Cloudberry (Rubus chamaemorus L.) Supplementation Attenuates the Development of Metabolic Inflammation in a High-Fat Diet Mouse Model of Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Study Design
2.2. Intraperitoneal Glucose Tolerance Test
2.3. Blood Samples and Analyses
2.4. RNA Extraction and Reverse Transcription Polymerase Chain Reaction
2.5. Statistics
3. Results
3.1. Weight Gain and Food Intake
3.2. Liver Function and Inflammation
3.3. Lipids and Adipokines
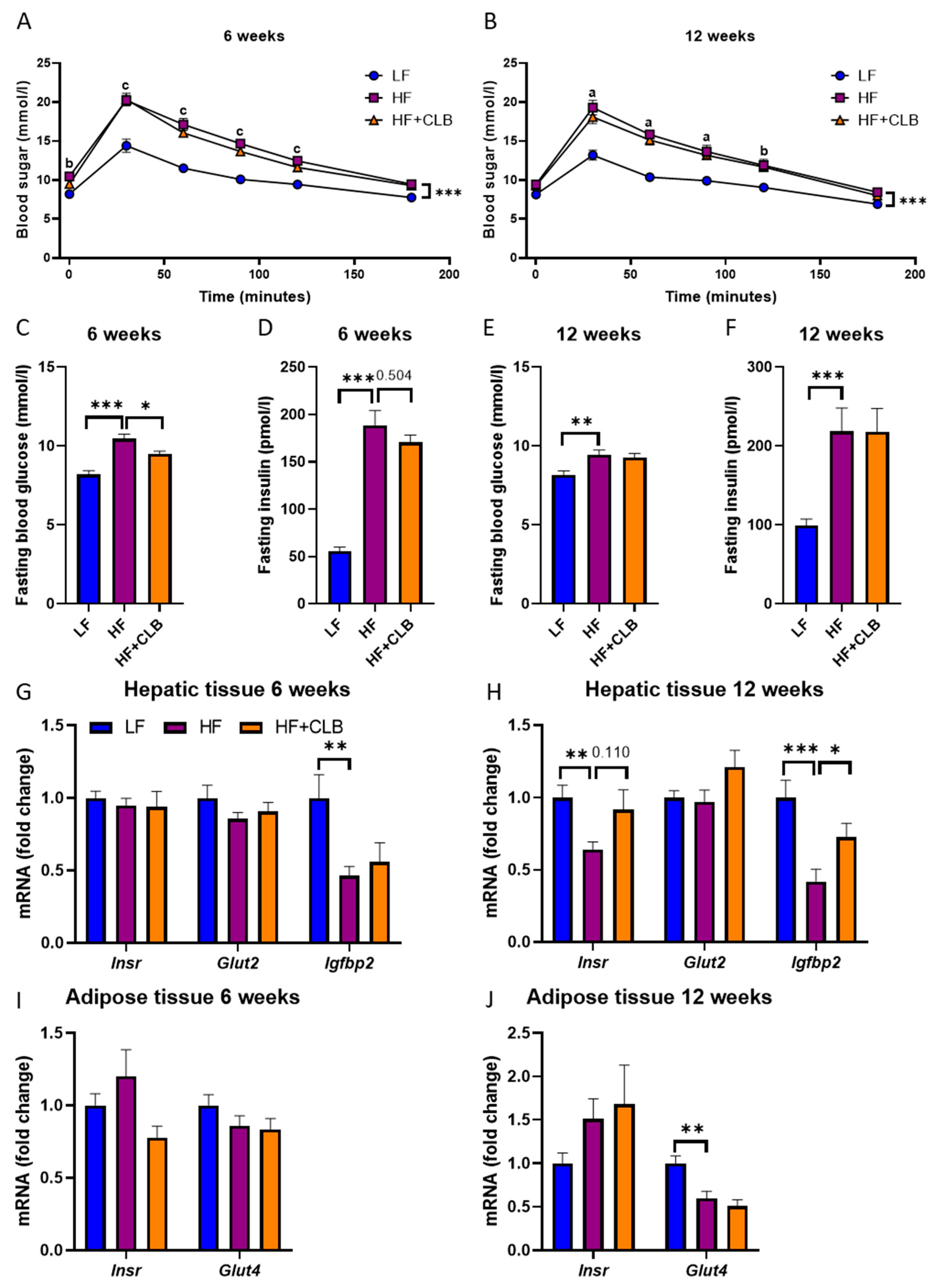
3.4. Glucose Metabolism
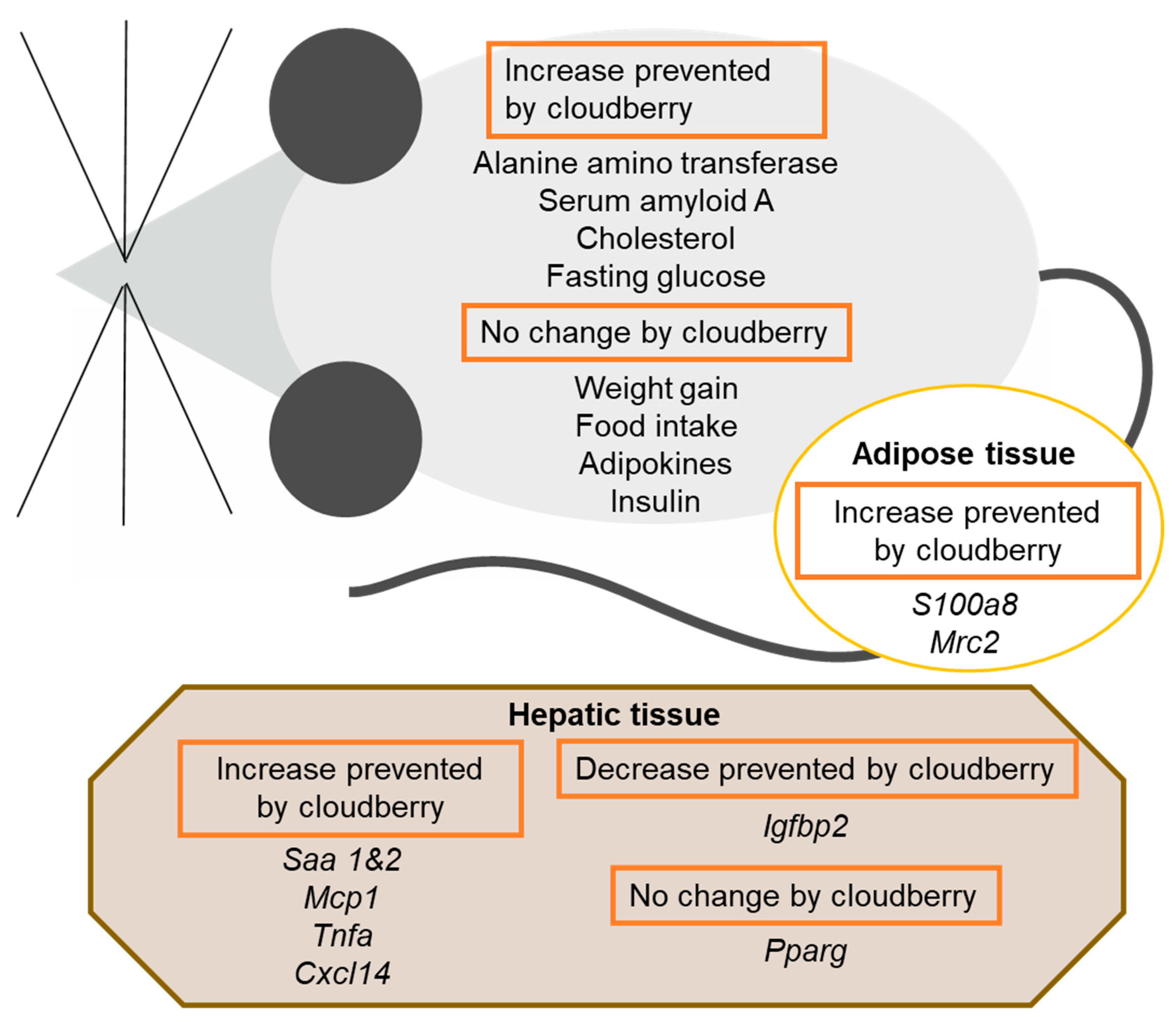
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charles-Messance, H.; Mitchelson, K.A.J.; De Marco Castro, E.; Sheedy, F.J.; Roche, H.M. Regulating metabolic inflammation by nutritional modulation. J. Allergy Clin. Immunol. 2020, 146, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Suganami, T.; Mieda, T.; Itoh, M.; Shimoda, Y.; Kamei, Y.; Ogawa, Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem. Biophys. Res. Commun. 2007, 354, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.-H.; Brickey, W.J.; Ting, J.P.-Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic Acids in Berries, Fruits, and Beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Hellström, J.K.; Törrönen, A.R.; Mattila, P.H. Proanthocyanidins in Common Food Products of Plant Origin. J. Agric. Food Chem. 2009, 57, 7899–7906. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, J.-L.; Lee, E.-S.; Han, S.-Y.; Gong, J.-H.; Kang, M.-K.; Kang, Y.-H. Dietary ellagic acid attenuates oxidized LDL uptake and stimulates cholesterol efflux in murine macrophages. J. Nutr. 2011, 141, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Rønning, S.B.; Voldvik, V.; Bergum, S.K.; Aaby, K.; Borge, G.I.A. Ellagic acid and urolithin A modulate the immune response in LPS-stimulated U937 monocytic cells and THP-1 differentiated macrophages. Food Funct. 