Evaluation of a Supervised Adapted Physical Activity Program Associated or Not with Oral Supplementation with Arginine and Leucine in Subjects with Obesity and Metabolic Syndrome: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Trial Design
2.3. Amino Acid Supplementation
2.4. Adapted Physical Activity
2.5. Anthropometric Measures, Body Composition, and Resting Energy Expenditure Assessments
2.6. Quality of Life Questionnaire
2.7. Cardiopulmonary Exercise Test (CPET)
2.8. Blood Parameters
2.9. Statistical Analysis
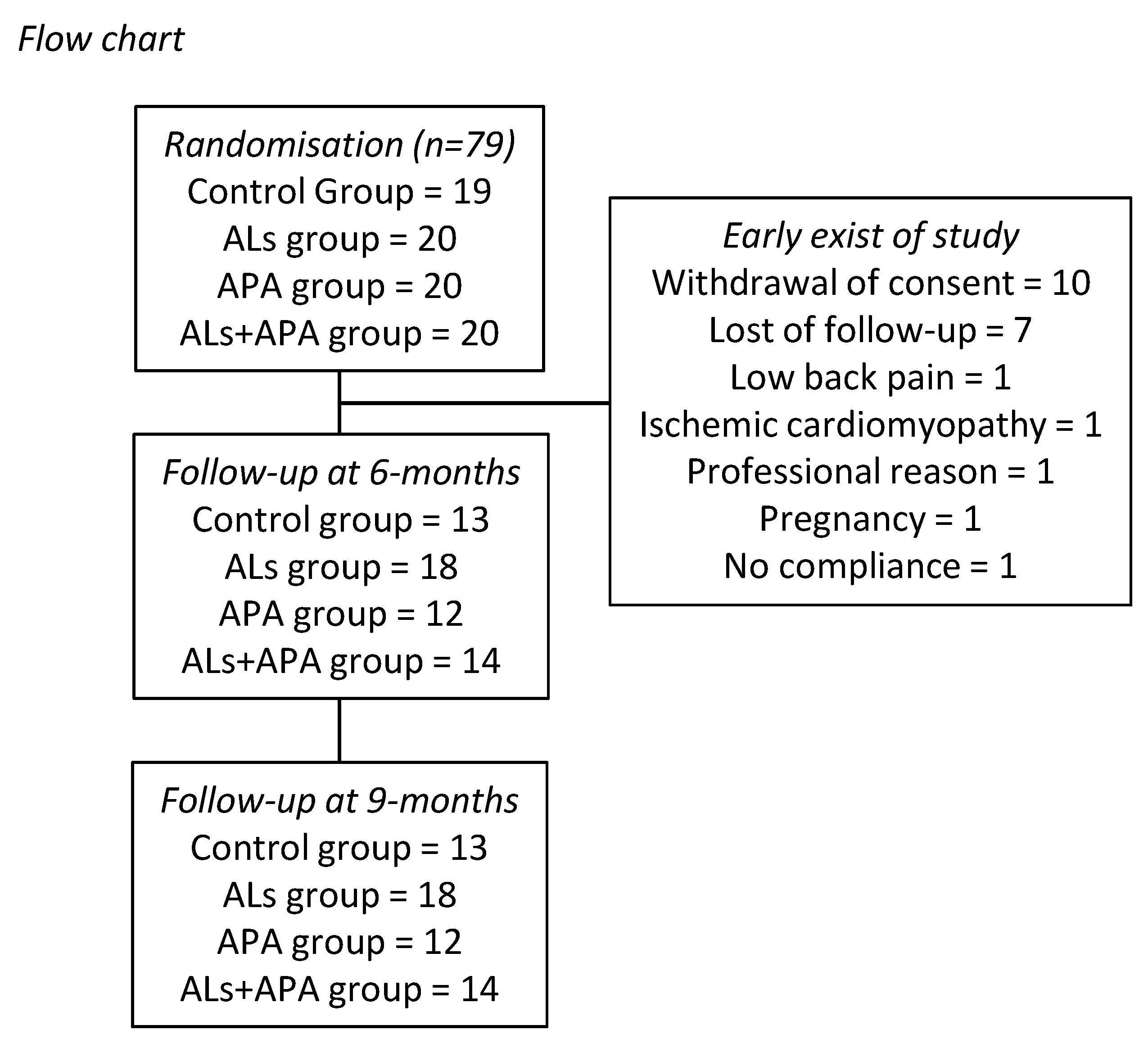
3. Results
3.1. At Baseline
3.2. At the End of the Program
3.3. Post-Test (+3 Months)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidell, J.C.; Halberstadt, J. The global burden of obesity and the challenges of prevention. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bodirsky, B.L.; Dietrich, J.P.; Martinelli, E.; Stenstad, A.; Pradhan, P.; Gabrysch, S.; Mishra, A.; Weindl, I.; Le Mouël, C.; Rolinski, S.; et al. The ongoing nutrition transition thwarts long-term targets for food security, public health and environmental protection. Sci. Rep. 2020, 10, 19778. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Haboubi, H.; Haboubi, N. Adult obesity complications: Challenges and clinical impact. Adv. Endocrinol. Metab. 2020, 11, 2042018820934955. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Folope, V.; Chapelle, C.; Grigioni, S.; Coëffier, M.; Déchelotte, P.J. Impact of eating disorders and psychological distress on the quality of life of obese people. Nutrition 2012, 28, e7–e13. [Google Scholar] [CrossRef]
- Folope, V. Grossophobia in the care sector, a reality to be fought. Soins 2021, 66, 22–24. [Google Scholar] [CrossRef]
- Poehlman, E.T.; Horton, E.S. The impact of food intake and exercise on energy expenditure. Nutr. Rev. 1989, 47, 129–137. [Google Scholar] [CrossRef]
- O’Leary, V.B.; Marchetti, C.M.; Krishnan, R.K.; Stetzer, B.P.; Gonzalez, F.; Kirwan, J.P. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J. Appl. Physiol. 2006, 100, 1584–1589. [Google Scholar] [CrossRef]
- Hill, J.O.; Sparling, P.B.; Shields, T.W.; Heller, P.A. Effects of exercise and food restriction on body composition and metabolic rate in obese women. Am. J. Clin. Nutr. 1987, 46, 622–630. [Google Scholar] [CrossRef]
- van Namen, M.; Prendergast, L.; Peiris, C. Supervised lifestyle intervention for people with metabolic syndrome improves outcomes and reduces individual risk factors of metabolic syndrome: A systematic review and meta-analysis. Metabolism 2019, 101, 153988. [Google Scholar] [CrossRef] [Green Version]
