Nutritional Predictors of Mortality after 10 Years of Follow-Up in Patients with Chronic Kidney Disease at a Multidisciplinary Unit of Advanced Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Data Collection
2.3. Malnutrition-Inflammation Score
2.4. Anthropometric Measures and Body Composition Analysis
2.5. Laboratory Parameters
2.6. Statistical Analysis
3. Results
3.1. Global Data and Comparison between Groups
3.2. Anthropometric Measures, Body Composition Analysis, and Laboratory Data
3.3. Factors Predicting Mortality
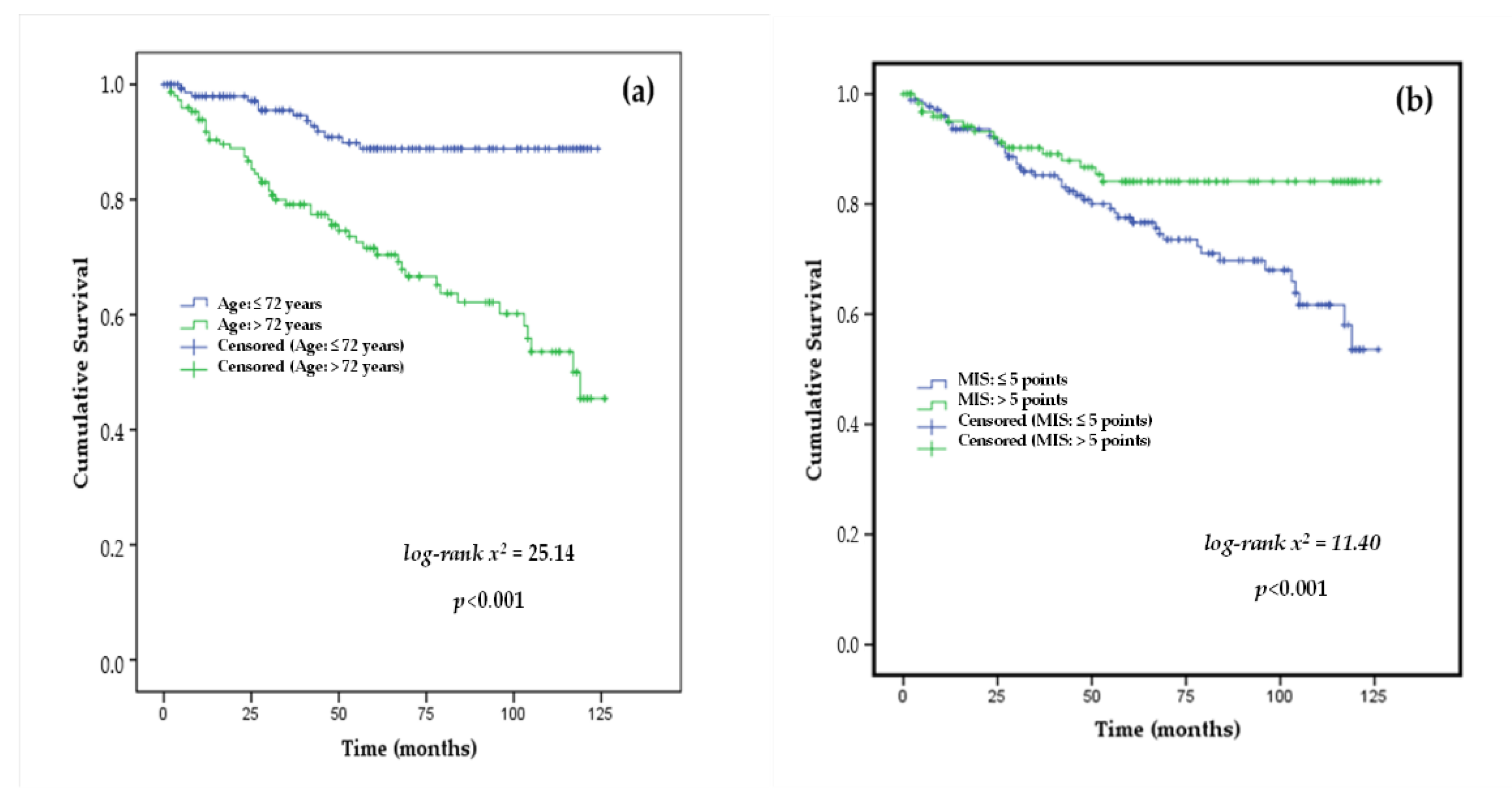
3.4. Cox Proportional Hazards Analysis of Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Minutolo, R.; Sasso, F.C.; Chiodini, P.; Cianciaruso, B.; Carbonara, O.; Zamboli, P.; Tirino, G.; Pota, A.; Torella, R.; Conte, G.; et al. Management of cardiovascular risk factors in advanced type 2 diabetic nephropathy: A comparative analysis in nephrology, diabetology and primary care settings. J. Hypertens. 2006, 24, 1655–1661. [Google Scholar] [CrossRef]
- Sasso, F.C.; De Nicola, L.; Carbonara, O.; Nasti, R.; Minutolo, R.; Salvatore, T.; Conte, G.; Torella, R. Cardiovascular risk factors and disease management in type 2 diabetic patients with diabetic nephropathy. Diabetes Care 2006, 29, 498–503. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; Terwee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef]
- Amparo, F.C.; Kamimura, M.A.; Molnar, M.Z.; Cuppari, L.; Lindholm, B.; Amodeo, C.; Carrero, J.J.; Cordeiro, A.C. Diagnostic validation and prognostic significance of the Malnutrition-Inflammation Score in nondialyzed chronic kidney disease patients. Nephrol. Dial. Transplant. 2015, 30, 821–828. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cuppari, L. The 2020 Updated KDOQI Clinical Practice Guidelines for Nutrition in Chronic Kidney Disease. Blood Purif. 2021, 50, 667–671. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Murray, D.C.; Barre, P.E. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J. Am. Soc. Nephrol. 1996, 7, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.C.; Sun, J.; Qureshi, A.R.; Dai, L.; Snaedal, S.; Barany, P.; Heimburger, O.; Lindholm, B.; Stenvinkel, P. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS ONE 2018, 13, e0190410. [Google Scholar] [CrossRef] [PubMed]
