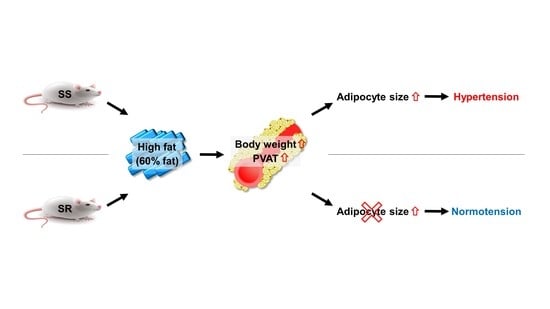
Dahl Salt-Resistant Rat Is Protected against Hypertension during Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Pressure Measurements
2.3. Histological Analysis
2.4. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5. Measurement of Cytokine Levels
2.6. Statistics
3. Results
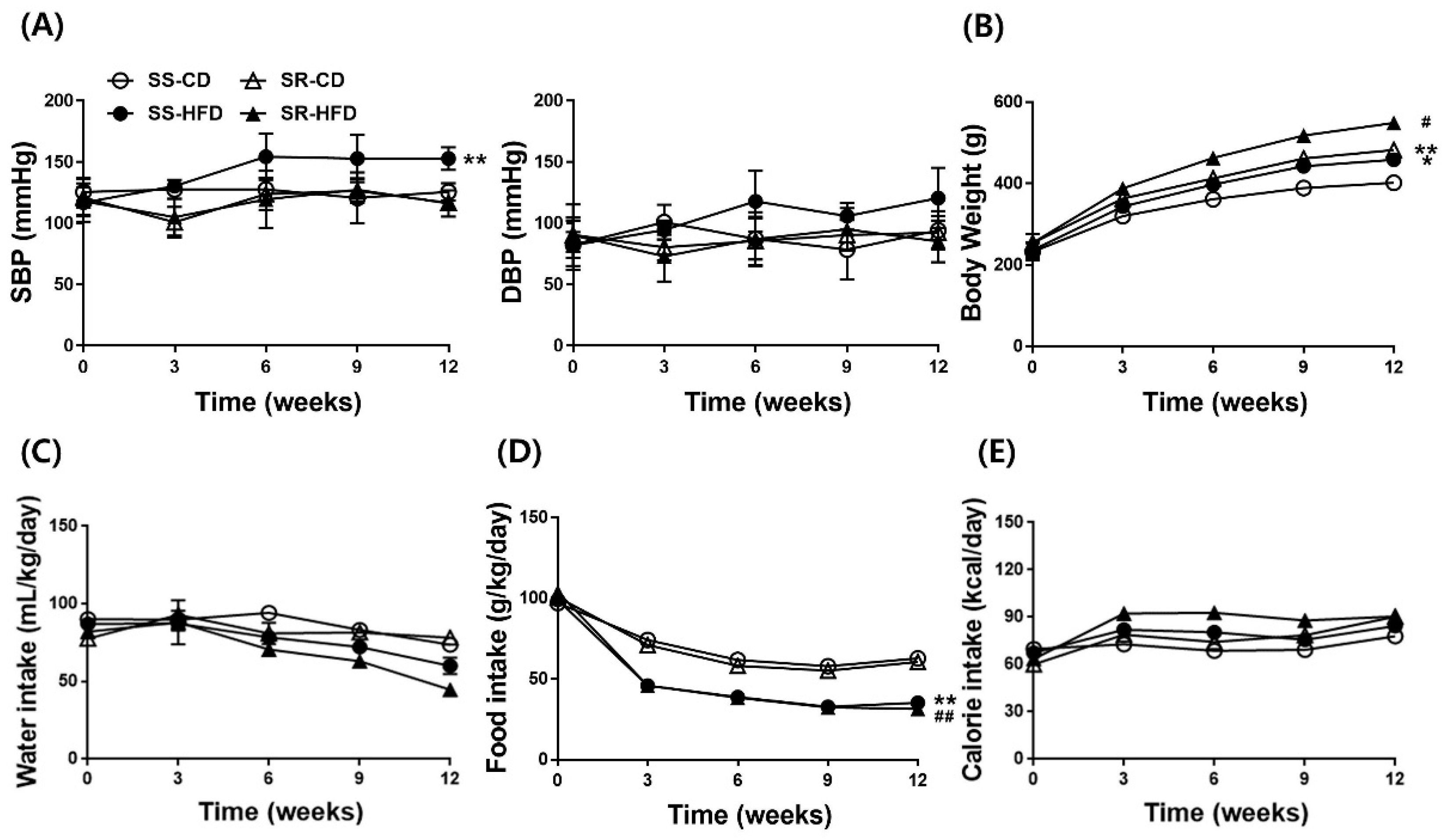
3.1. HFD Increased Blood Pressure Only in SS Rats Regardless of Accelerated Body Weight Gain in Both Rats
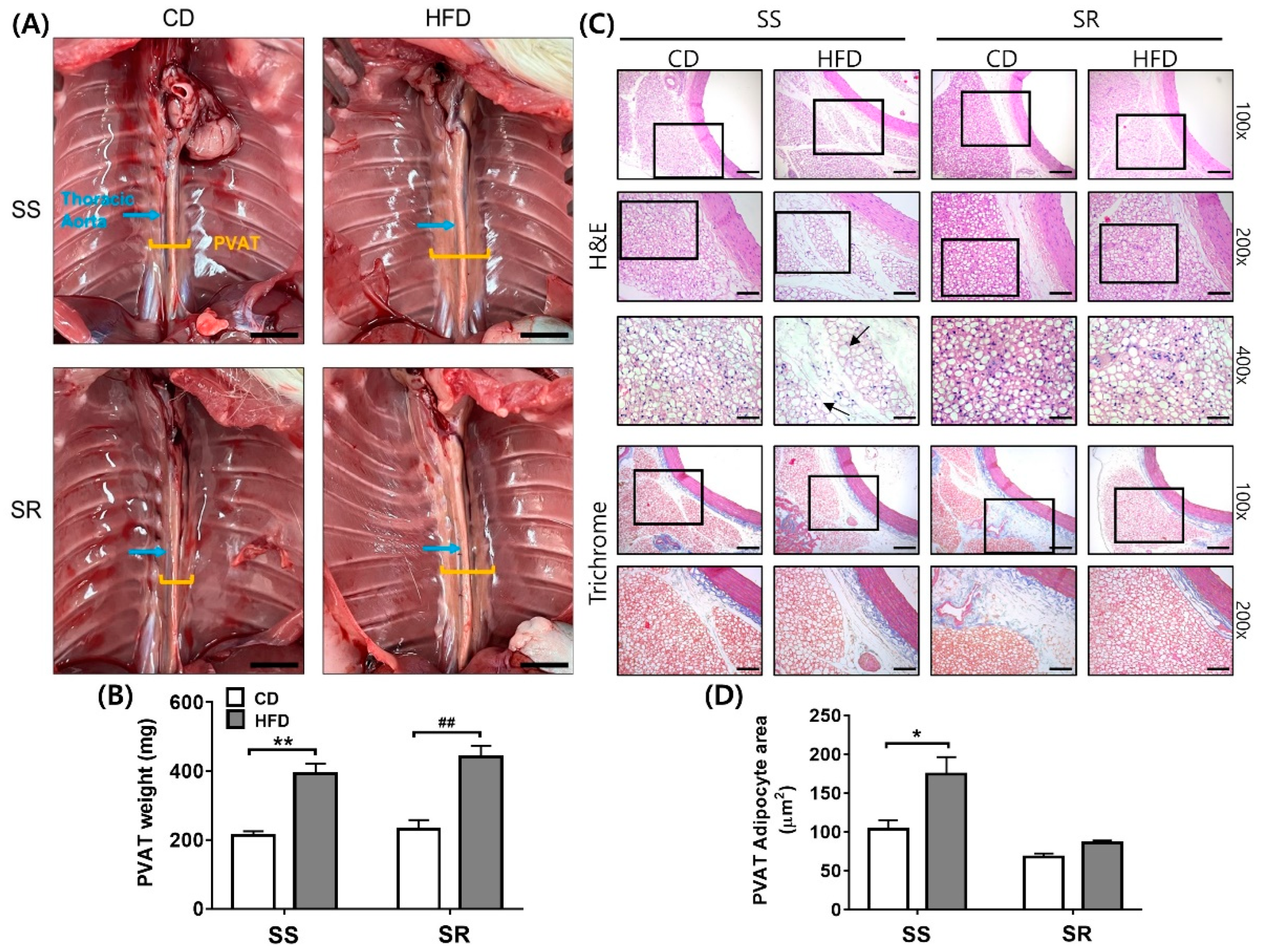
3.2. HFD Increased the Adipocyte Area in the SS Rats Regardless of Increased tPVAT Weight in Both Rats
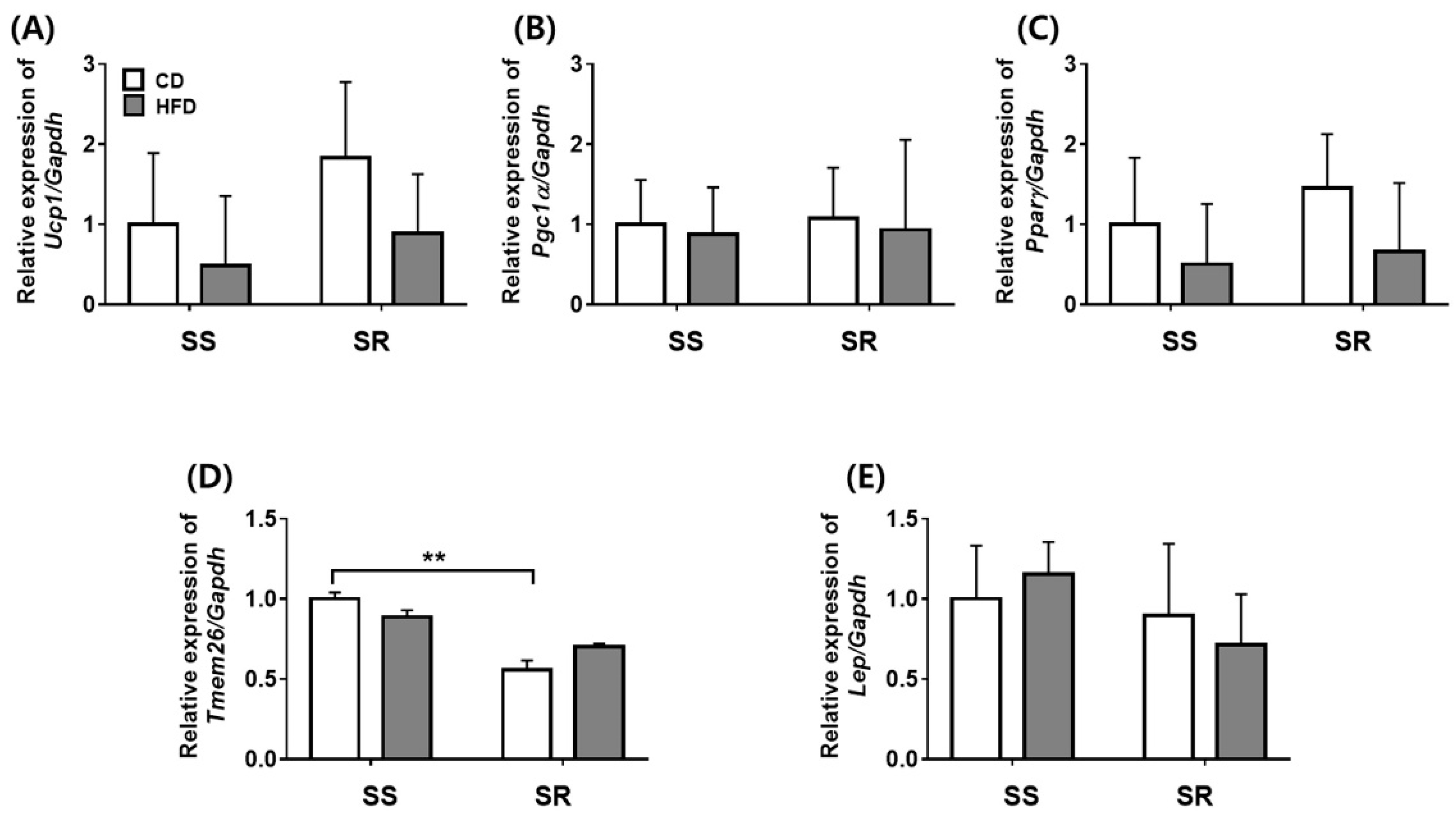
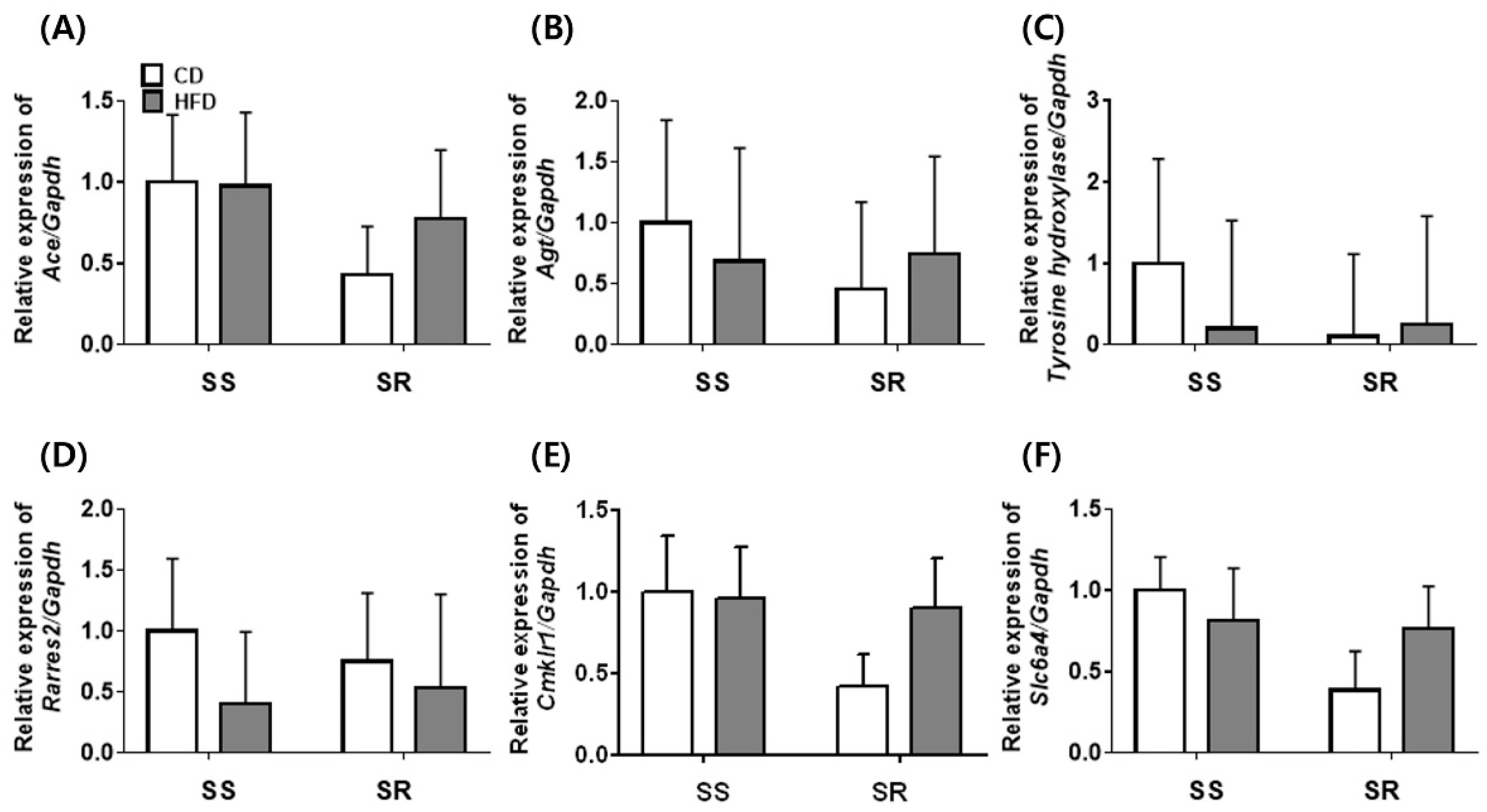
3.3. HFD Did Not Affect the Expression of Genes Related to Adipocyte Markers in tPVAT of SS and SR Rats
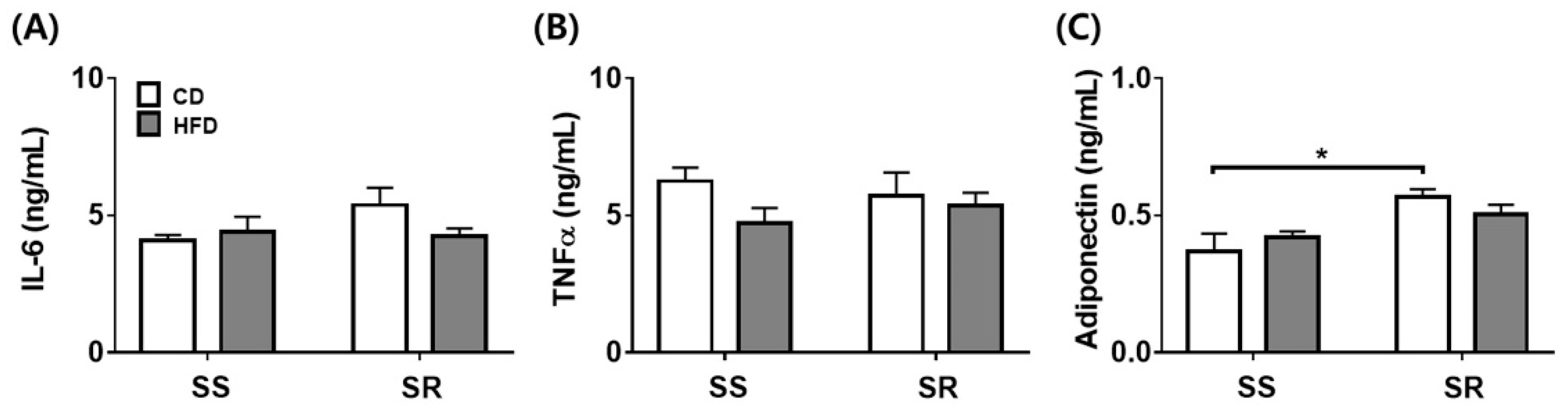
3.4. HFD Did Not Affect the Expression of the Inflammatory or Contractile Factors in tPVAT of SS and SR Rats
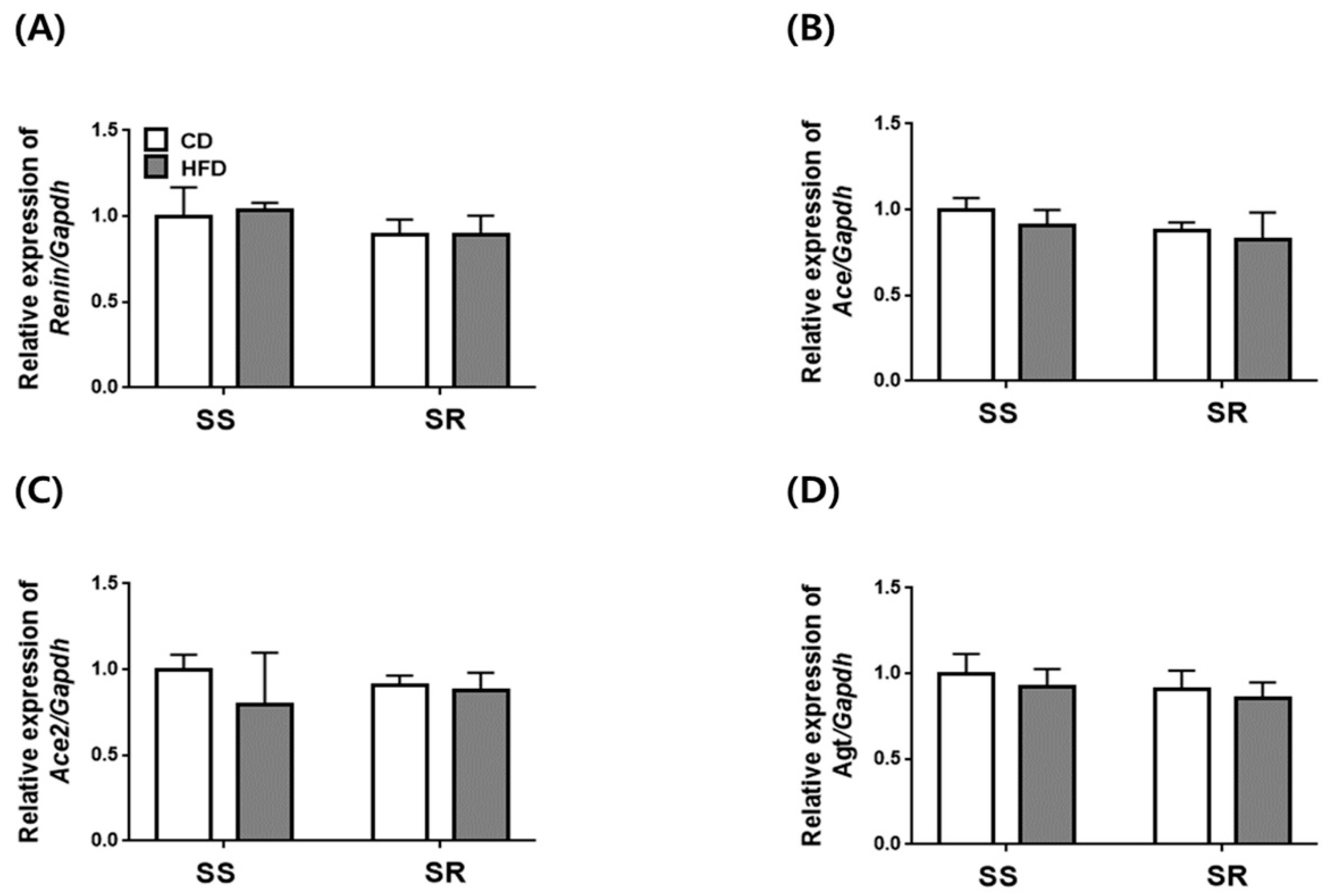
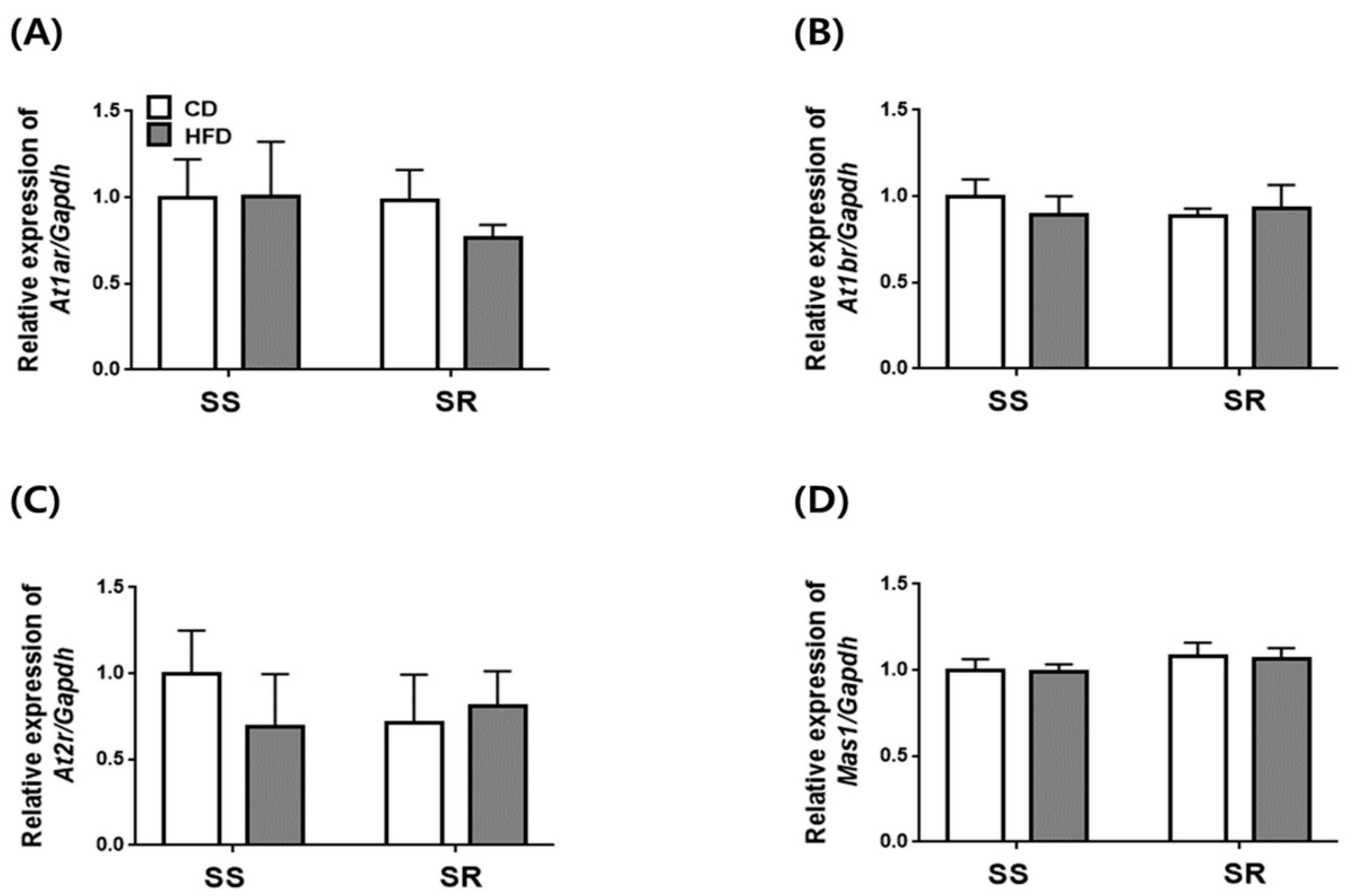
3.5. HFD Did Not Affect the Expression of Genes Related to RAAS in the Kidney of SS and SR Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Victorio, J.A.; Guizoni, D.M.; Freitas, I.N.; Araujo, T.R.; Davel, A.P. Effects of High-Fat and High-Fat/High-Sucrose Diet-Induced Obesity on PVAT Modulation of Vascular Function in Male and Female Mice. Front Pharmacol. 