2020, 11, 7946–7959. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus berries for the control of gastric inflammation: In vitro and in vivo studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef]
- Prabha, B.; Sini, S.; Priyadarshini, T.S.; Sasikumar, P.; Gopalan, G.; Joseph, J.P.; Jithin, M.M.; Sivan, V.V.; Jayamurthy, P.; Radhakrishnan, K.V. Anti-inflammatory effect and mechanism of action of ellagic acid-3,3′,4-trimethoxy-4′-O-α-L-rhamnopyranoside isolated from Hopea parviflora in lipopolysaccharide-stimulated RAW 264.7 macrophages. Nat. Prod. Res. 2021, 35, 3156–3160. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Nånberg, E.; Fjaeraa, C.; Wijkander, J. Ellagic acid inhibits lipopolysaccharide-induced expression of enzymes involved in the synthesis of prostaglandin E2 in human monocytes. Br. J. Nutr. 2010, 103, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.Y.; Bandiera, R.; Serrels, A.; Martínez-Estrada, O.M.; Qing, W.; Lee, M.; Slight, J.; Thornburn, A.; Berry, R.; Mchaffie, S.; et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 2014, 16, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef]
- Kellogg, J.; Wang, J.; Flint, C.; Ribnicky, D.; Kuhn, P.; De Mejia, E.G.; Raskin, I.; Lila, M.A. Alaskan Wild Berry Resources and Human Health under the Cloud of Climate Change. J. Agric. Food Chem. 2010, 58, 3884–3900. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, J.E.; Lee, M.; Hardwick, J.P. Hepatic lipid homeostasis by peroxisome proliferator-activated receptor gamma 2. Liver Res. 2018, 2, 209–215. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta-Galloyl-Glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed]