- Carroll, S.; Borkoles, E.; Polman, R. Short-term effects of a non-dieting lifestyle intervention program on weight management, fitness, metabolic risk, and psychological well-being in obese premenopausal females with the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 125–142. [Google Scholar] [CrossRef]
- Oh, E.G.; Bang, S.Y.; Hyun, S.S.; Kim, S.H.; Chu, S.H.; Jeon, J.Y.; Im, J.A.; Lee, M.K.; Lee, J.E. Effects of a 6-month lifestyle modification intervention on the cardiometabolic risk factors and health-related qualities of life in women with metabolic syndrome. Metabolism 2010, 59, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.G.; Hyun, S.S.; Kim, S.H.; Bang, S.Y.; Chu, S.H.; Jeon, J.Y.; Kang, M.S. A randomized controlled trial of therapeutic lifestyle modification in rural women with metabolic syndrome: A pilot study. Metabolism 2008, 57, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Saboya, P.P.; Bodanese, L.C.; Zimmermann, P.R.; Gustavo, A.D.; Macagnan, F.E.; Feoli, A.P.; Oliveira, M.D. Lifestyle Intervention on Metabolic Syndrome and its Impact on Quality of Life: A Randomized Controlled Trial. Arq. Bras. Cardiol. 2017, 108, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.D.; James, A.P.; Lee, A.H.; Jancey, J.; Howat, P.A.; Thi Phuong Mai, L. Effectiveness of a Community-Based Physical Activity and Nutrition Behavior Intervention on Features of the Metabolic Syndrome: A Cluster-Randomized Controlled Trial. Metab. Syndr. Relat. Disord. 2017, 15, 63–71. [Google Scholar] [CrossRef]
- Parker, B.; Noakes, M.; Luscombe, N.; Clifton, P. Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care 2002, 25, 425–430. [Google Scholar] [CrossRef]
- Farnsworth, E.; Luscombe, N.D.; Noakes, M.; Wittert, G.; Argyiou, E.; Clifton, P.M. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am. J. Clin. Nutr. 2003, 78, 31–39. [Google Scholar] [CrossRef]
- Layman, D.K. Role of leucine in protein metabolism during exercise and recovery. Can. J. Appl. Physiol. 2002, 27, 646–663. [Google Scholar] [CrossRef]
- Rieu, I.; Sornet, C.; Bayle, G.; Prugnaud, J.; Pouyet, C.; Balage, M.; Papet, I.; Grizard, J.; Dardevet, D. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J. Nutr. 2003, 133, 1198–1205. [Google Scholar] [CrossRef]
