Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review
Abstract
:1. Introduction
2. Literature Searching Strategy
3. Ferulic Acid: General Aspects
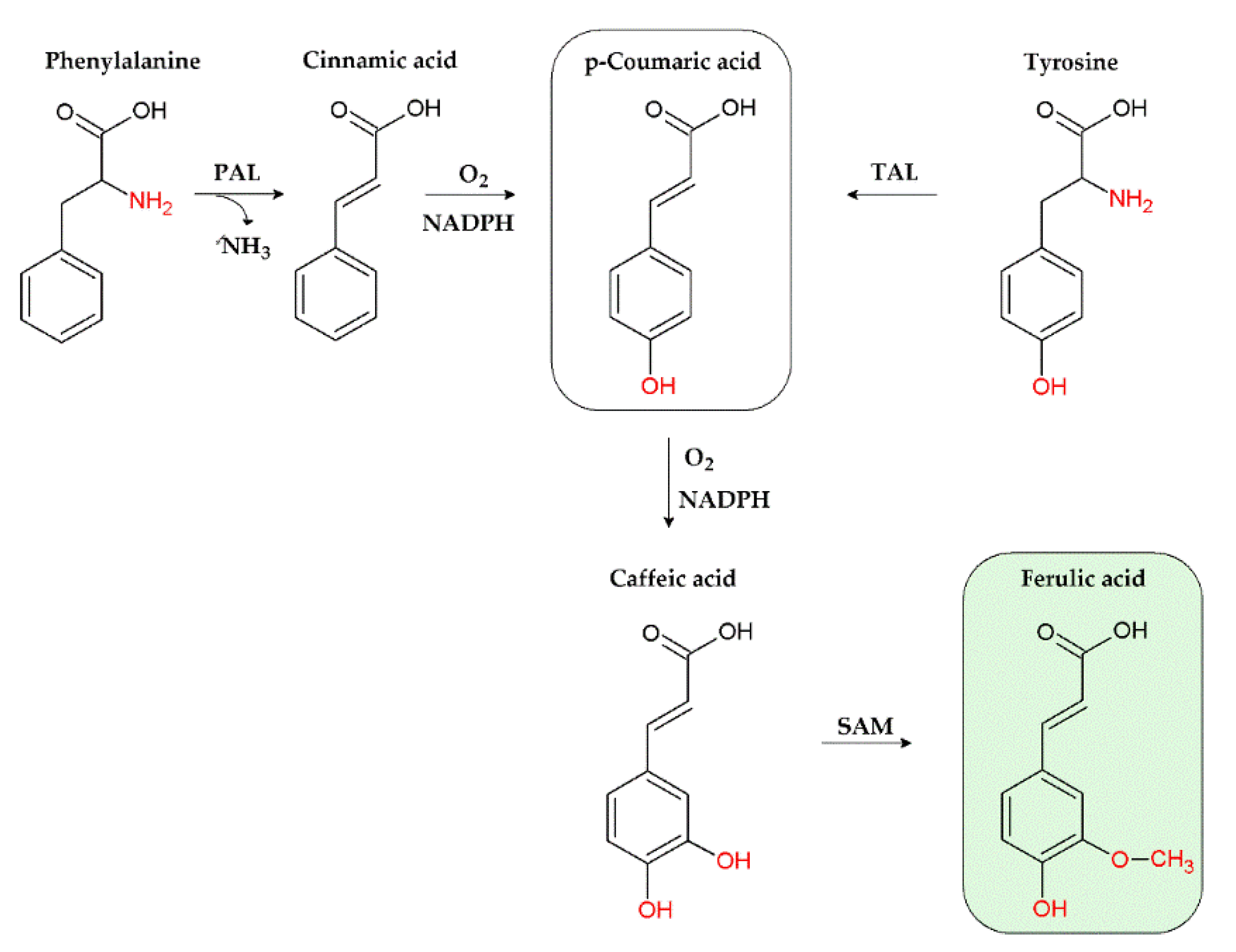
3.1. Natural Occurrence
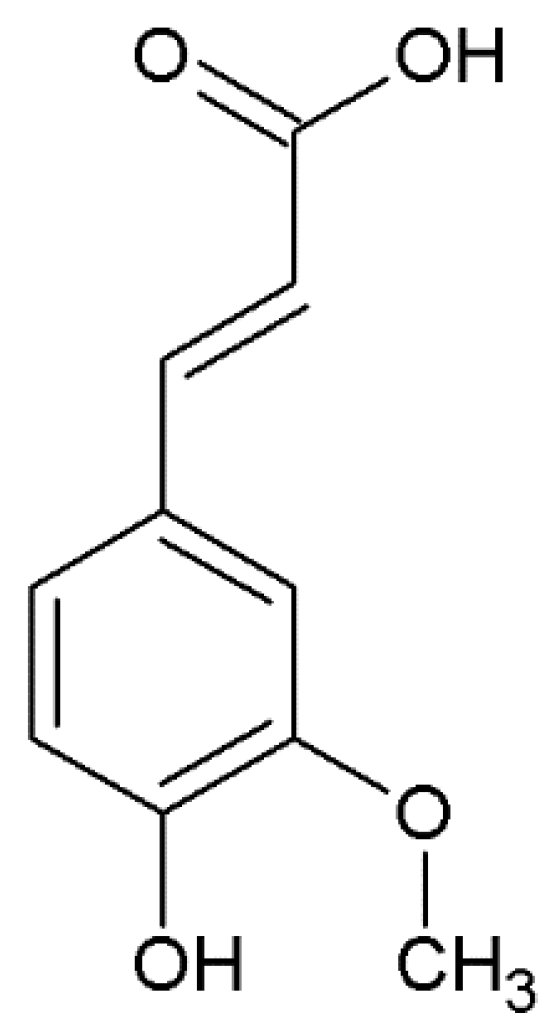
3.2. Chemical Features
4. Pharmacology of Ferulic Acid
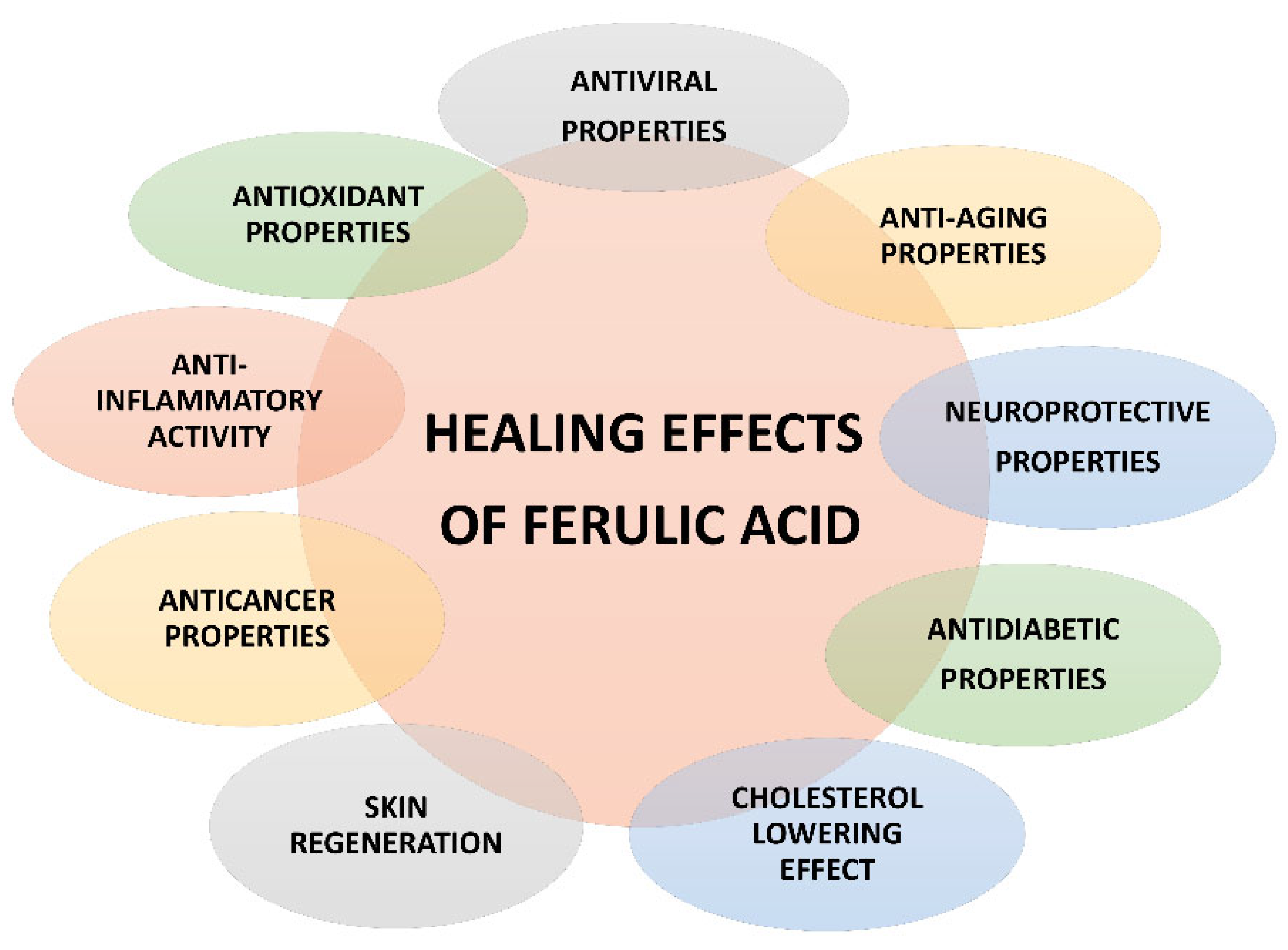
4.1. Pharmacological Activities and Mechanisms of Action
4.1.1. Anti-Inflammatory Properties
4.1.2. Antioxidant Properties
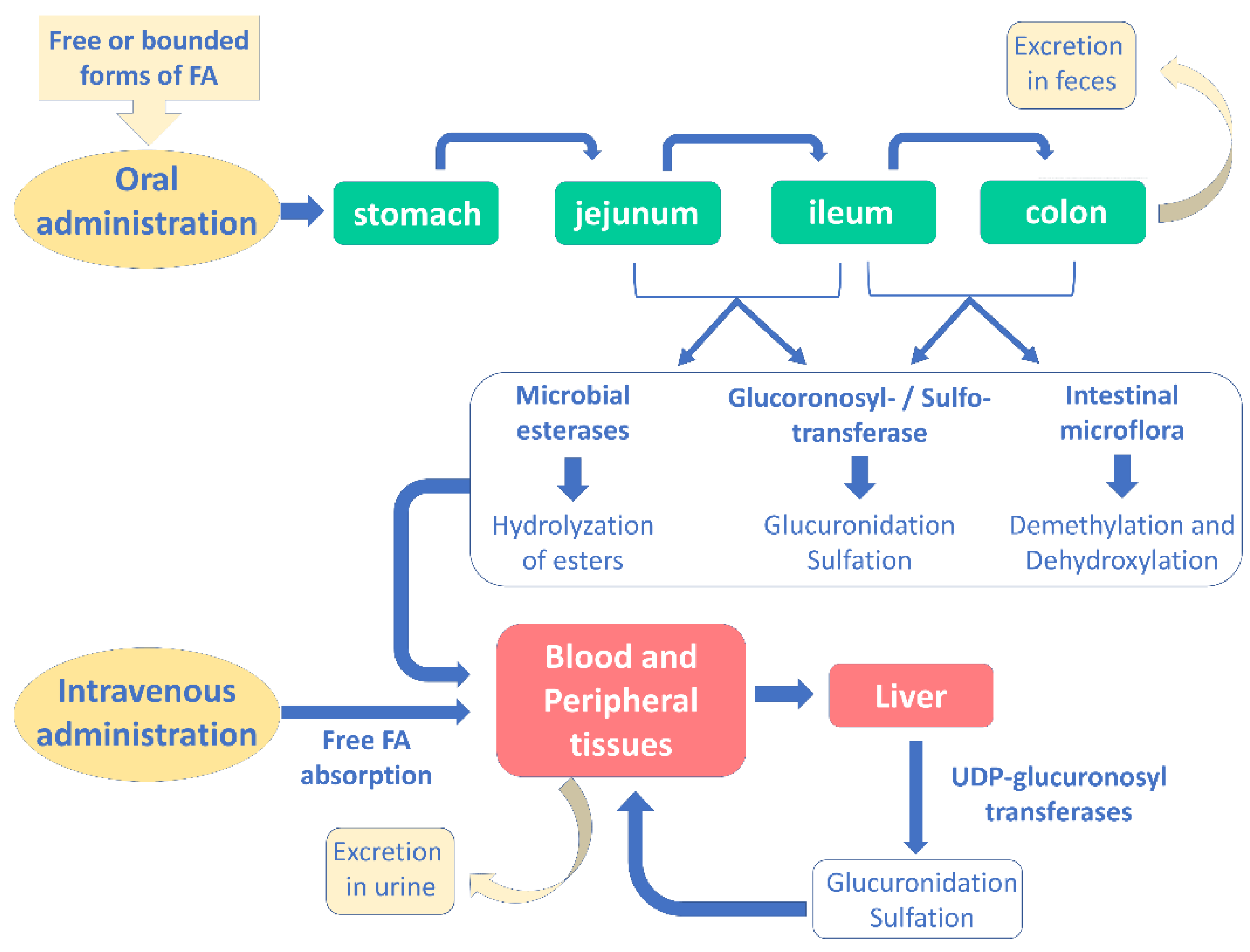
4.2. Pharmacokinetic Properties
5. Ferulic Acid and Alzheimer’s Disease (AD)
5.1. In Vitro Studies
5.2. In Vivo Studies
5.3. Clinical Trials
6. Ferulic Acid as a Possible Strategy to Fight Type 3 Diabetes
7. Safety Profile
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carmona, S.; Hardy, J.; Guerreiro, R. The genetic landscape of Alzheimer disease. Handb. Clin. Neurol. 2018, 148, 395–408. [Google Scholar] [PubMed]
- Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures; Alzheimer’s Dementia: Chicago, IL, USA, 2021; Volume 17. [Google Scholar]
- Wang, E.J.; Wu, M.Y.; Lu, J.H. Ferulic acid in animal models of Alzheimer’s disease: A systematic review of preclinical studies. Cells 2021, 10, 2653. [Google Scholar] [CrossRef] [PubMed]
- Cacace, R.; Sleegers, K.; Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer’s disease revisited. J. Alzheimer’s Dis. 2016, 12, 733–748. [Google Scholar] [CrossRef]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s disease as type 3 diabetes: Common pathophysiological mechanisms between Alzheimer’s disease and type 2 diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef] [PubMed]
- Richard, R.; Mousa, S. Necroptosis in Alzheimer’s disease: Potential therapeutic target. Biomed. Pharmacother. 2022, 152, 113203. [Google Scholar] [CrossRef]
- Yang, J.J. Brain insulin resistance and the therapeutic value of insulin and insulin-sensitizing drugs in Alzheimer’s disease neuropathology. Acta Neurol. Belg. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Loures, C.; Alves, L.; de Souza, L.C.; Borges, K.; Carvalho, M. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Kivipelto, M.; von Strauss, E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialog. Clin. Neurosci. 2009, 11, 111–128. [Google Scholar] [CrossRef]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.; Hofman, A.; Breteler, M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chung, C.M.; Leu, H.B.; Lin, L.Y.; Chiu, C.C.; Hsu, C.Y.; Chiang, C.H.; Huang, P.H.; Chen, T.J.; Lin, S.J.; et al. Diabetes mellitus and the risk of Alzheimer’s disease: A nationwide population-based study. PLoS ONE 2014, 9, e87095. [Google Scholar] [CrossRef]
- Crane, P.K.; Walker, R.; Hubbard, R.A.; Li, G.; Nathan, D.M.; Zheng, H.; Haneuse, S.; Craft, S.; Montine, T.J.; Kahn, S.E.; et al. Glucose levels and risk of dementia. N. Engl. J. Med. 2013, 369, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Macauley, S.L.; Stanley, M.; Caesar, E.E.; Yamada, S.A.; Raichle, M.E.; Perez, R.; Mahan, T.E.; Sutphen, C.L.; Holtzman, D.M. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J. Clin. Investig. 2015, 125, 2463–2467. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M.; Tong, M.; Daiello, L.A.; Ott, B.R. Earlystage Alzheimer’s disease is associated with simultaneous systemic and central nervous system dysregulation of insulinlinked metabolic pathways. J. Alzheimer’s Dis. 2019, 68, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; Watson, G.S.; Frey, W.H., 2nd; Baker, L.D.; Cholerton, B.; Keeling, M.L.; Belongia, D.A.; Fishel, M.A.; Plymate, S.R.; Schellenberg, G.D.; et al. Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol. Aging 2006, 27, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, H.; Sato, T.; Kiuchi, A.; Sakurai, H.; Iwamoto, T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer’s disease and mild cognitive impairment with diabetes mellitus. J. Am. Geriatr. Soc. 2009, 57, 177–179. [Google Scholar] [CrossRef]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; Grosso, G.; et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2022, 232, 108013. