Association between Serum Spermidine and TyG Index: Results from a Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
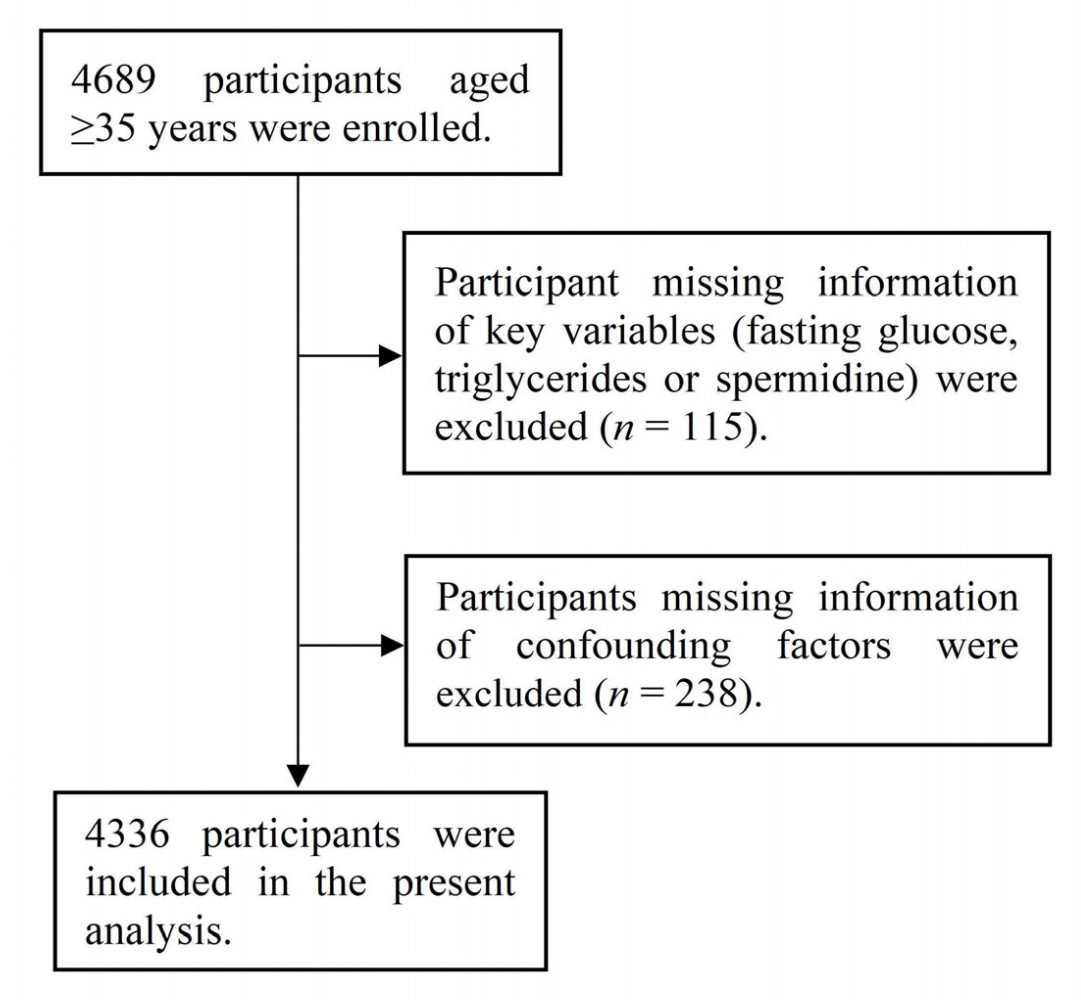
2.1. Study Population
2.2. Measurement of Serum SPD
2.3. Assessment and Definition of TyG Index
2.4. Assessment and Definition of Other Variables
2.5. Statistical Analysis
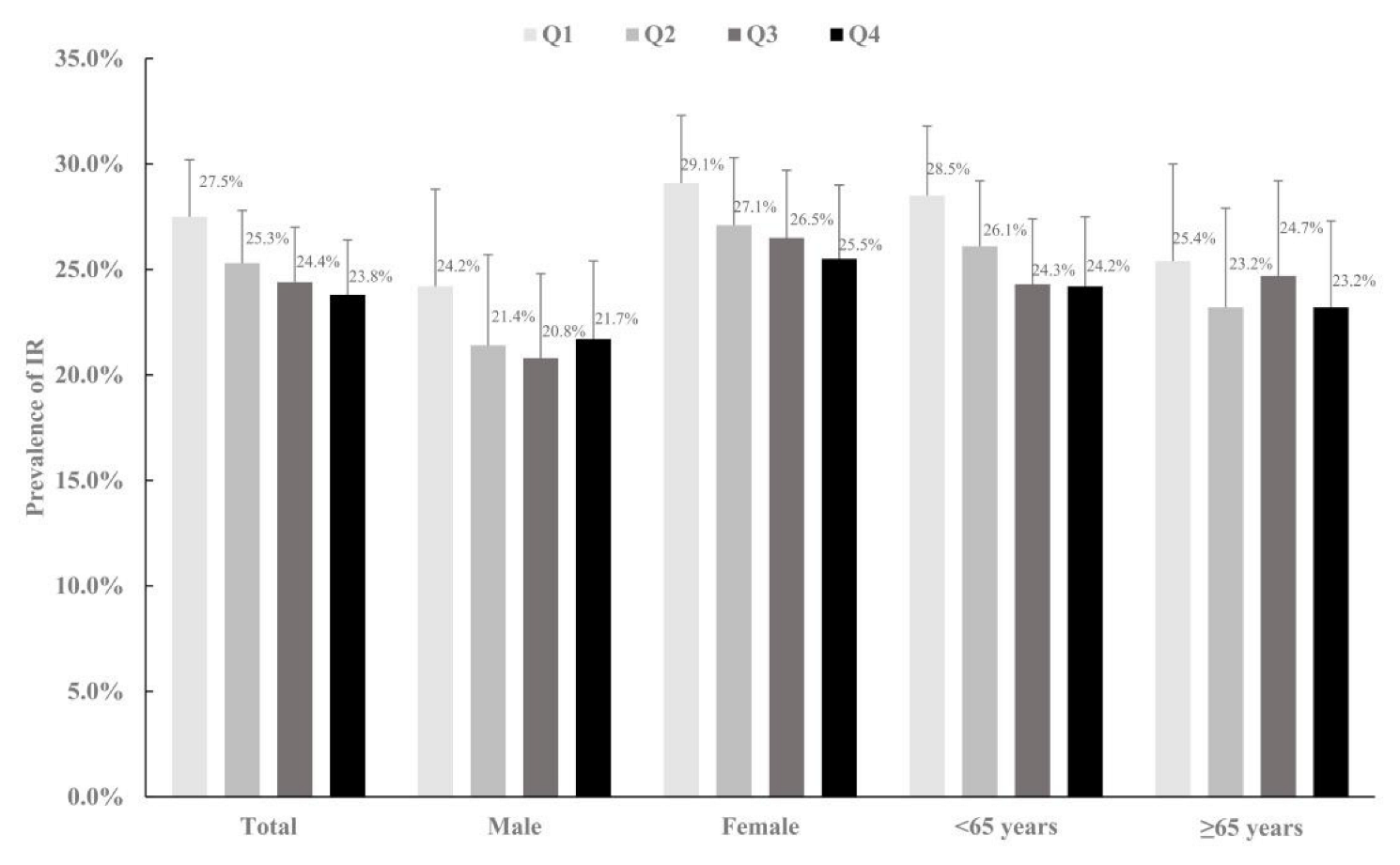
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed]
- Bardócz, S.; Duguid, T.J.; Brown, D.S.; Grant, G.; Pusztai, A.; White, A.; Ralph, A. The importance of dietary polyamines in cell regeneration and growth. Br. J. Nutr. 1995, 73, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamine. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef]
- Zheng, L.; Xie, Y.; Sun, Z.; Zhang, R.; Ma, Y.; Xu, J.; Zheng, J.; Xu, Q.; Li, Z.; Guo, X.; et al. Serum Spermidine in Relation to Risk of Stroke: A Multilevel Study. Front. Nutr. 2022, 9, 843616. [Google Scholar] [CrossRef]
- Els, T.; Bruckmann, J.; Röhn, G.; Daffertshofer, M.; Mönting, J.S.; Ernestus, R.-I.; Hennerici, M. Spermidine: A predictor for neurological outcome and infarct size in focal cerebral ischemia? Stroke 2001, 32, 43–46. [Google Scholar] [CrossRef]
- Méndez, J.D.; Balderas, F.L. Inhibition by L-arginine and spermidine of hemoglobin glycation and lipid peroxidation in rats with induced diabetes. Biomed. Pharmacother. 2006, 60, 26–31. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Q.; Xu, J.; Yuan, W.; Li, R.; Guo, H.; Gu, C.; Feng, W.; Ma, Y.; Sun, Z.; et al. Elevation of Serum Spermidine in Obese Patients: Results from a Cross-Sectional and Follow-Up Study. Nutrients 2022, 14, 2613. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-Wilhelmi, L.; Cardona, F.; Garrido-Sanchez, L.; Fernandez-Garcia, D.; Tinahones, F.J.; Ramos-Molina, B. Change in serum polyamine metabolome pattern after bariatric surgery in obese patients with metabolic syndrome. Surg. Obes. Relat. Dis. 2020, 16, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Yu, Z.; Jiao, Y.; Zhang, J.; Xu, Q.; Xu, J.; Li, R.; Yuan, W.; Guo, H.; Sun, Z.; Zheng, L. Effect of Serum Spermidine on the Prognosis in Patients with Acute Myocardial Infarction: A Cohort Study. Nutrients 2022, 14, 1394. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef]
- Wang, D.; Yin, J.; Zhou, Z.; Tao, Y.; Jia, Y.; Jie, H.; Zhao, J.; Li, R.; Li, Y.; Guo, C.; et al. Oral Spermidine Targets Brown Fat and Skeletal Muscle to Mitigate Diet-Induced Obesity and Metabolic Disorders. Mol. Nutr. Food Res. 2021, 65, e2100315. [Google Scholar] [CrossRef]
- Niiranen, K.; Keinänen, T.A.; Pirinen, E.; Heikkinen, S.; Tusa, M.; Fatrai, S.; Suppola, S.; Pietilä, M.; Uimari, A.; Laakso, M.; et al. Mice with targeted disruption of spermidine/spermine N1-acetyltransferase gene maintain nearly normal tissue polyamine homeostasis but show signs of insulin resistance upon aging. J. Cell. Mol. Med. 2006, 10, 933–945. [Google Scholar] [CrossRef]
- Böhm, A.; Halama, A.; Meile, T.; Zdichavsky, M.; Lehmann, R.; Weigert, C.; Fritsche, A.; Stefan, N.; Königsrainer, A.; Häring, H.-U.; et al. Metabolic Signatures of Cultured Human Adipocytes from Metabolically Healthy versus Unhealthy Obese Individuals. PLoS ONE 2014, 9, e93148. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Moraán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The Product of Fasting Glucose and Triglycerides as Surrogate for Identifying Insulin Resistance in Apparently Healthy Subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, J.; Zhang, Y.; Wu, L.; Yu, Z.; He, D.; Huang, H.; Qu, W.; Luo, X. Triglyceride glucose index influences platelet reactivity in acute ischemic stroke patients. BMC Neurol. 2021, 21, 409. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Petrie, J.; Littler, W.; de Swiet, M.; Padfield, P.L.; O’Malley, K.; Jamieson, M.; Altman, D.; Bland, M.; Atkins, N. The British hypertension society protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systems. J. Hypertens. 1990, 8, 607–619. [Google Scholar] [CrossRef]
- Ben Hassen, C.; Fayosse, A.; Landré, B.; Raggi, M.; Bloomberg, M.; Sabia, S.; Singh-Manoux, A. Association between age at onset of multimorbidity and incidence of dementia: 30 year follow-up in Whitehall II prospective cohort study. BMJ 2022, 376, e068005. [Google Scholar] [CrossRef]
- Wang, L.; Cong, H.-L.; Zhang, J.-X.; Hu, Y.-C.; Wei, A.; Zhang, Y.-Y.; Yang, H.; Ren, L.-B.; Qi, W.; Li, W.-Y.; et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc. Diabetol. 2020, 19, 80. [Google Scholar] [CrossRef]
- Sánchez-Escudero, V.; Lacalle, C.G.; Vergaz, A.G.; Mateo, L.R.; Cabrero, A.M. The triglyceride/glucose index as an insulin resistance marker in the pediatric population and its relation to eating habits and physical activity. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2021, 68, 296–303. [Google Scholar] [CrossRef]
- Pichiah, P.T.; Suriyakalaa, U.; Kamalakkannan, S.; Kokilavani, P.; Kalaiselvi, S.; SankarGanesh, D.; Gowri, J.; Archunan, G.; Cha, Y.-S.; Achiraman, S. Spermidine may decrease ER stress in pancreatic beta cells and may reduce apoptosis via activating AMPK dependent autophagy pathway. Med. Hypotheses 2011, 77, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Fetterman, J.L.; Holbrook, M.; Flint, N.; Feng, B.; Bretόn-Romero, R.; Linder, E.A.; Berk, B.D.; Duess, M.-A.; Farb, M.G.; Gokce, N.; et al. Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis 2016, 247, 207–217. [Google Scholar] [CrossRef]
| Characteristic | Total | Q1 | Q2 | Q3 | Q4 | p Values |
|---|---|---|---|---|---|---|