- Mourier, A.; Bigard, A.X.; de Kerviler, E.; Roger, B.; Legrand, H.; Guezennec, C.Y. Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int. J. Sports Med. 1997, 18, 47–55. [Google Scholar] [CrossRef]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. beta-Hydroxy-beta-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Bruckbauer, A.; Zemel, M.B.; Thorpe, T.; Akula, M.R.; Stuckey, A.C.; Osborne, D.; Martin, E.B.; Kennel, S.; Wall, J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. 2012, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Olveira, G.; Olveira, C.; Dona, E.; Palenque, F.J.; Porras, N.; Dorado, A.; Godoy, A.M.; Rubio-Martinez, E.; Rojo-Martinez, G.; Martin-Valero, R. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (Prospective, Randomised Study). Clin. Nutr. 2016, 35, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Lucotti, P.; Setola, E.; Monti, L.D.; Galluccio, E.; Costa, S.; Sandoli, E.P.; Fermo, I.; Rabaiotti, G.; Gatti, R.; Piatti, P. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E906–E912. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Sugita, M.; Maruyama, K. Amino acid mixture improves training efficiency in athletes. J. Nutr. 2006, 136, 538S–543S. [Google Scholar] [CrossRef]
- Kujawska-Luczak, M.; Suliburska, J.; Markuszewski, L.; Pupek-Musialik, D.; Jablecka, A.; Bogdanski, P. The effect of L-arginine and ascorbic acid on the visceral fat and the concentrations of metalloproteinases 2 and 9 in high-fat-diet rats. Endokrynol. Pol. 2015, 66, 526–532. [Google Scholar]
- Bogdanski, P.; Suliburska, J.; Grabanska, K.; Musialik, K.; Cieslewicz, A.; Skoluda, A.; Jablecka, A. Effect of 3-month L-arginine supplementation on insulin resistance and tumor necrosis factor activity in patients with visceral obesity. Eur. Rev. Med. Pharm. Sci. 2012, 16, 816–823. [Google Scholar]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar]
- Achamrah, N.; Colange, G.; Delay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Déchelotte, P.; Coëffier, M. Comparison of body composition assessment by DXA and BIA according to the body mass index: A retrospective study on 3655 measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef]
- Jésus, P.; Achamrah, N.; Grigioni, S.; Charles, J.; Rimbert, A.; Folope, V.; Petit, A.; Déchelotte, P.; Coëffier, M. Validity of predictive equations for resting energy expenditure according to the body mass index in a population of 1726 patients followed in a Nutrition Unit. Clin. Nutr. 2015, 34, 529–535. [Google Scholar] [CrossRef]