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and inflammation in cognitive ageing and alzheimer’s disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Bacci, A.; Runfola, M.; Sestito, S.; Rapposelli, S. Beyond antioxidant effects: Nature-based templates unveil new strategies for neurodegenerative diseases. Antioxidants 2021, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, M. Gut microbiome changes induced by a diet rich in fruits and vegetables. Int. J. Food Sci. Nutr. 2021, 72, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic acids and prevention of cognitive decline: Polyphenols with a neuroprotective role in cognitive disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Arslan, M.E.; Barboza, J.N.; Kahraman, C.Y.; de Sousa, D.P.; Mardinoğlu, A. Therapeutic potential of ferulic acid in Alzheimer’s disease. Curr. Drug. Deliv. 2022, 19, 860–873. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P. Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sonika Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic acid: A promising therapeutic phytochemical and recent patents advances. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic acid from plant biomass: A phytochemical with promising antiviral properties. Front. Nutr. 2022, 8, 777576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Appl. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Niazmand, R.; Razavizadeh, B.M. Ferula asafoetida: Chemical composition, thermal behavior, antioxidant and antimicrobial activities of leaf and gum hydroalcoholic extracts. Food Sci. Technol. 2021, 58, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Iranshahy, M.; Iranshahi, M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)-a review. J. Ethnopharmacol. 2011, 134, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.L.; Huang, L.F. Simultaneous determination of ferulic acid and phthalides of Angelica sinensis based on UPLC-Q-TOF/MS. Molecules 2015, 20, 4681–4694. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, M.; Jin, L.; Xie, X.; Li, M.; Wei, J. Cool temperature enhances growth, ferulic acid and flavonoid biosynthesis while inhibiting polysaccharide biosynthesis in Angelica sinensis. Molecules 2022, 27, 320. [Google Scholar] [CrossRef]
- Ran, X.; Ma, L.; Peng, C.; Zhang, H.; Qin, L.P. Ligusticum chuanxiong Hort: A review of chemistry and pharmacology. Pharm. Biol. 2011, 49, 1180–1189. [Google Scholar] [CrossRef]
- Zgórka, G.; Głowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Kapalavavi, B.; Doctor, N.; Zhang, B.; Yang, Y. Subcritical Water Extraction of Salvia miltiorrhiza. Molecules 2021, 26, 1634. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yin, T.; Wang, X.; Zhang, F.; Pan, G.; Lv, H.; Wang, X.; Owoicho Orgah, J.; Zhu, Y.; Wu, H. Traditional uses, phytochemistry, pharmacology and toxicology of the genus Cimicifuga: A review. J. Ethnopharmacol. 2017, 209, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, X.; Ren, X.; Liu, X.; Wang, Y.; Dong, Y.; Wang, X.; Sun, S.; Xu, X.; Li, X.; et al. Sparganii Rhizoma: A review of traditional clinical application, processing, phytochemistry, pharmacology, and toxicity. J. Ethnopharmacol. 2021, 268, 113571. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.N.; Wu, Y.; Wu, C.; Yue, W.; Liang, Q. Determination of seven phenolic compounds in Rhizoma Sparganii by RP-HPLC. J. Chromatograph. Sci. 2013, 51, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sood, A.; Lang, D.K.; Arora, R.; Kumar, N.; Diwan, V.; Saini, B. Natural products as sources of multitarget compounds: Advances in the development of ferulic acid as multitarget therapeutic. Curr. Top. Med. Chem. 2022, 22, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Babbar, R.; Dhiman, S.; Grover, R.; Kaur, A.; Arora, S. A comprehensive review on therapeutic applications of ferulic acid and its novel analogues: A brief literature. Mini. Rev. Med. Chem. 2021, 21, 1578–1593. [Google Scholar] [CrossRef]
- Rong, N.; Ausman, L.M.; Nicolosi, R.J. Oryzanol decreases cholesterol absorption and aortic fatty streaks in hamsters. Lipids 1997, 32, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Nankar, R.; Prabhakar, P.K.; Doble, M. Hybrid drug combination: Combination of ferulic acid and metformin as anti-diabetic therapy. Phytomedicine 2017, 37, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Talukdar, A.D.; Nath, R.; Sarker, S.D.; Nahar, L.; Sahu, J.; Choudhury, M.D. Role of natural phenolics in hepatoprotection: A mechanistic review and analysis of the regulatory network of associated genes. Front. Pharmacol. 2019, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Zuo, S.; Wu, R.M.; Deng, K.S.; Lu, S.; Zhu, J.J.; Zou, G.L.; Yang, J.; Cheng, M.L.; Zhao, X.K. Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway. Drug Des. Devel. Ther. 2018, 12, 4107–4115. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, K.; Lv, L.; Wu, S.; Guo, Z. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE-/-mice. Biomed. Pharmacother. 2019, 113, 108753. [Google Scholar] [CrossRef]