| (<13.56 ng/mL) | (13.56–25.17 ng/mL) | (25.17–50.49 ng/mL) | (≥50.49 ng/mL) | |||
| Female, n (%) | 2786 (64.3) | 740 (68.6) | 743 (68.2) | 699 (64.2) | 604 (56.0) | <0.001 |
| Age, y | 59.2 ± 9.9 | 59.2 ± 9.7 | 58.5 ± 9.8 | 59.0 ± 10.1 | 60.1 ± 10.1 | 0.001 |
| Ethnicity, n (%) | 0.196 | |||||
| Han ethnicity | 2840 (65.5) | 690 (63.9) | 697 (64.0) | 720 (66.1) | 733 (67.9) | |
| Mongolian | 1321 (30.5) | 350 (32.4) | 351 (32.2) | 320 (29.4) | 300 (27.8) | |
| Others | 175 (4.0) | 39 (3.6) | 41 (3.8) | 49 (4.5) | 46 (4.3) | |
| Current drinking, n (%) | 1306 (30.1) | 294 (27.2) | 308 (28.3) | 327 (30.0) | 377 (34.9) | <0.001 |
| Current smoking, n (%) | 1594 (36.8) | 380 (35.2) | 363 (33.3) | 401 (36.8) | 450 (41.7) | <0.001 |
| Physical activity, n (%) | 0.088 | |||||
| Low | 1403 (32.4) | 375 (34.8) | 337 (30.9) | 337 (30.9) | 254 (32.8) | |
| Middle | 2774 (64.0) | 675 (62.6) | 711 (65.3) | 714 (65.6) | 674 (62.5) | |
| High | 159 (3.7) | 29 (2.7) | 41 (3.8) | 38 (3.5) | 51 (4.7) | |
| Hypoglycemic drugs or insulin use, n (%) | 354 (8.2) | 104 (9.6) | 71 (6.5) | 87 (8.0) | 92 (8.5) | 0.063 |
| Hypolipidemic drugs use, n (%) | 175 (4.0) | 42 (3.9) | 53 (4.9) | 43 (3.9) | 37 (3.4) | 0.385 |
| Antihypertensive drugs use, n (%) | 855 (19.7) | 205 (19.0) | 236 (21.7) | 205 (18.8) | 209 (19.4) | 0.307 |
| BMI, kg/m2 | 24.7 ± 3.7 | 24.5 ± 3.8 | 24.5 ± 3.6 | 24.8 ± 3.7 | 25.2 ± 3.8 | <0.001 |
| Waistline, cm | 84.2 ± 10.0 | 83.2 ± 9.7 | 83.7 ± 10.1 | 84.2 ± 10.1 | 85.8 ± 9.7 | <0.001 |
| SBP, mmHg | 134.8 ± 21.5 | 134.0 ± 21.4 | 134.0 ± 21.7 | 134.8 ± 21.1 | 136.5 ± 21.7 | 0.025 |
| DBP, mmHg | 80.9 ± 11.2 | 80.3 ± 11.0 | 80.7 ± 11.1 | 81.1 ± 11.1 | 81.5 ± 11.6 | 0.067 |
| Fasting glucose, mmol/L | 6.0 ± 1.8 | 6.0 ± 2.2 | 5.9 ± 1.6 | 6.0 ± 1.8 | 5.9 ± 1.8 | 0.294 |
| TG, mmol/L | 1.6 ± 1.5 | 1.7 ± 1.9 | 1.7 ± 1.9 | 1.5 ± 1.0 | 1.5 ± 1.1 | <0.001 |