- Achamrah, N.; Jésus, P.; Grigioni, S.; Rimbert, A.; Petit, A.; Déchelotte, P.; Folope, V.; Coëffier, M. Validity of Predictive Equations for Resting Energy Expenditure Developed for Obese Patients: Impact of Body Composition Method. Nutrients 2018, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The RAND 36-Item Health Survey 1.0. Health Econ. 1993, 2, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Tous-Espelosin, M.; Gorostegi-Anduaga, I.; Corres, P.; MartinezAguirre-Betolaza, A.; Maldonado-Martin, S. Impact on Health-Related Quality of Life after Different Aerobic Exercise Programs in Physically Inactive Adults with Overweight/Obesity and Primary Hypertension: Data from the EXERDIET-HTA Study. Int. J. Environ. Res. Public Health 2020, 17, 9349. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Debeaumont, D.; Tardif, C.; Folope, V.; Castres, I.; Lemaitre, F.; Tourny, C.; Dechelotte, P.; Thill, C.; Darmon, A.; Coquart, J.B. A specific prediction equation is necessary to estimate peak oxygen uptake in obese patients with metabolic syndrome. J. Endocrinol. Investig. 2016, 39, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Stringer, W.W.; Casaburi, R.; Koike, A.; Cooper, C.B. Determination of the anaerobic threshold by gas exchange: Biochemical considerations, methodology and physiological effects. Z. Kardiol. 1994, 83 (Suppl 3), 1–12. [Google Scholar]
- Leone, N.; Courbon, D.; Thomas, F.; Bean, K.; Jego, B.; Leynaert, B.; Guize, L.; Zureik, M. Lung function impairment and metabolic syndrome: The critical role of abdominal obesity. Am. J. Respir. Crit. Care Med. 2009, 179, 509–516. [Google Scholar] [CrossRef]
- Parameswaran, K.; Todd, D.C.; Soth, M. Altered respiratory physiology in obesity. Can. Respir. J. 2006, 13, 203–210. [Google Scholar] [CrossRef]
- Kaufman, C.; Kelly, A.S.; Kaiser, D.R.; Steinberger, J.; Dengel, D.R. Aerobic-exercise training improves ventilatory efficiency in overweight children. Pediatr. Exerc. Sci. 2007, 19, 82–92. [Google Scholar] [CrossRef]
- Castres, I.; Lemaitre, F.; Tardif, C.; Beuret-Blanquart, F.; Tourny-Chollet, C. Dynamic cardiorespiratory changes in obese women. J. Sports Med. Phys. Fitness 2011, 51, 283–291. [Google Scholar]
- Ross, R.; Janssen, I.; Dawson, J.; Kungl, A.M.; Kuk, J.L.; Wong, S.L.; Nguyen-Duy, T.B.; Lee, S.; Kilpatrick, K.; Hudson, R. Exercise-induced reduction in obesity and insulin resistance in women: A randomized controlled trial. Obes. Res. 2004, 12, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.; Marshall, P.; Borkoles, E.; Ingle, L.; Barker, D.; Tan, L.B. Efficacy of lifestyle intervention on peak exercise cardiac power output and reserve in premenopausal obese females: A randomised pilot study. Int. J. Cardiol. 