- Girsang, E.; Lister, I.N.; Ginting, C.N.; Bethasari, M.; Amalia, A.; Widowati, W. Comparison of antiaging and antioxidant activities of protocatechuic and ferulic acids. Mol. Cell Biomed. Sci. 2020, 4, 68–75. [Google Scholar] [CrossRef]
- Drăgan, M.; Tătăringă, G.; Mircea, C.; Cioancă, O.; Dragostin, O.; Iacob, A.T.; Profire, L.; Stan, C.D. Ferulic acid-a versatile molecule. Acta Biol. Marisiensis 2018, 1, 53–60. [Google Scholar] [CrossRef]
- Bhowmik, D.; Nandi, R.; Jagadeesan, R.; Kumar, N.; Prakash, A.; Kumar, D. Identification of potential inhibitors against SARSCoV-2 by targeting proteins responsible for envelope formation and virion assembly using docking based virtual screening, and pharmacokinetics approaches. Infect. Genet. Evol. 2020, 84, 104451. [Google Scholar] [CrossRef]
- Kaur, S.; Dhiman, M.; Mantha, A.K. Ferulic Acid: A natural antioxidant with application towards neuroprotection against Alzheimer’s disease. In Functional Food and Human Health; Rani, V., Yadav, U., Eds.; Springer: Singapore, 2018. [Google Scholar]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative Stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Shen, J.D.; Xu, L.P.; Li, H.B.; Li, Y.C.; Yi, L.T. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ali, T.; Alam, S.I.; Ullah, R.; Zeb, A.; Lee, K.W.; Rutten, B.; Kim, M.O. Ferulic acid rescues LPS-induced neurotoxicity via modulation of the TLR4 receptor in the mouse hippocampus. Mol. Neurobiol. 2019, 56, 2774–2790. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Go, E.K.; Kim, J.Y.; Yu, B.P.; Chung, H.Y. Suppression of age-related renal changes in NF-kappaB and its target gene expression by dietary ferulate. J. Nutr. Biochem. 2009, 20, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Su, S.Y.; Tang, N.Y.; Ho, T.Y.; Chiang, S.Y.; Hsieh, C.L. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008, 1209, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.C.; Hong, Q.; Wang, Y.G.; Tan, H.L.; Xiao, C.R.; Liang, Q.D.; Cai, S.H.; Gao, Y. Ferulic acid attenuates adhesion molecule expression in gamma-radiated human umbilical vascular endothelial cells. Biol. Pharm. Bull. 2010, 33, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Zhang, Z.; Li, J.; Shi, Y.; Jin, N.; Zou, W.; Gao, Q.; Wang, W.; Liu, F. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NK-κB and MAPK pathway. Res. Vet. Sci. 2019, 126, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ge, K.; Mu, J.; Rong, J.; Zhang, L.; Wang, B.; Wan, J.; Xia, G. Ferulic acid attenuated acetaminophen-induced hepatotoxicity though down-regulating the cytochrome P 2E1 and inhibiting toll-like receptor 4 signaling-mediated inflammation in mice. Am. J. Transl. Res. 2016, 8, 4205–4214. [Google Scholar] [PubMed]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Helal, S.A. Upregulation of PPAR-γ mediates the renoprotective effect of omega-3 PUFA and ferulic acid in gentamicin-intoxicated rats. Biomed. Pharmacother. 2018, 99, 504–510. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Abd El-Twab, S.M.; Hozayen, W.G. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct. 2019, 10, 4593–4607. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F.L.; Munteanu, M.A.; Brisc, M.C.; et al. Current trends in neurodegeneration: Cross talks between oxidative stress, cell death, and inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Gao, X.; Chen, G.; Wang, Z. Probing the structure-antioxidant activity relationships of four cinnamic acids porous starch esters. Carbohydr. Polym. 2021, 256, 117428. [Google Scholar] [CrossRef] [PubMed]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Di Sotto, A.; Locatelli, M.; Macone, A.; Toniolo, C.; Cesa, S.; Carradori, S.; Eufemi, M.; Mazzanti, G.; Di Giacomo, S. Hypoglycemic, antiglycation, and cytoprotective properties of a phenol-rich extract from waste peel of Punica granatum L. var. Dente di Cavallo DC2. Molecules 2019, 24, 3103. [Google Scholar] [CrossRef] [Green Version]