2021, 12, 720224. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.E.; Bousquet-Santos, K.; Robles-Osorio, L.; Montagnani, V.; Soodini, G.; Porramatikul, S.; Hamdy, O.; Nobrega, A.C.; Horton, E.S. Overweight Latino children and adolescents have marked endothelial dysfunction and subclinical vascular inflammation in association with excess body fat and insulin resistance. Diabetes Care 2008, 31, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.K.; Zhou, Z.; Zhang, J.; Zeng, R.; Wu, J.; Eitzman, D.T.; Chen, Y.E.; Chang, L. Perivascular adipose tissue in vascular function and disease: A review of current research and animal models. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Szasz, T.; Bomfim, G.F.; Webb, R.C. The influence of perivascular adipose tissue on vascular homeostasis. Vasc. Health Risk Manag. 2013, 9, 105–116. [Google Scholar] [CrossRef]
- Chang, L.; Garcia-Barrio, M.T.; Chen, Y.E. Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1094–1109. [Google Scholar] [CrossRef]
- da Costa, R.M.; Fais, R.S.; Dechandt, C.R.P.; Louzada-Junior, P.; Alberici, L.C.; Lobato, N.S.; Tostes, R.C. Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice. Br. J. Pharmacol. 2017, 174, 3527–3541. [Google Scholar] [CrossRef]
- Knight, S.F.; Quigley, J.E.; Yuan, J.; Roy, S.S.; Elmarakby, A.; Imig, J.D. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension 2008, 51, 352–359. [Google Scholar] [CrossRef]