2007, 119, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Coquart, J.B.; Boitel, G.; Borel, B.; Duhamel, A.; Matran, R.; Delsart, P.; Mounier-Vehier, C.; Garcin, M. Exercise training at the crossover point improves bodily and cardiorespiratory data but not quality of life in women with metabolic syndrome. J. Sports Med. Phys. Fitness 2017, 57, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Ben Abderrahman, A.; Khodamoradi, A.; Saeidi, A.; Jayavel, A.; Hackney, A.C.; Laher, I.; Algotar, A.M.; Jabbour, G. Effects of physical training on anthropometrics, physical and physiological capacities in individuals with obesity: A systematic review. Obes. Rev. 2020, 21, e13039. [Google Scholar] [CrossRef]
- Maris, S.A.; Quintanilla, D.; Taetzsch, A.; Picard, A.; Letendre, J.; Mahler, L.; Lofgren, I.; Xu, F.; Delmonico, M.J. The combined effects of tai chi, resistance training, and diet on physical function and body composition in obese older women. J. Aging Res. 2014, 2014, 657851. [Google Scholar] [CrossRef] [Green Version]
- Dalzill, C.; Nigam, A.; Juneau, M.; Guilbeault, V.; Latour, E.; Mauriege, P.; Gayda, M. Intensive lifestyle intervention improves cardiometabolic and exercise parameters in metabolically healthy obese and metabolically unhealthy obese individuals. Can. J. Cardiol. 2014, 30, 434–440. [Google Scholar] [CrossRef]
- Dutheil, F.; Lac, G.; Lesourd, B.; Chapier, R.; Walther, G.; Vinet, A.; Sapin, V.; Verney, J.; Ouchchane, L.; Duclos, M.; et al. Different modalities of exercise to reduce visceral fat mass and cardiovascular risk in metabolic syndrome: The RESOLVE randomized trial. Int. J. Cardiol. 2013, 168, 3634–3642. [Google Scholar] [CrossRef]
- Kroeger, C.M.; Hoddy, K.K.; Varady, K.A. Impact of weight regain on metabolic disease risk: A review of human trials. J. Obes. 2014, 2014, 614519. [Google Scholar] [CrossRef]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575 Pt 1, 305–315. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Hu, W.; Liang, Y.; Zheng, L.; Zheng, J.; Wang, B.; Guo, X. Different Effects of Leucine Supplementation and/or Exercise on Systemic Insulin Sensitivity in Mice. Front. Endocrinol. 2021, 12, 651303. [Google Scholar] [CrossRef]
- Bagheri, R.; Forbes, S.C.; Candow, D.G.; Wong, A. Effects of branched-chain amino acid supplementation and resistance training in postmenopausal women. Exp. Gerontol. 2021, 144, 111185. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Gachon, P.; Beaufrère, B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am. J. Clin. Nutr. 1997, 65, 489–495. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 19) | ALs (n = 20) | APA (n = 20) | Als + APA (n = 20) | p Values | |
|---|---|---|---|---|---|