- Amor, A.J.; Gómez-Guerrero, C.; Ortega, E.; Sala-Vila, A.; Lázaro, I. Ellagic Acid as a Tool to Limit the Diabetes Burden: Updated Evidence. Antioxidants 2020, 9, 1226. [Google Scholar] [CrossRef]
- Pae, H.-O.; Oh, G.-S.; Jeong, S.-O.; Jeong, G.-S.; Lee, B.-S.; Choi, B.-M.; Lee, H.-S.; Chung, H.-T. 1,2,3,4,6-penta-O-galloyl-β-D-glucose up-regulates heme oxygenase-1 expression by stimulating Nrf2 nuclear translocation in an extracellular signal-regulated kinase-dependent manner in HepG2 cells. World J. Gastroenterol. 2006, 12, 214. [Google Scholar] [CrossRef]
- Sack, G.H. Serum amyloid A—A review. Mol. Med. 2018, 24, 46. [Google Scholar] [CrossRef]
- Marra, F.; Lotersztajn, S. Pathophysiology of NASH: Perspectives for a Targeted Treatment. Curr. Pharm. Des. 2013, 19, 5250–5269. [Google Scholar] [CrossRef]
- Liu, W.; Baker, R.D.; Bhatia, T.; Zhu, L.; Baker, S.S. Pathogenesis of nonalcoholic steatohepatitis. Cell. Mol. Life Sci. 2016, 73, 1969–1987. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Danesi, F.; Ferguson, L. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 2019, 70, 459–466. [Google Scholar] [CrossRef]
- Lu, J.; Chatterjee, M.; Schmid, H.; Beck, S.; Gawaz, M. CXCL14 as an emerging immune and inflammatory modulator. J. Inflamm. 2016, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Nara, N.; Nakayama, Y.; Okamoto, S.; Tamura, H.; Kiyono, M.; Muraoka, M.; Tanaka, K.; Taya, C.; Shitara, H.; Ishii, R.; et al. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J. Biol. Chem. 2007, 282, 30794–30803. [Google Scholar] [CrossRef]
- Cereijo, R.; Gavaldà-Navarro, A.; Cairó, M.; Quesada-López, T.; Villarroya, J.; Morón-Ros, S.; Sánchez-Infantes, D.; Peyrou, M.; Iglesias, R.; Mampel, T.; et al. CXCL14, a Brown Adipokine that Mediates Brown-Fat-to-Macrophage Communication in Thermogenic Adaptation. Cell Metab. 2018, 28, 750–763. [Google Scholar] [CrossRef]