- Mittman, N.; Avram, M.M.; Oo, K.K.; Chattopadhyay, J. Serum prealbumin predicts survival in hemodialysis and peritoneal dialysis: 10 years of prospective observation. Am. J. Kidney Dis. 2001, 38, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.P.; Hsieh, Y.P.; Kor, C.T.; Chiu, P.F. Association between Albumin-Globulin Ratio and Mortality in Patients with Chronic Kidney Disease. J. Clin. Med. 2019, 8, 1991. [Google Scholar] [CrossRef] [PubMed]
- Yeun, J.Y.; Levine, R.A.; Mantadilok, V.; Kaysen, G.A. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am. J. Kidney Dis. 2000, 35, 469–476. [Google Scholar] [CrossRef]
- Menon, V.; Greene, T.; Wang, X.; Pereira, A.A.; Marcovina, S.M.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Levey, A.S.; Sarnak, M.J. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005, 68, 766–772. [Google Scholar] [CrossRef]
- Chan, M.; Kelly, J.; Batterham, M.; Tapsell, L. Malnutrition (subjective global assessment) scores and serum albumin levels, but not body mass index values, at initiation of dialysis are independent predictors of mortality: A 10-year clinical cohort study. J. Ren. Nutr. 2012, 22, 547–557. [Google Scholar] [CrossRef]
- Bansal, N.; Zelnick, L.R.; Himmelfarb, J.; Chertow, G.M. Bioelectrical Impedance Analysis Measures and Clinical Outcomes in CKD. Am. J. Kidney Dis. 2018, 72, 662–672. [Google Scholar] [CrossRef]
- Han, B.G.; Lee, J.Y.; Kim, J.S.; Yang, J.W. Decreased Bioimpedance Phase Angle in Patients with Diabetic Chronic Kidney Disease Stage 5. Nutrients 2019, 11, 2874. [Google Scholar] [CrossRef]
- Bauer, J.; Morley, J.E.; Schols, A.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Vettoretti, S.; Caldiroli, L.; Armelloni, S.; Ferrari, C.; Cesari, M.; Messa, P. Sarcopenia is Associated with Malnutrition but Not with Systemic Inflammation in Older Persons with Advanced CKD. Nutrients 2019, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.A.; Oliveira, D.; Barbosa, S.R.; Correa, J.; Colugnati, F.A.B.; Mansur, H.N.; Fernandes, N.; Bastos, M.G. Sarcopenia in patients with chronic kidney disease not yet on dialysis: Analysis of the prevalence and associated factors. PLoS ONE 2017, 12, e0176230. [Google Scholar] [CrossRef]
- Roshanravan, B.; Robinson-Cohen, C.; Patel, K.V.; Ayers, E.; Littman, A.J.; de Boer, I.H.; Ikizler, T.A.; Himmelfarb, J.; Katzel, L.I.; Kestenbaum, B.; et al. Association between physical performance and all-cause mortality in CKD. J. Am. Soc. Nephrol. 2013, 24, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef]