| Age (years) | 40 [36; 45] | 39 [32; 44] | 44 [34.5; 49.5] | 44 [40; 52] | 0.2323 |
| Females (n/%) | 13 (68.4) | 12 (60) | 14 (70) | 15 (75) | 0.7822 |
| Body weight (kg) | 115.6 [110.4; 124.5] | 105.7 [95.9; 122.9] | 103.4 [87.1; 117.1] | 109.8 [89.1; 115.8] | 0.0572 |
| BMI (kg.m−2) | 41 [35.8; 46.5] | 38.2 [35.2; 41.1] | 35.8 [32.5; 41] | 36.9 [34.5; 39.2] | 0.0647 |
| Waist circumference (cm) | 122 [117; 133] | 118 [111.8; 127.5] | 113 [111; 123] | 114.8 [110; 121] | 0.0325 |
| Hip circumference (cm) | 133 [111; 147] | 121 [113; 128.5] | 117 [109; 126] | 124 [115; 130] | 0.2182 |
| Waist/hip ratio | 0.97 [0.89; 1.02] | 0.98 [0.93; 1.05] | 1 [0.93; 1.02] | 0.94 [0.91; 1.03] | 0.5924 |
| Fat mass (kg) | 55.4 [33.1; 62.9] | 43.6 [37.6; 48.1] | 36.8 [30.7; 51.6] | 40.7 [36.1; 50.8] | 0.2539 |
| Fat mass (%) | 47.3 [30.8; 51.1] | 41.6 [31.4; 48.6] | 43.3 [27.3; 46.5] | 42.8 [35.5; 46] | 0.4951 |
| Fat free mass (kg) | 62 [58.3; 75.5] | 62.1 [57.7; 74.2] | 61.6 [52.3; 72.1] | 62.5 [52.8; 67.7] | 0.6649 |
| Fat free mass (%) | 52.7 [48.9; 69.2] | 59 [51.8; 68.7] | 56.8 [53.5; 72.8] | 57.3 [54; 64.5] | 0.4579 |
| Abdominal fat mass (kg) | 5.4 [4.2; 6.6] | 5.1 [4.3; 6.5] | 4.5 [3.8; 5] | 4.8 [4.1; 5.8] | 0.2820 |
| Control (n = 13) | ALs (n = 18) | APA (n = 12) | Als + APA (n = 14) | p Values | |
|---|---|---|---|---|---|
| Body weight (kg) | −1.7 [−7.3; 0] | −3.65 [−7; 0] | −2.8 [−5.85; −1.3] | −4.05 [−6.2; −0.8] | 0.9816 |
| BMI (kg.m−2) | −0.64 [−2.41; −0.01] | −1.28 [−2.46; 0] | −0.91 [−2.1; −0.49] | −1.45 [−2.65; −0.3] | 0.9887 |
| Waist circumference (cm) | −5 [−10; −2] | −4 [−10; −3] | −5.5 [−10; −4] | −5 [−9; −3] | 0.9340 |
| Hip circumference (cm) | −6 [−11; −4] | −2 [−10; 0] | −1.5 [−9; 0] | −6 [−10; −3] | 0.4373 |
| Waist/hip ratio | 0 [−0.02; 0.03] | −0.02 [−0.03; −0.01] | 0 [−0.04; 0] | 0.01 [−0.02; 0.03] | 0.2758 |
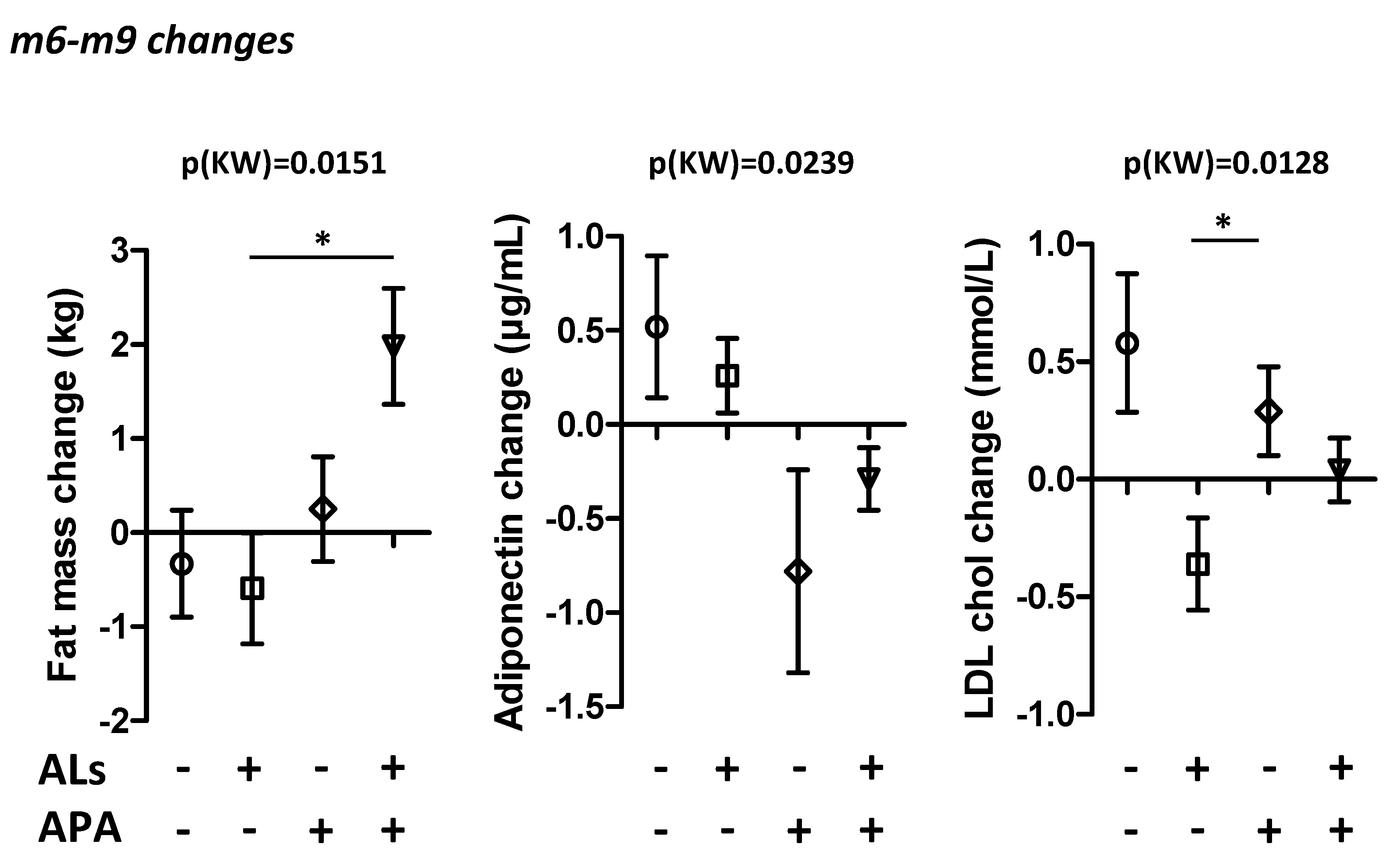
| Fat mass (kg) | −0.6 [−5.3; 0.3] | −0.9 [−4.7; −0.2] | −1.3 [−3.25; 0.2] | −3.15 [−6; −1.9] | 0.4338 |
| Fat mass (%) | −0.1 [−2.7; 1] | −0.95 [−5; 0.3] | −0.75 [−1.7; 0.25] | −2.7 [−3.3; −1] | 0.2773 |
| Fat free mass (kg) | −1.5 [−2.1; −0.2] | −0.6 [−3.2; 0.4] | −1.1 [−3.45; −0.05] | −1.05 [−1.4; 1.1] | 0.8868 |
| Fat free mass (%) | 0.1 [−1; 2.7] | 0.95 [−0.3; 5] | 0.25 [0.05; 1.7] | 2.7 [1; 3.3] | 0.2638 |
| Abdominal fat mass (kg) | −0.2 [−0.44; 0.24] | −0.2 [−1.2; 0] | −0.05 [−0.5; 0.18] | −0.7 [−0.9; −0.2] | 0.1771 |
| Control (n = 13) | ALs (n = 18) | APA (n = 12) | Als + APA (n = 14) | p Values | |
|---|---|---|---|---|---|