- Kramer, H.; Luke, A.; Bidani, A.; Cao, G.; Cooper, R.; McGee, D. Obesity and prevalent and incident CKD: The Hypertension Detection and Follow-Up Program. Am. J. Kidney Dis. 2005, 46, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Park, C.W.; Shin, S.J.; Lim, J.H.; Chung, H.W.; Youn, D.Y.; Kim, H.W.; Kim, B.S.; Lee, J.H.; Kim, G.H.; et al. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol. Dial. Transplant. 2010, 25, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Ecelbarger, C.M.; Rash, A.; Sinha, R.K.; Tiwari, S. The effect of chronic candesartan therapy on the metabolic profile and renal tissue cytokine levels in the obese Zucker rat. Mediat. Inflamm. 2010, 2010, 841343. [Google Scholar] [CrossRef]
- Griffin, K.A.; Kramer, H.; Bidani, A.K. Adverse renal consequences of obesity. Am. J. Physiol. Ren. Physiol. 2008, 294, F685–F696. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Bakris, G.L. Non-esterified fatty acids and blood pressure elevation: A mechanism for hypertension in subjects with obesity/insulin resistance? J. Hum. Hypertens. 2007, 21, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Pathophysiology of obesity hypertension. Curr. Hypertens. Rep. 2000, 2, 139–147. [Google Scholar] [CrossRef]