- Ruperto, M.; Sanchez-Muniz, F.J.; Barril, G. Predictors of protein-energy wasting in haemodialysis patients: A cross-sectional study. J. Hum. Nutr. Diet. 2014, 29, 38–47. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef]
- Rosler, A.; Lehmann, F.; Krause, T.; Wirth, R.; von Renteln-Kruse, W. Nutritional and hydration status in elderly subjects: Clinical rating versus bioimpedance analysis. Arch. Gerontol. Geriatr. 2010, 50, e81–e85. [Google Scholar] [CrossRef]
- Tabinor, M.; Elphick, E.; Dudson, M.; Kwok, C.S.; Lambie, M.; Davies, S.J. Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): Systematic review and subgroup meta-analysis. Sci. Rep. 2018, 8, 4441. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Miksza, J.; Yates, T.; Lightfoot, C.J.; Baker, L.A.; Watson, E.L.; Zaccardi, F.; Smith, A.C. Association of sarcopenia with mortality and end-stage renal disease in those with chronic kidney disease: A UK Biobank study. J. Cachexia Sarcopenia Muscle 2021, 12, 586–598. [Google Scholar] [CrossRef]
- Lopes, M.B.; Silva, L.F.; Dantas, M.A.; Matos, C.M.; Lopes, G.B.; Lopes, A.A. Sex-age-specific handgrip strength and mortality in an incident hemodialysis cohort: The risk explained by nutrition and comorbidities. Int. J. Artif. Organs 2018, 41, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, J.S.; Jung, S.W.; Hwang, H.S.; Moon, J.Y.; Jeong, K.H.; Lee, S.H.; Lee, S.Y.; Ko, G.J.; Lee, D.Y.; et al. Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol. 2020, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- McGinlay, J.M.; Payne, R.B. Serum albumin by dye-binding: Bromocresol green or bromocresol purple? The case for conservatism. Ann. Clin. Biochem. 1988, 25 Pt 4, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardu, C.; et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef]
- Kooman, J.P.; Broers, N.J.; Usvyat, L.; Thijssen, S.; van der Sande, F.M.; Cornelis, T.; Levin, N.W.; Leunissen, K.M.; Kotanko, P. Out of control: Accelerated aging in uremia. Nephrol. Dial. Transplant. 2013, 28, 48–54. [Google Scholar] [CrossRef]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef]
- Lodebo, B.T.; Shah, A.; Kopple, J.D. Is it Important to Prevent and Treat Protein-Energy Wasting in Chronic Kidney Disease and Chronic Dialysis Patients? J. Ren. Nutr. 2018, 28, 369–379. [Google Scholar] [CrossRef]