- Alim, Z.; Kilinç, N.; Sengül, B.; Beydemir, Ş. Inhibition behaviours of some phenolic acids on rat kidney aldose reductase enzyme: An in vitro study. J. Enzyme Inhib. Med. Chem. 2017, 32, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Badawy, D.; El-Bassossy, H.M.; Fahmy, A.; Azhar, A. Aldose reductase inhibitors zopolrestat and ferulic acid alleviate hypertension associated with diabetes: Effect on vascular reactivity. Can. J. Physiol. Pharmacol. 2013, 91, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.Y.; Guo, J.; Majeed, H.; Zhu, K.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Ferulic acid renders protection to HEK293 cells against oxidative damage and apoptosis induced by hydrogen peroxide. Vitr. Cell Dev. Biol. Anim. 2015, 51, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.C.; Hong, Q.; Wang, Y.G.; Tan, H.L.; Xiao, C.R.; Liang, Q.D.; Zhang, B.L.; Gao, Y. Ferulic acid protects human umbilical vein endothelial cells from radiation induced oxidative stress by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Biol. Pharm. Bull. 2010, 33, 29–34. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic acid sugar esters are recovered in rat plasma and urine mainly as the sulfoglucuronide of ferulic acid. J. Nutr. 2003, 133, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Wolffram, S.; Weber, T.; Grenacher, B.; Scharrer, E. A Na(+)-dependent mechanismis involved in mucosal uptake of cinnamic acid across the jejunal brush border in rats. J. Nutr. 1995, 125, 1300–1308. [Google Scholar]
- Konishi, Y.; Zhao, Z.; Shimizu, M. Phenolic acids are absorbed from the rat stomach with different absorption rates. J. Agric. Food Chem. 2006, 54, 7539–7543. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.F.; Gao, M.Y.; Zhu, Q.H.; Fan Mo, H.L.L.; Gao, S. Studies on pharmacokinetics and metabolism of ferulic acid. Asian J. Pharm. Pharm. 2009, 9, 135–143. [Google Scholar]
- Li, Y.; Liu, C.; Zhang, Y.; Mi, S.; Wang, N. Pharmacokinetics of ferulic acid and potential interactions with Honghua and clopidogrel in rats. J. Ethnopharmacol. 2011, 137, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.X.; Xu, L.Y.; Tao, J.S.; Feng, Y. Metabolism and pharmacokinetics of ferulic acid in rats. China J. Chin. Mater. Med. 1993, 18, 300–319. [Google Scholar]
- Rondini, L.; Peyrat-Maillard, M.N.; Marsset-Baglieri, A.; Berset, C. Sulfated ferulic acid is the main in vivo metabolite found after short-term ingestion of free ferulic acid in rats. J. Agric. Food Chem. 2002, 50, 3037–3041. [Google Scholar] [CrossRef]
- Panwar, R.; Raghuwanshi, N.; Srivastava, A.K.; Sharma, A.K.; Pruthi, V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater. Sci. Eng. C 2018, 92, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Phenolic antioxidants richly contained in corn bran are slightly bioavailable in rats. J. Agric. Food Chem. 2005, 53, 5030–5035. [Google Scholar] [CrossRef] [PubMed]
- Rondini, L.; Peyrat-Maillard, M.N.; Marsset-Baglieri, A.; Fromentin, G.; Durand, P. Bound ferulic acid frombran is more bioavailable than the free compoundin rat. J. Agric. Food Chem. 2004, 52, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, U.; Kaur, U.; Chakrabarti, S.S.; Sharma, P.; Agrawal, B.K.; Saso, L.; Chakrabarti, S. Oxidative stress, neuroinflammation, and NADPH oxidase: Implications in the pathogenesis and treatment of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2021, 2021, 7086512. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, M.; Tsutsuki, H.; Ida, T.; Nakajima, H.; Ihara, H.; Sakamoto, T. Water-soluble ferulic acid derivatives improve amyloidbeta-induced neuronal cell death and dysmnesia through inhibition of amyloid-beta aggregation. Biosci. Biotechnol. Biochem. 2016, 80, 547–553. [Google Scholar] [CrossRef]
- Huang, F.; Deng, H.M.; Zhu, M.M.; Xiao, F.; Yang, L.; Zhang, Z.J.; Xiao, Y.; Nie, H. Inhibitory effect of ferulic acid on inflammatory response in microglia induced by lipopolysaccharides. Zool. Res. 2011, 32, 311–316. [Google Scholar]
- Dong, G.C.; Kuan, C.Y.; Subramaniam, S.; Zhao, J.Y.; Sivasubramaniam, S.; Chang, H.Y.; Lin, F.H. A potent inhibition of oxidative stress induced gene expression in neural cells by sustained ferulic acid release from chitosan-based hydrogel. Mater. Sci. Eng. C 2015, 49, 691–699. [Google Scholar] [CrossRef]
- Huang, H.; Hong, Q.; Tan, H.-L.; Xiao, C.-R.; Gao, Y. Ferulic acid prevents LPS-induced up-regulation of PDE4B and stimulates the cAMP/CREB signaling pathway in PC12 cells. Acta Pharmacol. Sin. 2016, 37, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Peng, Y.F.; Hou, C.W. Ferulic acid protects PC12 neurons against hypoxia by inhibiting the p-MAPKs and COX-2 pathways. Iran. J. Basic Med. Sci. 2015, 18, 478–484. [Google Scholar] [PubMed]
