyroxine therapy: A nested study within a randomized placebo controlled trial. Bone 2022, 161, 116404. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R. Subclinical hypothyroidism in older individuals. Lancet Diabetes Endocrinol. 2022, 10, 129–141. [Google Scholar] [CrossRef]
- Burgos, N.; Toloza, F.J.; Singh Ospina, N.M.; Brito, J.P.; Salloum, R.G.; Hassett, L.C.; Maraka, S. Clinical Outcomes After Discontinuation of Thyroid Hormone Replacement: A Systematic Review and Meta-Analysis. Thyroid 2021, 31, 740–751. [Google Scholar] [CrossRef]
- Virili, C.; Brusca, N.; Capriello, S.; Centanni, M. Levothyroxine therapy in gastric malabsorptive disorders. Front. Endocrinol. 2020, 11, 621616. [Google Scholar] [CrossRef]
- Ribichini, D.; Fiorini, G.; Repaci, A.; Castelli, V.; Gatta, L.; Vaira, D.; Pasquali, R. Tablet and oral liquid L-thyroxine formulation in the treatment of naïve hypothyroid patients with Helicobacter pylori infection. Endocrine 2017, 57, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Bugdaci, M.S.; Zuhur, S.S.; Sokmen, M.; Toksoy, B.; Bayraktar, B.; Altuntas, Y. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter 2011, 16, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Centanni, M.; Gargano, L.; Canettieri, G.; Viceconti, N.; Franchi, A.; Fave, G.D.; Annibale, B. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. New Engl. J. Med. 2006, 354, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Bohinc Henderson, B. Levothyroxine Sodium Oral Solution Normalizes Thyroid Function in a Patient with Hashimoto’s Disease, Gastritis, Diabetic Gastroparesis, and Small Intestinal Bacterial Overgrowth (SIBO). Int. Med. Case Rep. J. 2021, 14, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Asik, M.; Gunes, F.; Binnetoglu, E.; Eroglu, M.; Bozkurt, N.; Sen, H.; Ukinc, K. Decrease in TSH levels after lactose restriction in Hashimoto’s thyroiditis patients with lactose intolerance. Endocrine 2014, 46, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Bassotti, G.; Santaguida, M.G.; Iuorio, R.; Del Duca, S.C.; Mercuri, V.; Centanni, M. Atypical celiac disease as cause of increased need for thyroxine: A systematic study. J. Clin. Endocrinol. Metab. 2012, 97, E419–E422. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Stramazzo, I.; Centanni, M. Gut microbiome and thyroid autoimmunity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101506. [Google Scholar] [CrossRef]
- Zangiabadian, M.; Mirsaeidi, M.; Pooyafar, M.H.; Goudarzi, M.; Nasiri, M.J. Associations of Yersinia Enterocolitica Infection with Autoimmune Thyroid Diseases: A Systematic Review and Meta-Analysis. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 682–687. [Google Scholar] [CrossRef]
- Wenzel, B.E.; Heesemann, J.; Wenzel, K.W.; Scriba, P.C. Patients with autoimmune thyroid diseases have antibodies to plasmid encoded proteins of enteropathogenic Yersinia. J. Endocrinol. Investig. 1988, 11, 139–140. [Google Scholar] [CrossRef]
- Asari, S.; Amino, N.; Horikawa, M.; Miyai, K. Incidences of antibodies to Yersinia enterocolitica: High incidence of serotype O5 in autoimmune thyroid diseases in Japan. Endocrinol. Jpn. 1989, 36, 381–386. [Google Scholar] [CrossRef]
- Polkowska-Pruszyńska, B.; Gerkowicz, A.; Szczepanik-Kułak, P.; Krasowska, D. Small intestinal bacterial overgrowth in systemic sclerosis: A review of the literature. Arch. Dermatol. Res. 2019, 311, 1–8. [Google Scholar] [CrossRef]
- Bertalot, G.; Montresor, G.; Tampieri, M.; Spasiano, A.; Pedroni, M.; Milanesi, B.; Negrini, R. Decrease in thyroid autoantibodies after eradication of Helicobacter pylori infection. Clin. Endocrinol. 2004, 61, 650–652. [Google Scholar] [CrossRef]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; Vaghef-Mehrabany, L.; Asghari-Jafarabadi, M.; Mohtadi-Nia, J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: A randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar] [CrossRef]
- Zamani, B.; Golkar, H.R.; Farshbaf, S.; Emadi-Baygi, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Int. J. Rheum. Dis. 2016, 19, 869–879. [Google Scholar] [CrossRef]
- Podas, T.; Nightingale, J.M.D.; Oldham, R.; Roy, S.; Sheehan, N.J.; Mayberry, J.F. Is rheumatoid arthritis a disease that starts in the intestine? A pilot study comparing an elemental diet with oral prednisolone. Postgrad. Med. J. 2007, 83, 128–131. [Google Scholar] [CrossRef]
- Huang, C.; Yi, P.; Zhu, M.; Zhou, W.; Zhang, B.; Yi, X.; Lu, Q. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: An EXPLORER trial. J. Autoimmun. 2022, 130, 102844. [Google Scholar] [CrossRef] [PubMed]
- Wartofsky, L. Combination L-T3 and L-T4 therapy for hypothyroidism. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 460–466. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruscio, M.; Guard, G.; Piedrahita, G.; D’Adamo, C.R. The Relationship between Gastrointestinal Health, Micronutrient Concentrations, and Autoimmunity: A Focus on the Thyroid. Nutrients 2022, 14, 3572. https://doi.org/10.3390/nu14173572
Ruscio M, Guard G, Piedrahita G, D’Adamo CR. The Relationship between Gastrointestinal Health, Micronutrient Concentrations, and Autoimmunity: A Focus on the Thyroid. Nutrients. 2022; 14(17):3572. https://doi.org/10.3390/nu14173572
Chicago/Turabian StyleRuscio, Michael, Gavin Guard, Gabriela Piedrahita, and Christopher R. D’Adamo. 2022. "The Relationship between Gastrointestinal Health, Micronutrient Concentrations, and Autoimmunity: A Focus on the Thyroid" Nutrients 14, no. 17: 3572. https://doi.org/10.3390/nu14173572
APA StyleRuscio, M., Guard, G., Piedrahita, G., & D’Adamo, C. R. (2022). The Relationship between Gastrointestinal Health, Micronutrient Concentrations, and Autoimmunity: A Focus on the Thyroid. Nutrients, 14(17), 3572. https://doi.org/10.3390/nu14173572