- Barril, G.; Nogueira, A.; Alvarez, G.; Nuñez, A.; Sanchez, C.; Romasco, P. Low Prevalence of Protein Energy Wasting in Advanced Chronic Kidney Disease Patients Managed with a Nutritional Monitoring Program. Acta Sci. Nutr. Health 2022, 6, 19–27. [Google Scholar] [CrossRef]
- Lu, J.L.; Kalantar-Zadeh, K.; Ma, J.Z.; Quarles, L.D.; Kovesdy, C.P. Association of body mass index with outcomes in patients with CKD. J. Am. Soc. Nephrol. 2014, 25, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Madero, M.; Sarnak, M.J.; Wang, X.; Sceppa, C.C.; Greene, T.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Levey, A.S.; Menon, V. Body mass index and mortality in CKD. Am. J. Kidney Dis. 2007, 50, 404–411. [Google Scholar] [CrossRef]
- Rymarz, A.; Bartoszewicz, Z.; Szamotulska, K.; Niemczyk, S. The Associations Between Body Cell Mass and Nutritional and Inflammatory Markers in Patients With Chronic Kidney Disease and in Subjects Without Kidney Disease. J. Ren. Nutr. 2016, 26, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Beberashvili, I.; Azar, A.; Sinuani, I.; Shapiro, G.; Feldman, L.; Stav, K.; Sandbank, J.; Averbukh, Z. Bioimpedance phase angle predicts muscle function, quality of life and clinical outcome in maintenance hemodialysis patients. Eur. J. Clin. Nutr. 2014, 68, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Kubrusly, M.; Mota, R.S.; Silva, C.A.; Choukroun, G.; Oliveira, V.N. The phase angle and mass body cell as markers of nutritional status in hemodialysis patients. J. Ren. Nutr. 2010, 20, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Han, B.G.; Lee, J.Y.; Kim, J.S.; Yang, J.W. Clinical Significance of Phase Angle in Non-Dialysis CKD Stage 5 and Peritoneal Dialysis Patients. Nutrients 2018, 10, 1331. [Google Scholar] [CrossRef]
- Tan, R.S.; Liang, D.H.; Liu, Y.; Zhong, X.S.; Zhang, D.S.; Ma, J. Bioelectrical Impedance Analysis-Derived Phase Angle Predicts Protein-Energy Wasting in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2019, 29, 295–301. [Google Scholar] [CrossRef]
- Bansal, N.; Matheny, M.E.; Greevy, R.A., Jr.; Eden, S.K.; Perkins, A.M.; Parr, S.K.; Fly, J.; Abdel-Kader, K.; Himmelfarb, J.; Hung, A.M.; et al. Acute Kidney Injury and Risk of Incident Heart Failure among US Veterans. Am. J. Kidney Dis. 2018, 71, 236–245. [Google Scholar] [CrossRef]
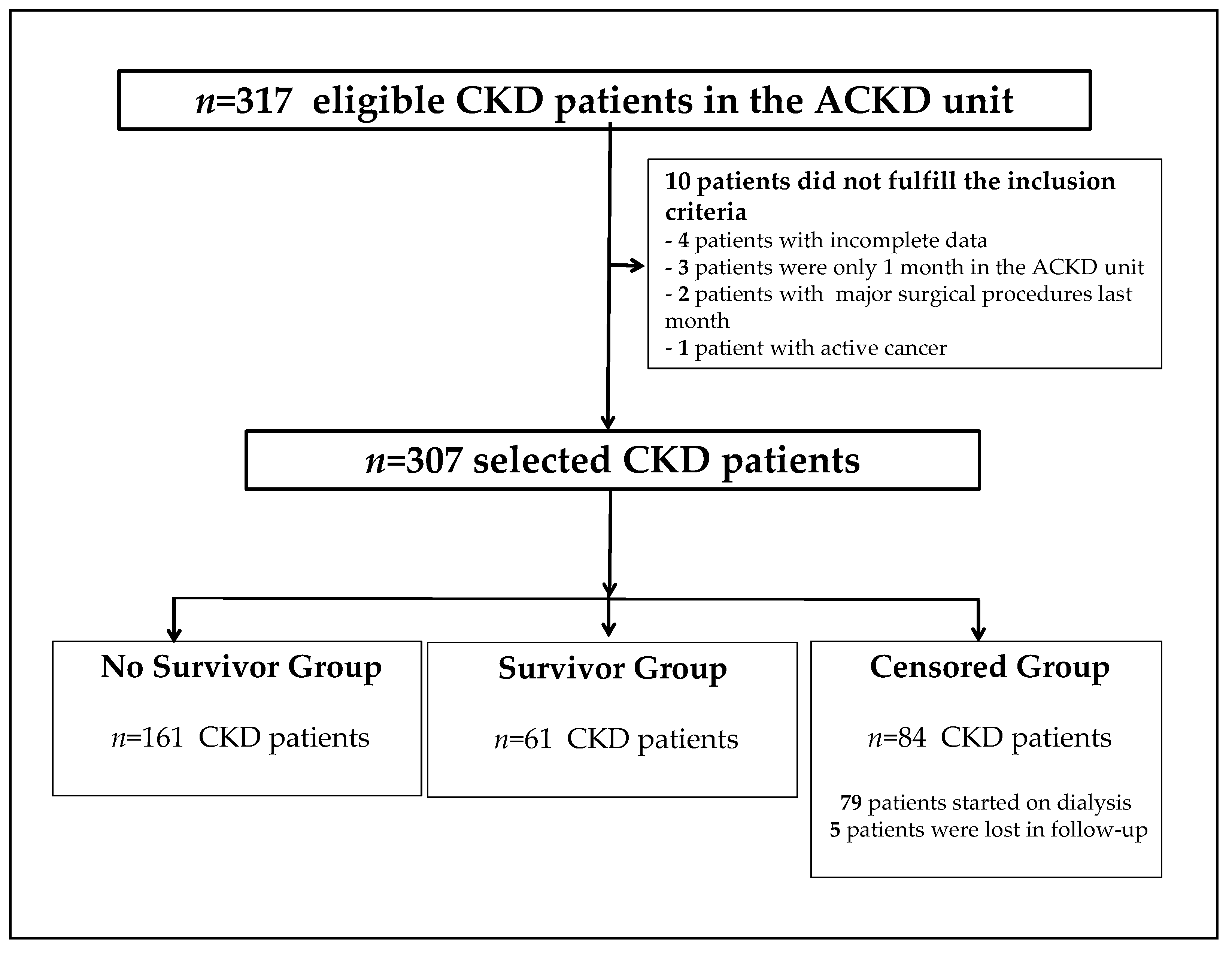

| Variables | Global n = 307 | No Survivors n = 62 | Survivors n = 161 | Censored n = 84 | p-Value |