- Riuzzi, F.; Chiappalupi, S.; Arcuri, C.; Giambanco, I.; Sorci, G.; Donato, R. S100 proteins in obesity: Liaisons dangereuses. Cell. Mol. Life Sci. 2020, 77, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Sturge, J.; Todd, S.K.; Kogianni, G.; McCarthy, A.; Isacke, C.M. Mannose receptor regulation of macrophage cell migration. J. Leukoc. Biol. 2007, 82, 585–593. [Google Scholar] [CrossRef]
- Wienke, D.; MacFadyen, J.R.; Isacke, C.M. Identification and characterization of the endocytic transmembrane glycoprotein Endo180 as a novel collagen receptor. Mol. Biol. Cell 2003, 14, 3592–3604. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Nguyen, T.K.Y.; Kase, E.T.; Tadesse, M.; Barsett, H.; Wangensteen, H. Enhanced Glucose Uptake in Human Liver Cells and Inhibition of Carbohydrate Hydrolyzing Enzymes by Nordic Berry Extracts. Molecules 2017, 22, 1806. [Google Scholar] [CrossRef]
- Haywood, N.J.; Slater, T.A.; Matthews, C.J.; Wheatcroft, S.B. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 2019, 19, 86–96. [Google Scholar] [CrossRef]
- McDougall, G.J.; Martinussen, I.; Junttila, O.; Verrall, S.; Stewart, D. Assessing the Influence of Genotype and Temperature on Polyphenol Composition in Cloudberry (Rubus chamaemorus L.) Using a Novel Mass Spectrometric Method. J. Agric. Food Chem. 2011, 59, 10860–10868. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Domínguez-Perles, R.; Gironés-Vilaplana, A.; Baenas, N.; García-Viguera, C.; Villaño, D. Flavan-3-ols, anthocyanins, and inflammation. IUBMB Life 2014, 66, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Marungruang, N.; Kostiuchenko, O.; Kravchenko, N.; Burleigh, S.; Prykhodko, O.; Hållenius, F.F.; Heyman-Lindén, L. Identification of Nordic Berries with Beneficial Effects on Cognitive Outcomes and Gut Microbiota in High-Fat-Fed Middle-Aged C57BL/6J Mice. Nutrients 2022, 14, 2734. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Pilon, G.; Dudonné, S.; Trottier, J.; St-Pierre, P.; Harris, C.S.; Lucas, M.; Lemire, M.; et al. Arctic berry extracts target the gut–liver axis to alleviate metabolic endotoxaemia, insulin resistance and hepatic steatosis in diet-induced obese mice. Diabetologia 2018, 61, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Seppänen-Laakso, T.; Kankainen, M.; Maukonen, J.; Törrönen, R.; Kolehmainen, M.; Leppänen, T.; Moilanen, E.; Nohynek, L.; Aura, A.-M.; et al. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol. Nutr. Food Res. 2013, 57, 2258–2263. [Google Scholar] [CrossRef]
- Törrönen, R.; Kolehmainen, M.; Sarkkinen, E.; Poutanen, K.; Mykkänen, H.; Niskanen, L. Berries reduce postprandial insulin responses to wheat and rye breads in healthy women. J. Nutr. 2013, 143, 430–436. [Google Scholar] [CrossRef]
- Ryyti, R.; Pemmari, A.; Peltola, R.; Hämäläinen, M.; Moilanen, E. Effects of Lingonberry (Vaccinium vitis-idaea L.) Supplementation on Hepatic Gene Expression in High-Fat Diet Fed Mice. Nutrients 2021, 13, 3693. [Google Scholar] [CrossRef]
- Ryyti, R.; Hämäläinen, M.; Peltola, R.; Moilanen, E. Beneficial effects of lingonberry (Vaccinium vitis-idaea L.) supplementation on metabolic and inflammatory adverse effects induced by high-fat diet in a mouse model of obesity. PLoS ONE 2020, 15, e0232605. [Google Scholar] [CrossRef]
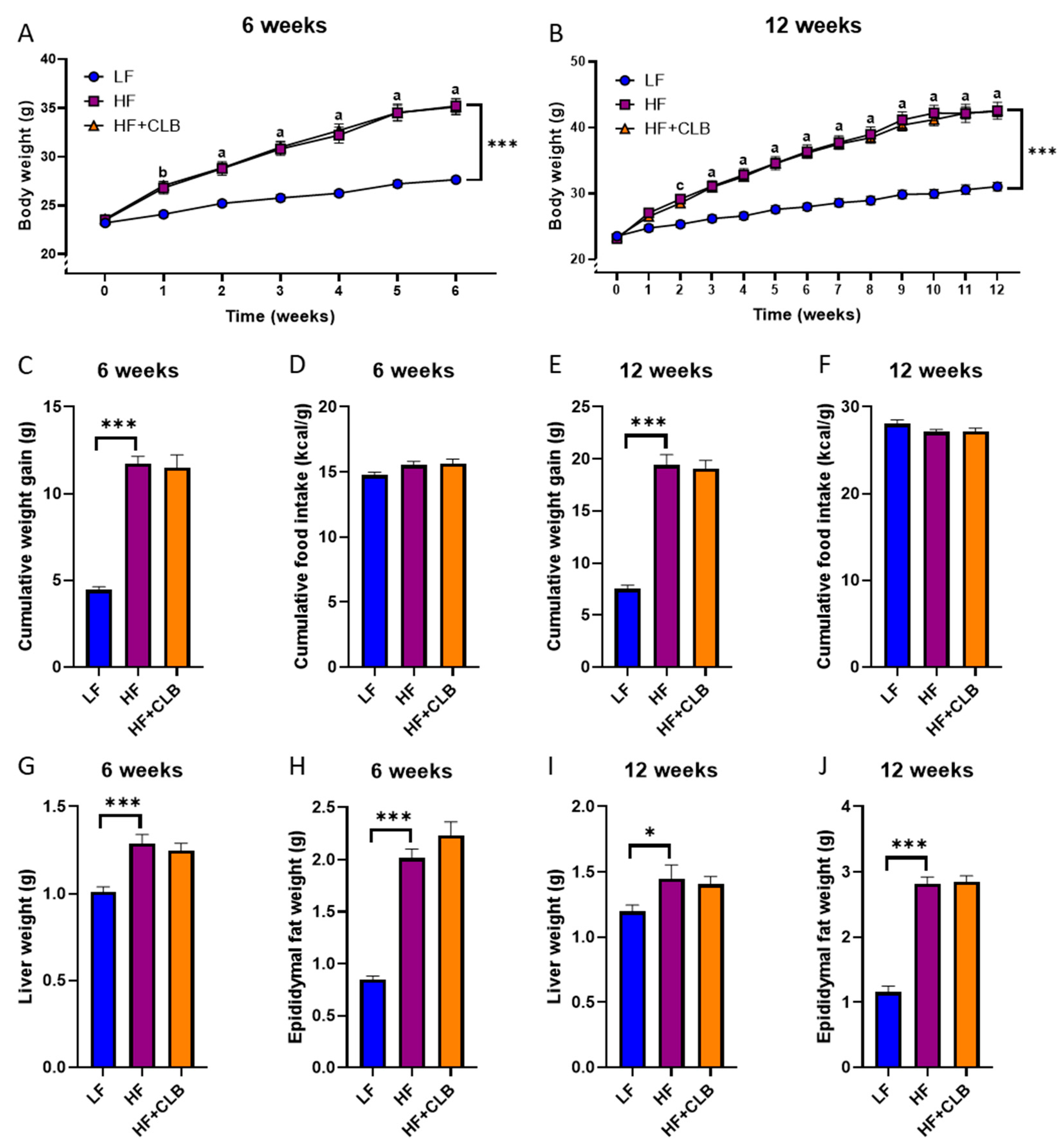

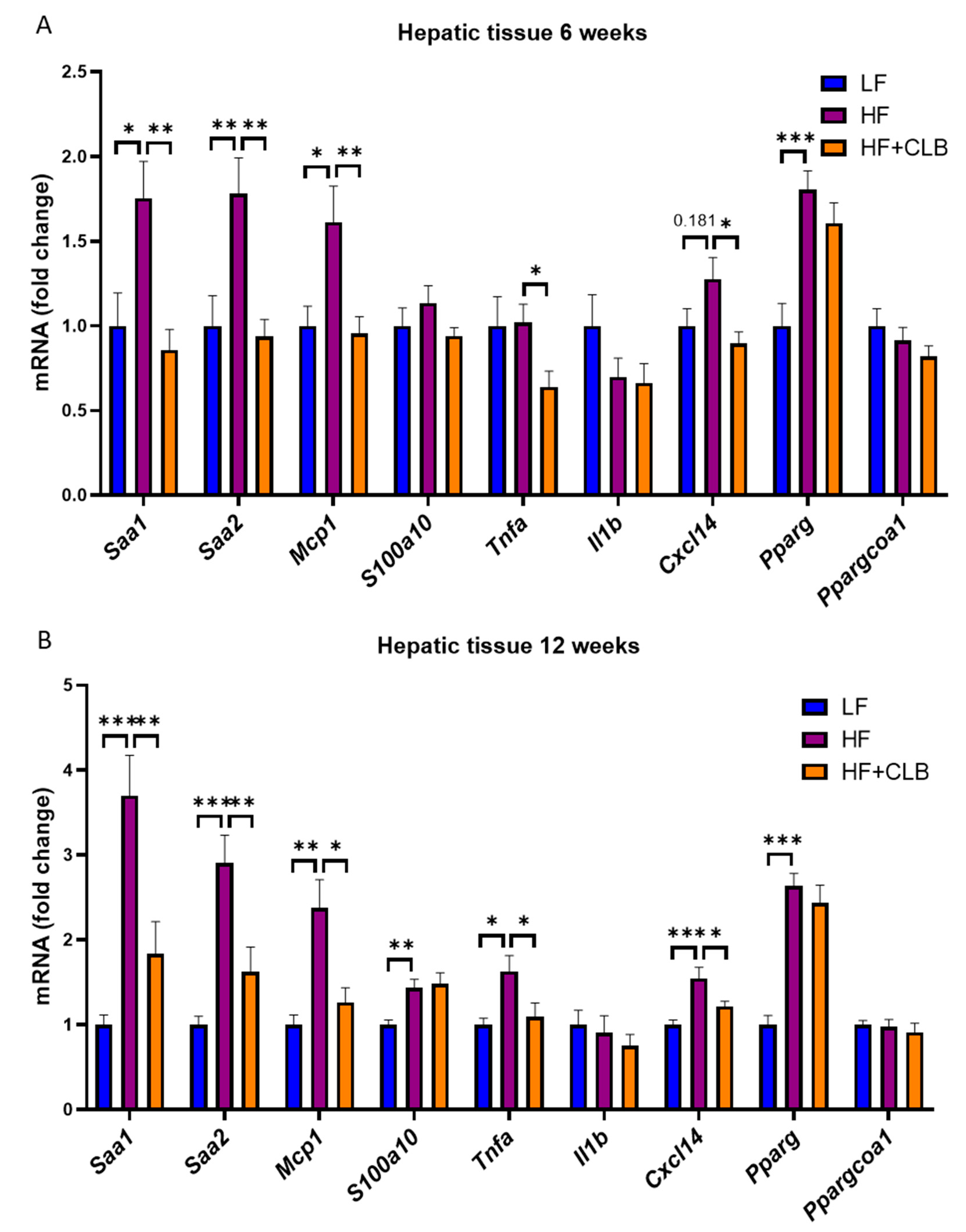
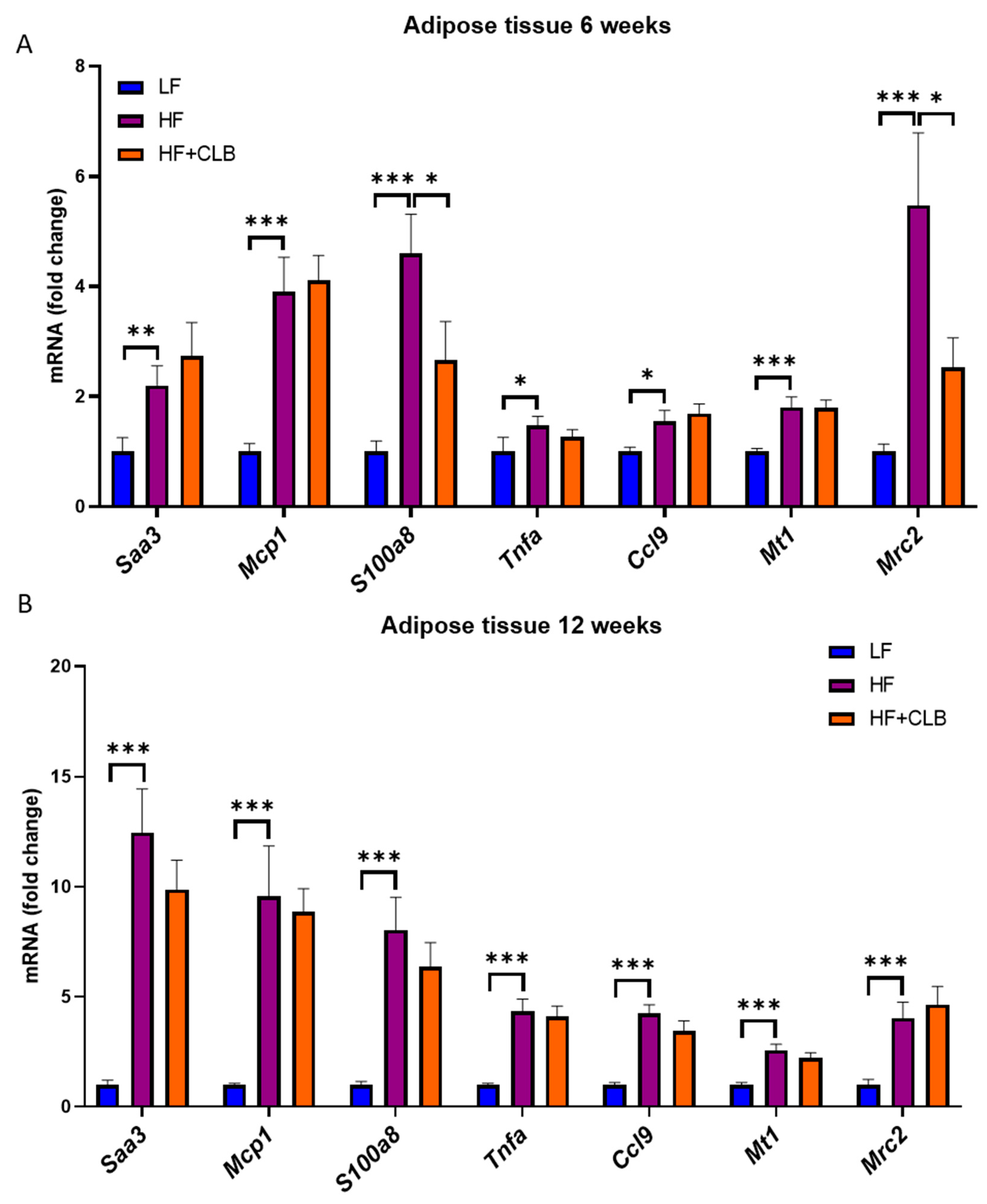
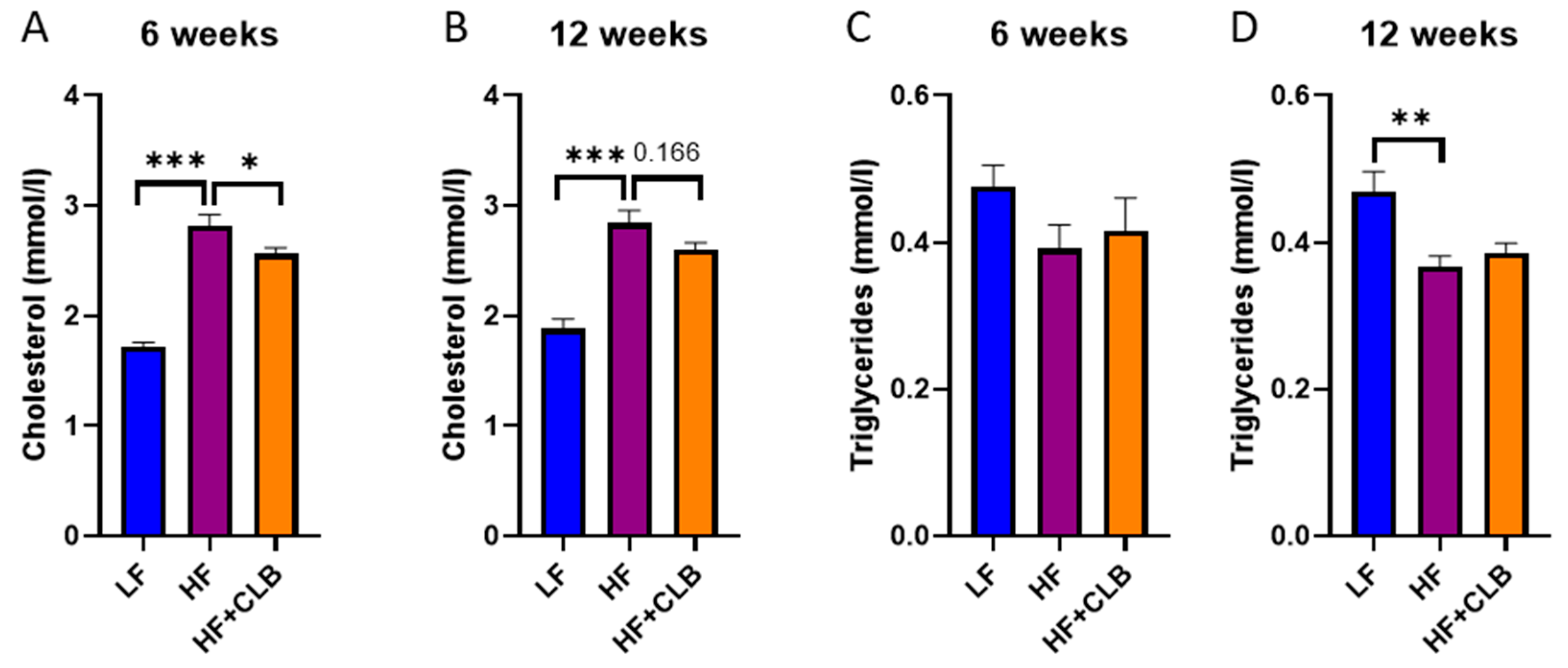