- Rosini, M.; Simoni, E.; Caporaso, R.; Basagni, F.; Catanzaro, M.; Abu, I.F.; Fagiani, F.; Fusco, F.; Masuzzo, S.; Albani, D.; et al. Merging memantine and ferulic acid to probe connections between NMDA receptors, oxidative stress and amyloid-β peptide in Alzheimer’s disease. Eur. J. Med. Chem. 2019, 180, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Cho, J.Y.; Kim, H.S.; Kim, K.L.; Jung, J.S.; Huh, S.O.; Suh, H.W.; Kim, Y.H.; Song, D.K. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharmacol. 2001, 133, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cho, J.Y.; Kim, D.H.; Yan, J.J.; Lee, H.K.; Suh, H.W.; Song, D.K. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of β-amyloid peptide (1–42) in mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.Y.; Kim, H.S.; Kim, D.H.; Yan, J.J.; Suh, H.W.; Song, D.K. Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of beta-amyloid peptide (1–42) in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 901–907. [Google Scholar] [CrossRef]
- Jin, Y.; Yan, E.Z.; Fan, Y.; Zong, Z.H.; Qi, Z.M.; Li, Z. Sodium ferulate prevents amyloid-beta-induced neurotoxicity through suppression of p38 MAPK and upregulation of ERK-1/2 and Akt/protein kinase B in rat hippocampus. Acta Pharmacol. Sin. 2005, 26, 943–951. [Google Scholar] [CrossRef]
- Jin, Y.; Fan, Y.; Yan, E.Z.; Liu, Z.; Zong, Z.H.; Qi, Z.M. Effects of sodium ferulate on amyloid-beta-induced MKK3/MKK6-p38 MAPK-Hsp27 signal pathway and apoptosis in rat hippocampus. Acta Pharmacol. Sin. 2006, 27, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Choi, S.J.; Lim, S.T.; Kim, H.K.; Heo, H.J.; Kim, E.K.; Jun, W.J.; Cho, H.Y.; Kim, Y.J.; Shin, D.H. Ferulic acid supplementation prevents trimethyltininduced cognitive deficits in mice. Biosci. Biotechnol. Biochem. 2007, 71, 1063–1068. [Google Scholar] [CrossRef]
- Mamiya, T.; Kise, M.; Morikawa, K. Ferulic acid attenuated cognitive deficits and increase in carbonyl proteins induced by buthionine-sulfoximine in mice. Neurosci. Lett. 2008, 430, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yan, E.Z.; Li, X.M.; Fan, Y.; Zhao, Y.J.; Liu, Z.; Liu, W.Z. Neuroprotective effect of sodium ferulate and signal transduction mechanisms in the aged rat hippocampus. Acta Pharmacol. Sin. 2008, 29, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenol.lic compounds prevent Alzheimer’s pathology through different effects on the amyloid-beta aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Beibei, J.; Qin, C.; Qinglin, C. Effect of Ferulic Acid on Learing-memory and Expression of GFAP in the Hippocampus Tissue of Alzheimer’s Disease-like Modle Mice. Acta Laser Biol. Sin. 2011, 20, 484–489. [Google Scholar]
- Yan, J.J.; Jung, J.S.; Kim, T.K.; Hasan, A.; Hong, C.W.; Nam, J.S.; Song, D.K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical beta-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.S.; Wu, L.Y.; Yang, S.E.; Cheng, H.Y.; Tsai, C.C.; Wu, C.R.; Lin, L.W. Ferulic acid reverses the cognitive dysfunction caused by amyloid beta peptide 1-40 through anti-oxidant activity and cholinergic activation in rats. Am. J. Chin. Med. 2015, 43, 319–335. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 11310–11325. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Xu, W.; Song, Y.U.; Chun, W. Effects of ferulic acid on oxidative stress and apoptosis related proteins in Alzheimer’s disease transgenic mice. Nat. Prod. Res. Dev. 2017, 29, 762–766. [Google Scholar]
- Rui, M.; Yi-qing, C.; Qin, C. Effects of ferulic acid on g.glial activation and inflammatory cytokines expression in the cerebral cortex of Alzheimer’s disease like model mice. Chin. Hosp. Pharm. J. 2018, 38, 50–53. [Google Scholar]
- Zafeer, M.F.; Firdaus, F.; Anis, E.; Mobarak Hossain, M. Prolong treatment with Trans-ferulic acid mitigates bioenergetics loss and restores mitochondrial dynamics in streptozotocin-induced sporadic dementia of Alzheimer’s type. Neurotoxicology 2019, 73, 246–257. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combined treatment with the phenolics (-)-epigallocatechin-3-gallate and ferulic acid improves cognition and reduces Alzheimer-like pathology in mice. J. Biol. Chem. 2019, 294, 2714–2731. [Google Scholar] [CrossRef]
- Qian, W.; Wei-wei, Q.; Jie-wen, Z. Effect of ferulic acid on learning and memory impairment by the repairing of mitochondrial fission-fusion imbalance in AD Mice. Chin. Pharm. J. 2019, 54, 703–710. [Google Scholar]
- Wang, N.Y.; Li, J.N.; Liu, W.L.; Huang, Q.; Li, W.X.; Tan, Y.H.; Liu, F.; Song, Z.H.; Wang, M.Y.; Xie, N.; et al. Ferulic acid ameliorates Alzheimer’s disease-like pathology and repairs cognitive decline by preventing capillary hypofunction in APP/PS1 mice. Neurotherapeutics 2021, 18, 1064–1080. [Google Scholar] [CrossRef]