|---|---|---|---|---|---|
| Male n; (%) | 212 (61.90) | 36 (58.10) | 113 (70.20) | 63 (75.0) | 0.003 |
| Age (years) | 70.16 ± 12.46 | 68.72 ± 12.43 | 77.55 ± 9.66 | 67.10 ± 12.33 | <0.001 |
| DM n (%) | 142 (46.30) | 28 (45.20) | 76 (47.20) | 38 (45.20) | 0.940 |
| Time ACKD unit (mo.) | 58.89 ± 37.59 | 32.82 ± 24.93 | 64.09 ± 63.29 | 23.63 ± 19.93 | <0.001 |
| e-GFR (mL/min/1.73 m2) | 18.96 ± 8.24 | 19.38 ± 7.75 | 20.78 ± 8.85 | 15.16 ± 5.82 | <0.001 |
| nPna (g/kg/day) | 0.92 ± 0.24 | 0.90 ± 0.20 | 0.94 ± 0.28 | 0.90 ± 0.20 | 0.608 |
| PEW * n(%) | 83 (27.0%) | 24 (38.70%) | 37 (23.0%) | 22 (26.20) | 0.049 |
| Variables | Global n = 307 | No Survivors n = 62 | Survivors n = 161 | Censored n = 84 | p-Value |
|---|---|---|---|---|---|
| BW (kg) | 75.03 ± 16.35 | 71.61 ± 15.18 | 76.04 ± 17.17 | 75.60 ± 15.38 | 0.181 |
| BMI (kg/m2) | 27.04 ± 5.12 | 27.14 ± 5.20 | 27.67 ± 5.24 | 27.09 ± 4.88 | 0.634 |
| Exchangeable Na/K | 1.44 ± 0.49 | 1.57 ± 0.58 | 1.37 ± 0.45 | 1.47 ± 0.49 | 0.019 |
| TBW (%) | 53.59 ± 6.93 | 53.82 ± 8.08 | 52.91 ± 6.34 | 54.70 ± 7.04 | 0.152 |
| ECW (%) | 58.66 ± 8.27 | 56.66 ± 7.29 | 56.16 ± 8.57 | 56.61 ± 8.54 | 0.018 |
| ICW (%) | 42.99 ± 8.41 | 40.33 ± 7.29 | 43.81 ± 8.58 | 43.38 ± 8.54 | 0.019 |
| BCM (%) | 38.74 ± 9.37 | 36.51 ± 8.25 | 40.19 ± 9.79 | 37.62 ± 8.95 | 0.014 |
| PA (°) | 4.10 ±1.16 | 3.72 ± 0.97 | 4.24 ± 1.19 | 4.13 ± 1.19 | 0.012 |
| FM (%) | 32.11 ± 10.36 | 31.94 ± 9.91 | 32.58 ± 9.15 | 29.97 ± 9.57 | 0.123 |
| FFM (%) | 68.35 ± 9.48 | 67.88 ± 10.36 | 67.67 ± 8.99 | 70.01 ± 9.58 | 0.168 |
| MM (%) | 33.18 ± 7.88 | 32.02 ± 7.39 | 33.30 ± 8.21 | 33.81 ± 7.57 | 0.386 |
| Right-HGS (kg/m2) | 25.78 ± 9.90 | 21.04 ± 7.72 | 27.19 ± 10.27 | 26.51 ± 9.64 | <0.001 |
| s-Albumin (g/dL) | 4.10 ± 4.47 | 4.10 ± 0.39 | 4.21 ± 0.44 | 4.15 ± 0.45 | 0.231 |
| s-Prealbumin (mg/dL) | 28.30 ± 6.83 | 25.83 ± 5.21 | 28.61 ± 6.60 | 29.53 ± 7.81 | 0.005 |
| s-CRP (mg/dL) | 0.82 ± 1.49 | 0.59 ± 0.73 | 0.98 ± 1.86 | 0.71 ± 1.01 | 0.158 |
| Predictor Variable | HR (95%CI) | p-Value |
|---|---|---|
| Gender | 1.530 (0.926 to 2.528) | 0.097 |
| Age (years) | 1.071 (1.071 to 1.101) | <0.001 |
| DM n; (%) | 0.161 (0.549 to 1.486) | 0.688 |
| Time ACKD-unit (months) | 0.978 (0.969 to 0.986) | <0.001 |
| e-GFR (mL/min/1.73 m2) | 1.008 (0.979 to 1.038) | 0.587 |
| nPna (g/kg/day) | 0.529 (0.156 to 1.794) | 0.307 |