| Nutrient | Low-Fat Diet (LF) | High-Fat Diet (HF) | Cloudberry Supplemented High-Fat Diet (HF+CLB) |
|---|---|---|---|
| Calculated energy from protein, carbohydrate, and fat per gram of diet (kcal/g) | |||
| Protein | 0.7 | 0.8 | 0.8 |
| Carbohydrate | 2.7 | 1.6 | 1.6 |
| Fat | 0.4 | 2.0 | 2.1 |
| Total Energy | 3.7 | 4.6 | 4.4 |
| Calculated Energy (kcal %) | |||
| Protein | 29 | 18 | 18 |
| Carbohydrate | 72 | 36 | 36 |
| Fat | 10 | 46 | 46 |
| Total Energy | 100 | 100 | 100 |
| Ingredients (g) | |||
| Cloudberry powder (CLB, g) | 0 | 0 | 180 1 |
| Casein (protein) | 200 | 200 | 180 (+20 from CLB), total 200 |
| L-Cysteine | 3 | 3 | 3 |
| Corn Starch | 448 | 68 | 63 (+4 from CLB), total 67 |
| Maltodextrin 10 | 75 | 100 | 100 |
| Glucose | 28 | 28 | 6 (+22 from CLB), total 28 |
| Fructose | 38 | 38 | 11 (+27 from CLB), total 38 |
| Sucrose | 107 | 107 | 107 |
| Cellulose (insoluble fiber) | 54 | 54 | 0 (+54 from CLB), total 54 |
| Inulin (soluble fiber) | 12 | 12 | 0 (+12 from CLB), total 12 |
| Soybean oil | 25 | 25 | 8 (+11 from CLB), total 19 |
| Lard | 20 | 178 | 182 |
| Mineral Mix S10026 | 10 | 10 | 10 |
| DiCalcium Phosphate | 13 | 13 | 13 |
| Calcium Carbonate | 6 | 6 | 6 |
| Potassium Citrate | 17 | 17 | 17 |
| Vitamin Mix V10001 | 10 | 10 | 10 |
| Choline Bitartrate | 2 | 2 | 2 |
| Gene | Abbreviation | Assay ID |
|---|---|---|
| Adiponectin | Adipoq | Mm00456425_m1 |
| Adipsin | And | Mm01143935_g1 |
| Chemokine (C-C motif) ligand 9 | Ccl9 | Mm00441260_m1 |
| Chemokine (C-X-C) motif ligand 14 | Cxcl14 | Mm00444699_m1 |
| Glucose transporter type 2 | Glut2 | Mm00446229_m1 |
| Glucose transporter type 4 | Glut4 | Mm00436615_m1 |
| Insulin-like growth factor binding protein 2 | Igfbp2 | Mm00492632_m1 |
| Insulin receptor | Insr | Mm01211875_m1 |