- Kudoh, C.; Hori, T.; Yasaki, S.; Ubagai, R.; Tabira, T. Effects of ferulic acid and Angelica archangelica extract (Feru-guard®) on mild cognitive impairment: A multicenter, randomized, double-blind, placebo-controlled prospective trial. J. Alzheimer’s Dis. Rep. 2020, 4, 393–398. [Google Scholar] [CrossRef]
- Kimura, T.; Hayashida, H.; Murata, M.; Takamatsu, J. Effect of ferulic acid and Angelica archangelica extract on behavioral and psychological symptoms of dementia in frontotemporal lobar degeneration and dementia with Lewy bodies. Geriatr. Gerontol. Int. 2011, 11, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, K.; Yamamoto, Y.; Sora, I. Effect of Feru-guard 100M on amyloid-beta deposition in individuals with mild cognitive impairment. Psychogeriatrics 2020, 20, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Aisen, P.S.; Beckett, L.A.; Donohue, M.C.; Gamst, A.C.; Harvey, D.J.; Jack, C.R., Jr.; Jagust, W.J.; Shaw, L.M.; Toga, A.W.; et al. Alzheimer’s disease neuroimaging initiative (ADNI): Clinical characterization. Neurology 2010, 74, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.N.; Chorawala, M.R.; Shah, M.B.; Shah, K.C.; Dave, B.P.; Shah, M.P.; Patel, T.M. Emerging pathophysiological mechanisms linking diabetes mellitus and Alzheimer’s disease: An old wine in a new bottle. J. Alzheimer’s Dis. Rep. 2022, 6, 349–357. [Google Scholar] [CrossRef]
- Kroner, Z. The relationship between Alzheimer’s disease and diabetes: Type 3 diabetes? Altern. Med. Rev. 2009, 14, 373–379. [Google Scholar] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V.V. Type 3 diabetes and its role implications in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Lourenço, V.M.; Menezes, R.; Brites, C. Rice compounds with impact on diabetes control. Foods 2021, 10, 1992. [Google Scholar] [CrossRef] [PubMed]
- Nomura, E.; Kashiwada, A.; Hosoda, A.; Nakamura, K.; Morishita, H.; Tsuno, T.; Taniguchi, H. Synthesis of amide compounds of ferulic acid, and their stimulatory effects on insulin secretion in vitro. Bioorg. Med. Chem. 2003, 11, 3807–3813. [Google Scholar] [CrossRef]
- Park, S.; Moon, N.R.; Kang, S.; Kim, D.S. Ferulic acid and vinpocetine intake improves memory function by enhancing insulin sensitivity and reducing neuroinflammation and oxidative stress in type 2 diabetic animals with induced Alzheimer’s disease. J. Funct Foods 2022, 95, 105180. [Google Scholar] [CrossRef]
- Singh, Y.P.; Rai, H.; Singh, G.; Singh, G.K.; Mishra, S.; Kumar, S.; Srikrishna, S.; Modi, G. A review on ferulic acid and analogs based scaffolds for the management of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 215, 113278. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Carranza-Naval, M.J.; Vargas-Soria, M.; Hierro-Bujalance, C.; Baena-Nieto, G.; Garcia-Alloza, M.; Infante-Garcia, C.; Del Marco, A. Alzheimer’s disease and diabetes: Role of diet, microbiota and inflammation in preclinical models. Biomolecules 2021, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Park, J.K.; Kim, K.M.; Lee, H.J.; Kim, S. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef]
- Truzzi, F.; Valerii, M.C.; Tibaldi, C.; Zhang, Y.; Abduazizova, V.; Spisni, E.; Dinelli, G. Are supplements safe? Effects of gallic and ferulic acids on in vitro cell models. Nutrients 2020, 12, 1591. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, M.S. Ferulic acid modulates dysfunctional metabolic pathways and purinergic activities, while stalling redox imbalance and cholinergic Activities in oxidative brain injury. Neurotox. Res. 2020, 37, 944–955. [Google Scholar] [CrossRef]
- Tada, Y.; Tayama, K.; Aoki, N. Acute oral toxicity of ferulic acid, natural food additive, in rats. Ann. Rep. Tokyo Metr. Lab. 1999, 50, 311–313. [Google Scholar]
- Xu, J.; Li, Y.K.; Liang, Z.J. Effects of ligustrazine and ferulic acid on vascular smooth muscle, blood viscosity and acute toxicity. China J. Chin. Mater. Med. 1992, 17, 680–703. [Google Scholar]
- Wang, B.H.; Ou-Yang, J.P. Pharmacological actions of sodium ferulate in cardiovascular system. Cardiovasc. Drug Rev. 2005, 23, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Hsieh, C.L.; Wang, H.E.; Chung, J.Y.; Chen, K.C.; Peng, R.Y. Ferulic acid is nephrodamaging while gallic acid is renal protective in long term treatment of chronic kidney disease. Clin. Nutr. 2012, 31, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yoshida, D.; Ohara, T.; Hata, J.; Honda, T.; Hirakawa, Y.; Shibata, M.; Oishi, E.; Sakata, S.; Furuta, Y.; et al. Long-term association of vegetable and fruit intake with risk of dementia in Japanese older adults: The Hisayama study. BMC Geriatr. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