| SPD, median (IQR), ng/mL | 25.17 (13.56, 50.48) | 10.02 (8.24, 11.41) | 18.70 (15.95, 21.75) | 34.09 (28.80, 40.69) | 78.80 (61.97, 117.73) | |
| TyG | 8.7 ± 0.7 | 8.8 ± 0.7 | 8.7 ± 0.7 | 8.7 ± 0.6 | 8.7 ± 0.6 | 0.020 |
| Comordity, n (%) | 925 (21.3) | 223 (20.7) | 234 (21.5) | 226 (20.8) | 242 (22.4) | 0.732 |
| lnSPD | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β ± SE | p | β ± SE | p | |
| Total (n = 4336) | −0.015 ± 0.010 | 0.148 | −0.036 ± 0.009 | <0.001 |
| Male (n = 1550) | 0.005 ± 0.016 | 0.759 | −0.021 ± 0.015 | 0.160 |
| Female (n = 2786) | −0.027 ± 0.013 | 0.038 | −0.044 ± 0.011 | <0.001 |
| <65 years (n = 2902) | −0.032 ± 0.013 | 0.016 | −0.050 ± 0.012 | <0.001 |
| ≥65 years (n = 1434) | 0.023 ± 0.016 | 0.147 | −0.004 ± 0.014 | 0.772 |
| Excluding the population taking hypoglycemic drugs or insulin | ||||
| (n = 3982) | −0.013 ± 0.010 | 0.207 | −0.031 ± 0.009 | 0.001 |
| lnSPD | Q1 | Q2 | Q3 | Q4 | p Value for Trend | |
|---|---|---|---|---|---|---|
| Total, OR (95% CI) | ||||||
| Model 1 | 0.96 (0.90, 1.03) | 1.00 (Ref.) | 0.89 (0.74, 1.08) | 0.86 (0.71, 1.05) | 0.86 (0.70, 1.03) | 0.090 |
| Model 2 | 0.89 (0.83, 0.96) | 1.00 (Ref.) | 0.91 (0.73, 1.12) | 0.80 (0.65, 0.99) | 0.71 (0.57, 0.88) | 0.001 |
| Male, OR (95% CI) | ||||||
| Model 1 | 0.98 (0.87, 1.10) | 1.00 (Ref.) | 0.87 (0.62, 1.22) | 0.92 (0.65, 1.28) | 0.86 (0.61, 1.22) | 0.483 |
| Model 2 | 0.91 (0.80, 1.03) | 1.00 (Ref.) | 0.85 (0.59, 1.24) | 0.86 (0.60, 1.25) | 0.67 (0.46, 0.98) | 0.054 |
| Female, OR (95% CI) | ||||||
| Model 1 | 0.95 (0.87,1.04) | 1.00 (Ref.) | 1.02 (0.81, 1.29) | 0.87 (0.69, 1.11) | 0.86 (0.68, 1.10) | 0.123 |
| Model 2 | 0.89 (0.80, 0.98) | 1.00 (Ref.) | 0.99 (0.77, 1.28) | 0.81 (0.62, 1.05) | 0.73 (0.56, 0.95) | 0.007 |
| <65 years, OR (95% CI) | ||||||