| Interleukin 1β | Il1b | Mm00434228_m1 |
| Leptin | Lep | Mm00434759_m1 |
| Leptin receptor | Lepr | Mm00440181_m1 |
| Mannose receptor C type 2 | Mrc2 | Mm00485184_m1 |
| Metallothionein 1 | Mt1 | Mm00496660_g1 |
| Monocyte chemoattractant protein 1 | Mcp1 | Mm00441242_m1 |
| Peroxisome proliferator-activated receptor γ | Pparg | Mm01184322_m1 |
| Peroxisome proliferator-activated receptor γ coactivator 1 α | Ppargc1a | Mm01208835_m1 |
| Resistin | Retn | Mm00445641_m1 |
| S100 calcium-binding protein A8 | S100a8 | Mm00496696_g1 |
| S100 calcium-binding protein A10 | S100a10 | Mm00501458_g1 |
| Serum amyloid A1 | Saa1 | Mm00656927_g1 |
| Serum amyloid A2 | Saa2 | Mm04208126_mH |
| Serum amyloid A3 | Saa3 | Mm00441203_m1 |
| Adipokine | Week | LF | HF | HF+CLB | HF vs. LF p-Value | HF vs. HF+CLB p-Value |
|---|---|---|---|---|---|---|
| Serum | ||||||
| Adiponectin (mg/L) | 6 | 7.4 ± 0.20 | 6.2 ± 0.12 | 6.1 ± 0.10 | <0.001 | ns |
| 12 | 6.6 ± 0.12 | 6.4 ± 0.20 | 6.1 ± 0.11 | ns | ns | |
| Adipsin (mg/L) | 6 | 12 ± 0.38 | 7.5 ±0.33 | 7.3 ±0.43 | <0.001 | ns |
| 12 | 10 ± 0.38 | 6.9 ± 0.55 | 6.2 ± 0.27 | <0.001 | ns | |
| Leptin (μg/L) | 6 | 3.5 ± 0.29 | 26 ± 2.3 | 24 ± 2.3 | <0.001 | ns |
| 12 | 6.9 ± 1.1 | 39 ± 4.5 | 38 ± 3.3 | <0.001 | ns | |
| Resistin (μg/L) | 6 | 18 ± 0.69 | 17 ± 0.58 | 17 ± 0.39 | ns | ns |
| 12 | 19 ± 1.1 | 18 ± 0.63 | 17 ± 1.1 | ns | ns | |
| Adipose tissue, gene expression | ||||||
| Adiponectin | 6 | 1 ± 0.037 | 1.0 ± 0.093 | 1.0 ± 0.060 | ns | ns |
| 12 | 1 ± 0.049 | 0.85 ± 0.12 | 0.72 ± 0.094 | ns | ns | |
| Adipsin | 6 | 1 ± 0.071 | 0.57 ± 0.048 | 0.59 ± 0.040 | <0.001 | ns |
| 12 | 1 ± 0.077 | 0.41 ± 0.081 | 0.41 ± 0.061 | <0.001 | ns | |
| Leptin | 6 | 1 ± 0.094 | 3.8 ± 0.35 | 3.7 ± 0.24 | <0.001 | ns |
| 12 | 1 ± 0.15 | 2.5 ± 0.22 | 2.4 ± 0.21 | <0.001 | ns | |