- Taylor, L.E.; Gillis, E.E.; Musall, J.B.; Baban, B.; Sullivan, J.C. High-fat diet-induced hypertension is associated with a proinflammatory T cell profile in male and female Dahl salt-sensitive rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1713. [Google Scholar] [CrossRef]
- De Miguel, C.; Das, S.; Lund, H.; Mattson, D.L. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1136–R1142. [Google Scholar] [CrossRef]
- Lee, E.; Kim, N.; Kang, J.; Yoon, S.; Lee, H.A.; Jung, H.; Kim, S.H.; Kim, I. Activated pathogenic Th17 lymphocytes induce hypertension following high-fructose intake in Dahl salt-sensitive but not Dahl salt-resistant rats. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef]
- Kim, C.W.; Young Kim, J.; Lee, S.; Kim, I. Dahl salt-resistant rats are protected against angiotensin II-induced hypertension. Biochem. Pharmacol. 2022, 203, 115193. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, M.; Dolan, J.; He, J. Sodium sensitivity of blood pressure in Chinese populations. J. Hum. Hypertens. 2020, 34, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gu, D.; Huang, J.; Rao, D.C.; Jaquish, C.E.; Hixson, J.E.; Chen, C.S.; Chen, J.; Lu, F.; Hu, D.; et al. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: A dietary intervention study. Lancet 2009, 373, 829–835. [Google Scholar] [CrossRef]