| Source | mg of FA/100 g of Product | Reference | |
|---|---|---|---|
| Barley extract | 1358–2293 a | [31] | |
| Barley—whole grain flour | 25–34 b | [31] | |
| Corn—refined bran | 2610–3300 a | [31] | |
| Corn—dehulled kernels | 174 a | [31] | |
| Corn flour | 38 a | [31] | |
| Millet grits | 26 a | [26] | |
| Oat bran | 33 a | [31] | |
| Whole oat | 25–35 a | [31] | |
| Cereals | Rice—endosperm cell wall | 910 a | [31] |
| Rye bran | 280 a | [31] | |
| Rye—whole grain flour | 86 a | [31] | |
| Rye grain | 90–117 b | [27] | |
| Wheat—soft and hard bran | 1351–1456 a | [31] | |
| Wheat—fine bran | 530–540 a | [31] | |
| Wheat bran | 300 a | [26] | |
| Whole wheat flour | 89 a | [31] | |
| Barley—whole grain flour | 25–34 b | [31] | |
| Avocado | 1.1 a | [26] | |
| Broccoli | 4.1 a | [26] | |
| Carrot | 1.5 a | [26] | |
| Cauliflower | 0.35 a | [26] | |
| Eggplant | 7.3–35 a | [31] | |
| Garlic | 0.63 a | [26] | |
| Peanut | 8.7 a | [26] | |
| Red cabbage | 6.3 a | [26] | |
| Vegetables and Fruits | Soybean | 12 a | [26] |
| Spinach | 7.4 a | [31] | |
| Tomato | 0.29–6 a | [31] | |
| White cabbage | 0.27 a | [26] | |
| Banana | 5.4 a | [26] | |
| Blueberry fruits | 290–1697 a | [20] | |
| Grapefruit | 11.6 a | [26] | |
| Mandarin | 9.24 a | [26] | |
| Chokeberries | 0.01–2.8 a | [32] | |
| Orange | 0.3 a | [26] | |
| Plum | 1.47 a | [26] | |
| Strawberry | 12.17 a | [26] | |
| Apple juice | 0.1 a | [26] | |
| Beer | 0.95 a | [26] | |
| Beverages | Black tea | 0.16 a | [26] |
| Coffee | 9.1 a | [26] | |
| Orange juice | 4.7 a | [26] |
| Species | Family Name | Source | mg of FA/g of Product | Reference |
|---|---|---|---|---|
| Angelica sinensis (Oliv.) Diels | Umbelliferae | roots | 0.049–1.502 a | [38] |
| Angelica sinensis (Oliv.) Diels | Umbelliferae | roots b | 0.018–0.035 | [39] |
| Ferula assa-foetida L. | Umbelliferae | leaves c | 4.62 | [35] |
| Ferula assa-foetida L. | Umbelliferae | gum c | 3.72 | [35] |
| Hyssopus officinalis L. | Lamiaceae | herb | 0.45 d | [41] |
| Lavandula angustifolia Mill. | Lamiaceae | flowers b | 1.31 | [42] |
| Lavandula angustifolia Mill. | Lamiaceae | flowers | 0.1 d | [41] |
| Lavandula x intermedia Emeric ex Loisel | Lamiaceae | flowers b | 0.11 | [42] |
| Mentha x piperita L. | Lamiaceae | leaves b | 0.5 | [42] |
| Micromeria graeca L. | Lamiaceae | aerial parts b | 0.27 | [42] |
| Micromeria Juliana (L.) Benth. ex Rchb. | Lamiaceae | aerial parts b | 0.28 | [42] |
| Micromeria thymifolia (Scop.) Fritsch | Lamiaceae | aerial parts b | 0.93 | [42] |
| Origanum majorana L. | Lamiaceae | herb | 0.05 d | [41] |
| Rosmarinus officinalis L. | Lamiaceae | leaves | 0.05 d | [41] |
| Salvia officinalis L. | Lamiaceae | leaves b | 0.77 | [42] |
| Salvia officinalis L. | Lamiaceae | leaves | 0.05 d | [41] |
| Salvia miltiorrhiza B. | Lamiaceae | roots e | 8.32 a | [43] |
| Teucrium arduini L. | Lamiaceae | aerial parts b | 0.97 | [42] |
| Teucrium chamaedrys L. | Lamiaceae | aerial parts b | 1.37 | [42] |
| Teucrium montanum L. | Lamiaceae | aerial parts b | 1.70 | [42] |
| Teucrium polium L. | Lamiaceae | aerial parts b | 1.38 | [42] |
| Thymus vulgaris L. | Lamiaceae | aerial parts b | 0.49 | [42] |
| Study | Animal Models and Species (Number, Sex, Age) | Administration | Outcome | ||
|---|---|---|---|---|---|
| Behavioral Change | Neuropathological Change | Biochemical Change | |||
| Yan et al., 2001 [95] | i.c.v. injection of Aβ1–42 ICR mice (10, M, 4 weeks) | 0.002%, 0.004%, and 0.006% (w/v) Free drinking 1, 2, 3, or 4 weeks | ↑ memory | ↓ Hippocampus GFAP and IL-1β immunoreactivities | ↑ Cortex Acetylcholine level but not statistically significant |
| Kim et al., 2004 [96] | i.c.v. injection of Aβ1–42 ICR mice (6, M, 4 weeks) | 0.006% (w/v) Free drinking 4 weeks | NA | ↓ microglial activation | ↓ IFN-γ |
| Cho et al., 2005 [97] | i.c.v. injection of Aβ1–42 ICR mice (6, M, 4 weeks) | 0.006% (w/v) Free drinking 4 weeks | NA | ↓ astrocytes activation | ↓ hippocampal oxidative stress |
| Jin et al., 2005 [98] | i.c.v. injection of Aβ25–35 Sprague Dawley rats (7 weeks) | 50, 100 or 250 mg/kg ig 3 weeks | NA | ↓ astrocytes activation | ↓ IL-1β and FasL ↓ p-p38 MAPK and caspase-3 ↑ ERK-1/2 and Akt/PKB activation |
| Jin et al., 2006 [99] | i.c.v. injection of Aβ1–40 Sprague Dawley rats (8 weeks) | 100 or 200 mg/kg ig 3 weeks | NA | ↑ hippocampal CA1 pyramidal neurons | ↓ p-MKK3/MKK6, p-p-38 MAPK ↑ p-MAPKAPK-2, p-Hsp27, PARP ↓ IL-1β ↓ caspase-9, -3 and -7 activation |