| Model 1 | 0.94 (0.86, 1.03) | 1.00 (Ref.) | 0.95 (0.75, 1.19) | 0.78 (0.61, 0.98) | 0.81 (0.64, 1.03) | 0.029 |
| Model 2 | 0.88 (0.80, 0.97) | 1.00 (Ref.) | 0.97 (0.75, 1.24) | 0.75 (0.58, 0.97) | 0.71 (0.55, 0.92) | 0.002 |
| ≥65 years, OR (95% CI) | ||||||
| Model 1 | 1.03 (0.91, 1.16) | 1.00 (Ref.) | 0.88 (0.62, 1.25) | 1.03 (0.73, 1.45) | 1.04 (0.74, 1.48) | 0.628 |
| Model 2 | 0.93 (0.82, 1.07) | 1.00 (Ref.) | 0.86 (0.58, 1.26) | 0.91 (0.62, 1.33) | 0.79 (0.54, 1.16) | 0.299 |
| Excluding the population taking hypoglycemic drugs or insulin, OR (95% CI) | ||||||
| Model 1 | 0.94 (0.87,1.01) | 1.00 (Ref.) | 1.02 (0.82, 1.25) | 0.91 (0.73, 1.12) | 0.83 (0.67, 1.04) | 0.061 |
| Model 2 | 0.88 (0.81, 0.96) | 1.00 (Ref.) | 0.98 (0.79, 1.23) | 0.84 (0.67, 1.05) | 0.71 (0.56, 0.89) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Xu, J.; Li, R.; Yu, Z.; Yuan, W.; Gao, H.; Feng, W.; Gu, C.; Sun, Z.; Zheng, L. Association between Serum Spermidine and TyG Index: Results from a Cross-Sectional Study. Nutrients 2022, 14, 3847. https://doi.org/10.3390/nu14183847
Zhang R, Xu J, Li R, Yu Z, Yuan W, Gao H, Feng W, Gu C, Sun Z, Zheng L. Association between Serum Spermidine and TyG Index: Results from a Cross-Sectional Study. Nutrients. 2022; 14(18):3847. https://doi.org/10.3390/nu14183847
Chicago/Turabian StyleZhang, Rui, Jiahui Xu, Ruixue Li, Zhecong Yu, Wei Yuan, Hanshu Gao, Wenjing Feng, Cuiying Gu, Zhaoqing Sun, and Liqiang Zheng. 2022. "Association between Serum Spermidine and TyG Index: Results from a Cross-Sectional Study" Nutrients 14, no. 18: 3847. https://doi.org/10.3390/nu14183847
APA StyleZhang, R., Xu, J., Li, R., Yu, Z., Yuan, W., Gao, H., Feng, W., Gu, C., Sun, Z., & Zheng, L. (2022). Association between Serum Spermidine and TyG Index: Results from a Cross-Sectional Study. Nutrients, 14(18), 3847. https://doi.org/10.3390/nu14183847