| MIS (points) | 1.080 (1.005 to 1.161) | 0.036 |
| BW (kg) | 0.989 (0.973 to 1.005) | 0.178 |
| BMI (kg/m2) | 0.997 (0.949 to 1.047) | 0.899 |
| Exchangeable Na/K | 1.669 (1.169 to 2.385) | 0.005 |
| TBW (%) | 1.004 (0.968 to 1.041) | 0.838 |
| ECW (%) | 1.056 (1.026 to 1.087) | <0.001 |
| ICW (%) | 0.947 (0.920 to 0.975) | <0.001 |
| BCM (%) | 0.968 (0.942 to 0.995) | 0.021 |
| PA (°) | 0.650 (0.513 to 0.824) | <0.001 |
| FM (%) | 0.397 (0.982 to 1.035) | 0.529 |
| FFM (%) | 0.377 (0.966 to 1.018) | 0.539 |
| MM (%) | 0.969 (0.937 to 1.002) | 0.068 |
| Right-HGS (kg/m2) | 0.946 (0.919 to 0.973) | <0.001 |
| s-Albumin (g/dL) | 0.592 (0.355 to 0.987) | 0.045 |
| s-Prealbumin (mg/dL) | 0.936 (0.900 to 0.973) | 0.001 |
| s-CRP (mg/dL) | 0.954 (0.762 to 1.194) | 0.680 |
| Predictor Variable | HR (95% CI) | p-Value |
|---|---|---|
| Time of follow-up (months) | 0.975 (0.966 to 0.984) | <0.001 |
| s-Prealbumin (mg/dL) | 0.946 (0.911 to 0.982) | 0.003 |
| Right-HGS (kg/m2) | 0.953 (0.925 to 0.983) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barril, G.; Nogueira, A.; Alvarez-García, G.; Núñez, A.; Sánchez-González, C.; Ruperto, M. Nutritional Predictors of Mortality after 10 Years of Follow-Up in Patients with Chronic Kidney Disease at a Multidisciplinary Unit of Advanced Chronic Kidney Disease. Nutrients 2022, 14, 3848. https://doi.org/10.3390/nu14183848
Barril G, Nogueira A, Alvarez-García G, Núñez A, Sánchez-González C, Ruperto M. Nutritional Predictors of Mortality after 10 Years of Follow-Up in Patients with Chronic Kidney Disease at a Multidisciplinary Unit of Advanced Chronic Kidney Disease. Nutrients. 2022; 14(18):3848. https://doi.org/10.3390/nu14183848
Chicago/Turabian StyleBarril, Guillermina, Angel Nogueira, Graciela Alvarez-García, Almudena Núñez, Carmen Sánchez-González, and Mar Ruperto. 2022. "Nutritional Predictors of Mortality after 10 Years of Follow-Up in Patients with Chronic Kidney Disease at a Multidisciplinary Unit of Advanced Chronic Kidney Disease" Nutrients 14, no. 18: 3848. https://doi.org/10.3390/nu14183848
APA StyleBarril, G., Nogueira, A., Alvarez-García, G., Núñez, A., Sánchez-González, C., & Ruperto, M. (2022). Nutritional Predictors of Mortality after 10 Years of Follow-Up in Patients with Chronic Kidney Disease at a Multidisciplinary Unit of Advanced Chronic Kidney Disease. Nutrients, 14(18), 3848. https://doi.org/10.3390/nu14183848