| Leptin receptor | 6 | 1 ± 0.10 | 0.80 ± 0.11 | 0.67 ± 0.097 | ns | ns |
| 12 | 1 ± 0.18 | 0.87 ± 0.19 | 0.95 ± 0.37 | ns | ns | |
| Resistin | 6 | 1 ± 0.053 | 0.89 ± 0.081 | 0.99 ± 0.10 | ns | ns |
| 12 | 1 ± 0.063 | 0.54 ± 0.047 | 0.54 ± 0.084 | <0.001 | ns | |
| Hepatic tissue, gene expression | ||||||
| Adipsin | 6 | 1 ± 0.38 | 9.3 ± 1.6 | 7.6 ± 1.4 | <0.001 | ns |
| 12 | 1 ± 0.31 | 30 ± 6.9 | 53 ± 15 | <0.001 | ns | |
| Leptin receptor | 6 | 1 ± 0.12 | 0.18 ± 0.0091 | 0.092 ± 0.012 | <0.001 | ns |
| 12 | 1 ± 0.13 | 0.19 ± 0.027 | 0.12 ± 0.012 | <0.001 | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pemmari, T.; Hämäläinen, M.; Ryyti, R.; Peltola, R.; Moilanen, E. Cloudberry (Rubus chamaemorus L.) Supplementation Attenuates the Development of Metabolic Inflammation in a High-Fat Diet Mouse Model of Obesity. Nutrients 2022, 14, 3846. https://doi.org/10.3390/nu14183846
Pemmari T, Hämäläinen M, Ryyti R, Peltola R, Moilanen E. Cloudberry (Rubus chamaemorus L.) Supplementation Attenuates the Development of Metabolic Inflammation in a High-Fat Diet Mouse Model of Obesity. Nutrients. 2022; 14(18):3846. https://doi.org/10.3390/nu14183846
Chicago/Turabian StylePemmari, Toini, Mari Hämäläinen, Riitta Ryyti, Rainer Peltola, and Eeva Moilanen. 2022. "Cloudberry (Rubus chamaemorus L.) Supplementation Attenuates the Development of Metabolic Inflammation in a High-Fat Diet Mouse Model of Obesity" Nutrients 14, no. 18: 3846. https://doi.org/10.3390/nu14183846
APA StylePemmari, T., Hämäläinen, M., Ryyti, R., Peltola, R., & Moilanen, E. (2022). Cloudberry (Rubus chamaemorus L.) Supplementation Attenuates the Development of Metabolic Inflammation in a High-Fat Diet Mouse Model of Obesity. Nutrients, 14(18), 3846. https://doi.org/10.3390/nu14183846