| Kim et al., 2007 [100] | i.p. injection of TMT ICR mice (8, M) | 0.002% or 0.005% (w/v) Free drinking 28 days | ↓ memory impairment | NA | ↑ ChAT activity |
| Mamiya et al., 2008 [101] | i.c.v. injection of BSO ICR mice (10/15, M, 25 weeks) | 0.5, 1, or 5 mg/kg sc 6 days | ↑ recognition memory ↑ short-term memory | ↓ protein oxidation ↓ carbonyl protein levels in forebrains | NA |
| Jin et al., 2008 [102] | Aged Sprague Dawley rats (M, 21 months) | 100 or 200 mg/kg in food 4 weeks | NA | ↓ microglia and astrocytes activation in cortex and hippocampus Better arrangement of hippocampal CA1 pyramidal neurons | ↓ IL-1β, p-MKK4, p-JNK, p-c-Jun ↑ p-ERK-1/2, p-MEK 1/2 ↓ caspase -3 and -7 activation |
| Hamaguchi et al., 2009 [103] | Mice double mutation K670N-M671L Tg2576 mice (10, F, 5 months) | 0.5% in food 10 months | NA | ↓ Aβ deposits | NA |
| Beibei et al., 2011 [104] | Injected KA into hippocampus CA1 region KM mice (10, M and F, 6 weeks) | 20, 40 and 80 mg/kg ig 30 days | ↑ learning and cognitive skills | ↓ GFAP in hippocampal CA1 region | NA |
| Yan et al., 2013 [105] | APP/PS1 mice (5, F, 6 months) | 5.3 and 16 mg/kg/d Free drinking 6 months | ↑ memory | ↓ Aβ1–42 and Aβ1–40 levels | ↓ Il-1β |
| Mori et al., 2013 [106] | PSAPP C57BL/6J mice (12, M and F, 6 months) | 30 mg/kg ig 6 months | ↓ behavioral impairment | ↓ Aβ deposits ↓ neuroinflammation and oxidative stress ↓ microglial and astroglial activation | ↑ Iba1 ↓ TNF-a, IL-1β, Sod1, catalase, Gpx1 ↓ GFAP |
| Tsai et al., 2015 [107] | i.c.v. injection of Aβ1–40 SD rats (10–12, M, 9 weeks) | 50 and 100 mg/kg ig 2 weeks | ↓ cognitive function impairment ↑ improve memory | NA | ↑ GSH, SOD ↓ Zn-SOD and AChE Activity |
| Kikugawa et al., 2016 [89] | i.c.v. injection of Aβ25–35 C57BL/6 J mice (6, M, 6 weeks) | 0.1 µmol/g/day po 42 days | ↑ contextual freezing response impairment | ↑ neurons survival | NA |
| Mori et al., 2017 [108] | APP/PS1 C57BL/6J mice (8, M and F, 12 months) | 30 mg/kg ig 3 months | ↑ memory | ↓ Aβ deposits ↓ astrocytosis, microgliosis, synaptotoxicity ↓ neuroinflammation and oxidative stress | ↓ sAPP-α/holo-APP and β-oligomers ↑ ADAM10 and ↓ BACE1 ↑ synaptophysin ↓ TNF-α, IL-1β, SOD1, GPx1 |
| Yue et al., 2017 [109] | APP/PS1 C57BL/6 mice (10, 5 weeks) | 20, 40, 100 mg/kg ig 7 days | NA | ↓ apoptosis and oxidative stress | ↑ Bcl-2 and ↓ Bax, p-JNK, p-C-Jun, Caspase3 ↓ MDA and ↑ SOD |
| Rui et al., 2018 [110] | Injected KA into hippocampus CA1 region KM mice (M and F, 6 weeks) | 20, 40, and 80 mg/kg 30 days | N/A | ↓ positive GFAP cells in cerebral cortical glial cells | ↓ IL-1β, IL-6, and TNF-α |
| Zafeer et al., 2019 [111] | ICV-STZ Wistar rats (6, M, 2 months) | 100 mg/kg po 21 days | ↓ spatial memory and learning loss | ↓ oxidative stress ↓ mitochondrial damage | ↓ ROS ↑ Drp-1, PGC1-α ↓ Mfn2, BAX, Cytochrome-C, LPO, and DNA fragmentation |
| Mori et al., 2019 [112] | APP/PS1 mice (8, M and F, 12 months) | 30 mg/kg ig 3 months | ↑ memory | ↓ Aβ deposits ↓ astrocytosis and microgliosis ↓ neuroinflammation and oxidative stress ↓ synaptotoxicity | ↓ amyloidogenic APP cleavage ↓ ADAM10 and BACE1 ↓ GFAP and Iba1 ↓ TNF-α and IL-1β, ↓ SOD1 and GPx1 ↑ synaptophysin |
| Qian et al., 2019 [113] | Injecting Aβ1–42 into the lateral ventricle KM mice (10, M, 6 weeks) | 0.1 and 0.4 g/kg ig | ↑ spatial positioning memory | ↓ morphological changes ↓ Aβ generation | ↓ Drp1, CnAα, CnAβ, and BACE1 ↓ Tau and pS396 protein |
| Wang et al., 2021 [114] | APP/PS1 mice (6 months) | 20 mg/kg/day Free drinking 30 days | ↑ spatial memory | ↑ capillary density and diameter ↑ whole-brain blood vessels ↓ Aβ plaque deposits ↓ area of aggregative microglial cells ↓ ET1- mediated vasoconstriction and prevention of CBF reduction | ↓ BACE1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giacomo, S.; Percaccio, E.; Gullì, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients 2022, 14, 3709. https://doi.org/10.3390/nu14183709
Di Giacomo S, Percaccio E, Gullì M, Romano A, Vitalone A, Mazzanti G, Gaetani S, Di Sotto A. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients. 2022; 14(18):3709. https://doi.org/10.3390/nu14183709
Chicago/Turabian StyleDi Giacomo, Silvia, Ester Percaccio, Marco Gullì, Adele Romano, Annabella Vitalone, Gabriela Mazzanti, Silvana Gaetani, and Antonella Di Sotto. 2022. "Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review" Nutrients 14, no. 18: 3709. https://doi.org/10.3390/nu14183709