| Total cholesterol (mmol/L) | −0.2 [−0.3; 0.3] | −0.05 [−0.5; 0.2] | −0.2 [−0.45; 0.25] | −0.1 [−0.3; 0.2] | 0.9510 |
| HDL cholesterol (mmol/L) | −0.03 [−0.15; 0.04] | 0.02 [−0.12; 0.15] | 0.09 [−0.03; 0.27] | 0.06 [−0.02; 0.2] | 0.2066 |
| LDL cholesterol (mmol/L) | −0.11 [−0.57; 0.18] | 0.05 [−0.49; 0.39] | −0.27 [−0.56; 0.04] | −0.14 [−0.26; 0.21] | 0.5553 |
| Triglycerides (mmol/L) | 0.25 [−0.05; 0.36] | −0.29 [−0.66; 0.01] | −0.05 [−0.39; 0.17] | 0.06 [−0.36; 0.47] | 0.1939 |
| ASAT (UI/L) | −2 [−7; 2] | −0.5 [−7; 4] | −1 [−4; 6] | 0 [−3; 2] | 0.6919 |
| ALAT (UI/L) | −2 [−17; 1] | −0.5 [−18; 13] | −7 [−15; 3] | −4.5 [−12; 3] | 0.6029 |
| ALP (UI/L) | 0 [−5; 3] | 0 [−4; 7] | 0 [−3; 4] | −3 [−9; 1] | 0.5830 |
| γGT (UI/L) | 1 [−10; 4] | 0 [−6; 7] | −5 [−9; 0] | −3 [−19; −1] | 0.2764 |
| Fasting glycaemia (pmol/L) | −0.3 [−0.7; −0.2] | 0 [−0.4; 0.2] | −0.2 [−0.35; 0.1] | −0.1 [−0.3; 0.3] | 0.1514 |
| Fasting insulin (mUI/L) | −29 [−59; 7] | −17 [−53; 7] | −11 [−45; 50] | −23.5 [−44; −7] | 0.6825 |
| HOMA-IR | −7.86 [−21.04; 7.99] | −3.65 [−13.59; −0.6] | −3.05 [−10.8; 11.63] | −6.86 [−11.89; −1.71] | 0.7646 |
| Leptin (ng/mL) | −4.68 [−23.36; 15.19] | −10.45 [−26.53; 1.53] | −7.6 [−20.41; 10.86] | −10.36 [−33.72; −6.01] | 0.5381 |
| Adiponectin (µg/mL) | −0.42 [−0.99; 0.19] | −0.1 [−0.79; 1.11] | 0.62 [−0.26; 1.7] | 0.43 [−0.54; 0.74] | 0.2557 |
| IL-6 (pg/mL) | −0.21 [−0.32; 0.21] | −0.18 [−1; 0.32] | −0.21 [−0.36; 0.12] | −0.22 [−0.55; 0] | 0.8735 |
| TNFα (pg/mL) | 0.09 [−0.3; 0.76] | −0.09 [−0.48; 0.34] | 0.03 [−0.17; 0.78] | 0.31 [0.09; 0.48] | 0.3903 |
| CRP (mg/L) | −0.73 [−1.45; 2.8] | −0.33 [−0.86; 0.35] | −0.14 [−0.43; 1.2] | −0.45 [−0.93; −0.01] | 0.6269 |
| Control (n = 13) | ALs (n = 18) | APA (n = 12) | Als + APA (n = 14) | p Values | |
|---|---|---|---|---|---|
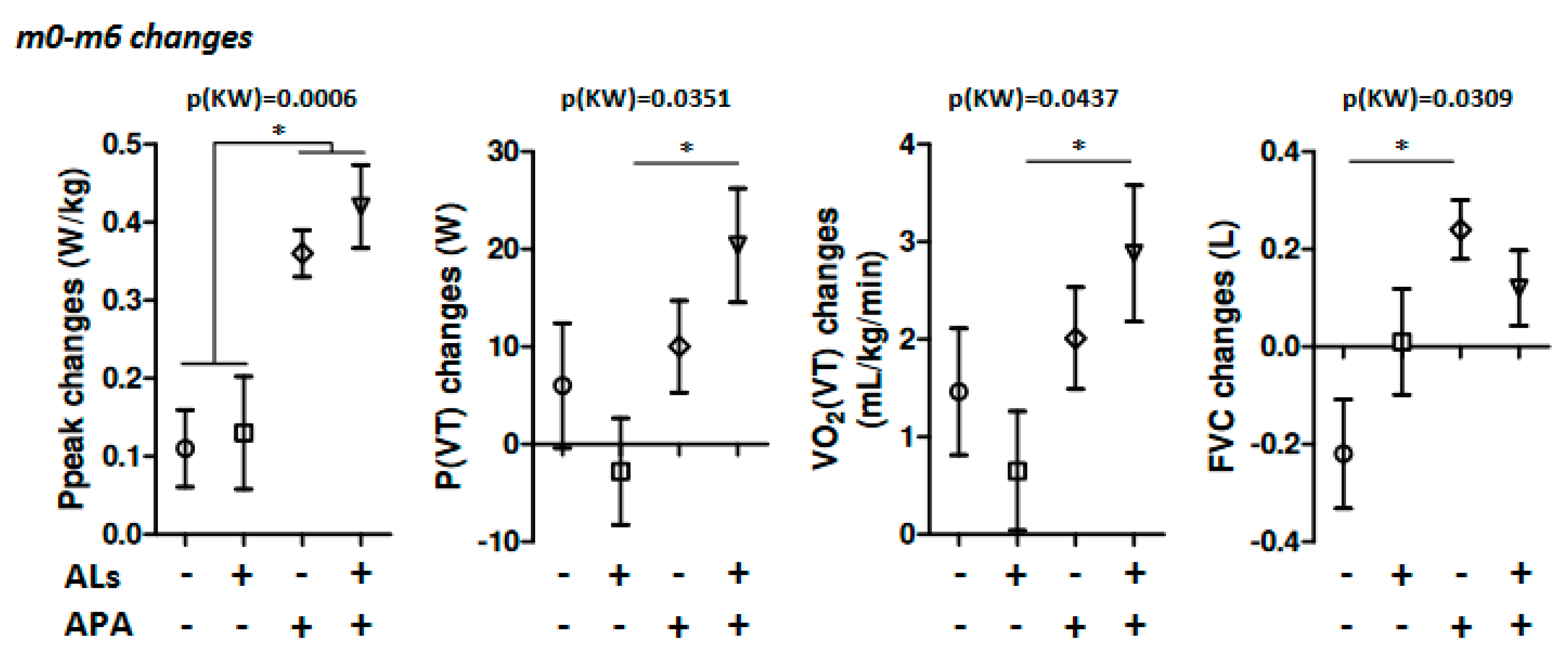
| Pmax (W) | 0 [0; 10] a | 7.5 [0; 15] a | 30 [20; 30] b | 25 [10; 40] b | 0.0005 |
| Ppeak (Pmax/poids) (W.kg−1) | 0.1 [0.03; 0.2] a | 0.2 [0; 0.3] a,b | 0.32 [0.3; 0.45] b,c | 0.4 [0.3; 0.6] c | 0.0006 |
| VO2 max (L.min−1) | 0.03 [−0.11; 0.1] | 0.04 [−0.16; 0.17] | 0.19 [0.02; 0.37] | 0.27 [0.06; 0.42] | 0.0708 |
| VO2 max (mL.kg−1.min−1) | 0.85 [−0.5; 3] | 0.5 [−0.8; 1.7] | 3.35 [1.15; 4.45] | 4.1 [0.6; 6.4] | 0.0965 |
| VCO2/VO2 max | 0.03 [−0.06; 0.1] | 0.04 [−0.01; 0.09] | 0 [−0.07; 0.07] | −0.01 [−0.1; 0.06] | 0.3808 |
| Heart rate max (bpm) | 3 [−8; 11] | 0.5 [−5; 6] | 3 [−1; 7.5] | 5 [−3; 13] | 0.6059 |
| P(VT) (W) | 5 [−10; 20] a,b | 0 [−10; 10] a | 10 [0; 20] a,b | 20 [10; 30] b | 0.0351 |
| VO2 (VT) (mL.kg−1.min−1) | 2.3 [0.6; 2.5] a,b | 0.2 [−0.8; 1.6] a | 1.9 [0.6; 3.7] a,b | 2.45 [1.2; 3.8] b | 0.0437 |