- Chung, H.W.; Lim, J.H.; Kim, M.Y.; Shin, S.J.; Chung, S.; Choi, B.S.; Kim, H.W.; Kim, Y.S.; Park, C.W.; Chang, Y.S. High-fat diet-induced renal cell apoptosis and oxidative stress in spontaneously hypertensive rat are ameliorated by fenofibrate through the PPARα-FoxO3a-PGC-1α pathway. Nephrol. Dial. Transplant. 2012, 27, 2213–2225. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.J. Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am. J. Physiol. 1986, 251, F57–F65. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Saez, F.; Monzon, C.M.; Asirwatham, J.; Garvin, J.L. Thick Ascending Limb Sodium Transport in the Pathogenesis of Hypertension. Physiol. Rev. 2019, 99, 235–309. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Stehouwer, C.D.; Emeis, J.J.; Coppack, S.W. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Sowers, J.R. Obesity as a cardiovascular risk factor. Am. J. Med. 2003, 115, 37–41. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Hall, J.E.; Brands, M.W.; Dixon, W.N.; Smith, M.J., Jr. Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension 1993, 22, 292–299. [Google Scholar] [CrossRef]
- Kihara, S.; Matsuzawa, Y. Fat distribution and cardiovascular disease risk. Curr. Cardiovasc. Risk Rep. 2015, 9, 8. [Google Scholar] [CrossRef]
- Huang Cao, Z.F.; Stoffel, E.; Cohen, P. Role of Perivascular Adipose Tissue in Vascular Physiology and Pathology. Hypertension 2017, 69, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Orshal, J.M.; Khalil, R.A. Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R1013–R1023. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Duranti, E.; Rossi, C.; Dell’Agnello, U.; Santini, E.; Anselmino, M.; Chiarugi, M.; Taddei, S.; Solini, A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: Role of perivascular adipose tissue. Eur. Heart J. 2015, 36, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Nagae, A.; Fujita, M.; Kawarazaki, H.; Matsui, H.; Ando, K.; Fujita, T. Effect of high fat loading in Dahl salt-sensitive rats. Clin. Exp. Hypertens. 2009, 31, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Culver, S.A.; Quadri, S.; Ledford, K.L.; Al-Share, Q.Y.; Ghadieh, H.E.; Najjar, S.M.; Siragy, H.M. High-fat diet amplifies renal renin angiotensin system expression, blood pressure elevation, and renal dysfunction caused by Ceacam1 null deletion. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E802–E810. [Google Scholar] [CrossRef] [PubMed]
- Deji, N.; Kume, S.; Araki, S.; Soumura, M.; Sugimoto, T.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Koya, D.; Haneda, M.; et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am. J. Physiol.-Ren. Physiol. 2009, 296, F118–F126. [Google Scholar] [CrossRef] [PubMed]
- Wind, S.; Beuerlein, K.; Armitage, M.E.; Taye, A.; Kumar, A.H.; Janowitz, D.; Neff, C.; Shah, A.M.; Wingler, K.; Schmidt, H.H. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 2010, 56, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y.; Abboud, H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. USA 2009, 106, 14385–14390. [Google Scholar] [CrossRef]
- Christensen, K.L.; Mulvany, M.J. Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J. Vasc. Res. 1993, 30, 73–79. [Google Scholar] [CrossRef]
- Tatchum-Talom, R.; Eyster, K.M.; Martin, D.S. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can. J. Physiol. Pharmacol. 2005, 83, 413–422. [Google Scholar] [CrossRef]
- Pratt, P.F.; Bonnet, S.; Ludwig, L.M.; Bonnet, P.; Rusch, N.J. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension 2002, 40, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.R.; Lee, R.P.; Wu, C.Y.; Chen, H.I. Nitric oxide in mesenteric vascular reactivity: A comparison between rats with normotension and hypertension. Clin. Exp. Pharmacol. Physiol. 2002, 29, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Beyer, A.M.; Raffai, G.; Weinberg, B.D.; Fredrich, K.; Rodgers, M.S.; Geurts, A.M.; Jacob, H.J.; Dwinell, M.R.; Lombard, J.H. Amelioration of salt-induced vascular dysfunction in mesenteric arteries of Dahl salt-sensitive rats by missense mutation of extracellular superoxide dismutase. Am. J. Physiol. Heart. Circ. Physiol. 2014, 306, H339–H347. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cantú, S.M.; Álvarez Primo, M.; Peredo, H.A.; Donoso, A.S.; Puyó, A.M.; Choi, M.R. Losartan prevents mesenteric vascular bed alterations in high-fat diet fed rats. Clin. Investig. Arterioscler. 2021, 33, 1–9. [Google Scholar] [CrossRef]
- Padilla, J.; Jenkins, N.T.; Vieira-Potter, V.J.; Laughlin, M.H. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R543–R552. [Google Scholar] [CrossRef]
- Fitzgibbons, T.P.; Kogan, S.; Aouadi, M.; Hendricks, G.M.; Straubhaar, J.; Czech, M.P. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, H1425–H1437. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Jang, S.; Kim, J.Y.; Kim, I. Dahl Salt-Resistant Rat Is Protected against Hypertension during Diet-Induced Obesity. Nutrients 2022, 14, 3843. https://doi.org/10.3390/nu14183843
Lee S, Jang S, Kim JY, Kim I. Dahl Salt-Resistant Rat Is Protected against Hypertension during Diet-Induced Obesity. Nutrients. 2022; 14(18):3843. https://doi.org/10.3390/nu14183843
Chicago/Turabian StyleLee, Soyung, Sungmin Jang, Jee Young Kim, and Inkyeom Kim. 2022. "Dahl Salt-Resistant Rat Is Protected against Hypertension during Diet-Induced Obesity" Nutrients 14, no. 18: 3843. https://doi.org/10.3390/nu14183843
APA StyleLee, S., Jang, S., Kim, J. Y., & Kim, I. (2022). Dahl Salt-Resistant Rat Is Protected against Hypertension during Diet-Induced Obesity. Nutrients, 14(18), 3843. https://doi.org/10.3390/nu14183843