| Heart rate (VT) (bpm) | −1.0 [−8.0; 14.0] | −1.5 [−8.0; 8.0] | 2.0 [−2.5; 7.0] | 4.0 [−9.0; 20.0] | 0.8214 |
| Slow VC (L) | −0.23 [−0.45; 0.22] | −0.04 [−0.48; 0.28] | 0.21 [−0.03; 0.35] | 0.27 [−0.2; 0.5] | 0.1091 |
| Forced VC (L) | −0.19 [−0.63; 0.1] a | 0.04 [−0.24; 0.22] a,b | 0.22 [0.07; 0.41] b | 0.18 [−0.11; 0.38] a,b | 0.0309 |
| Inspiratory VC (L) | −0.01 [−0.05; 0.11] | −0.1 [−0.26; 0.13] | −0.08 [−0.25; 0.02] | 0.16 [−0.23; 0.3] | 0.4904 |
| Expiratory reserve volume (L) | −0.02 [−0.46; 0.15] | −0.02 [−0.29; 0.59] | 0.35 [0.16; 0.62] | 0.17 [−0.11; 0.46] | 0.1554 |
| Forced expiratory volume (L) | −0.17 [−0.57; 0.07] | 0.01 [−0.30; 0.23] | 0.12 [−0.07; 0.34] | 0.12 [−0.07; 0.23] | 0.1276 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folope, V.; Meret, C.; Castres, I.; Tourny, C.; Houivet, E.; Grigioni, S.; Lelandais, H.; Petit, A.; Coquard, A.; Guérin, C.; et al. Evaluation of a Supervised Adapted Physical Activity Program Associated or Not with Oral Supplementation with Arginine and Leucine in Subjects with Obesity and Metabolic Syndrome: A Randomized Controlled Trial. Nutrients 2022, 14, 3708. https://doi.org/10.3390/nu14183708
Folope V, Meret C, Castres I, Tourny C, Houivet E, Grigioni S, Lelandais H, Petit A, Coquard A, Guérin C, et al. Evaluation of a Supervised Adapted Physical Activity Program Associated or Not with Oral Supplementation with Arginine and Leucine in Subjects with Obesity and Metabolic Syndrome: A Randomized Controlled Trial. Nutrients. 2022; 14(18):3708. https://doi.org/10.3390/nu14183708
Chicago/Turabian StyleFolope, Vanessa, Caroline Meret, Ingrid Castres, Claire Tourny, Estelle Houivet, Sébastien Grigioni, Hélène Lelandais, André Petit, Aude Coquard, Charlène Guérin, and et al. 2022. "Evaluation of a Supervised Adapted Physical Activity Program Associated or Not with Oral Supplementation with Arginine and Leucine in Subjects with Obesity and Metabolic Syndrome: A Randomized Controlled Trial" Nutrients 14, no. 18: 3708. https://doi.org/10.3390/nu14183708
APA StyleFolope, V., Meret, C., Castres, I., Tourny, C., Houivet, E., Grigioni, S., Lelandais, H., Petit, A., Coquard, A., Guérin, C., Quillard, M., Bôle-Feysot, C., Déchelotte, P., Achamrah, N., & Coëffier, M. (2022). Evaluation of a Supervised Adapted Physical Activity Program Associated or Not with Oral Supplementation with Arginine and Leucine in Subjects with Obesity and Metabolic Syndrome: A Randomized Controlled Trial. Nutrients, 14(18), 3708. https://doi.org/10.3390/nu14183708







