Resveratrol and Some of Its Derivatives as Promising Prophylactic Treatments for Neonatal Hypoxia-Ischemia
Abstract
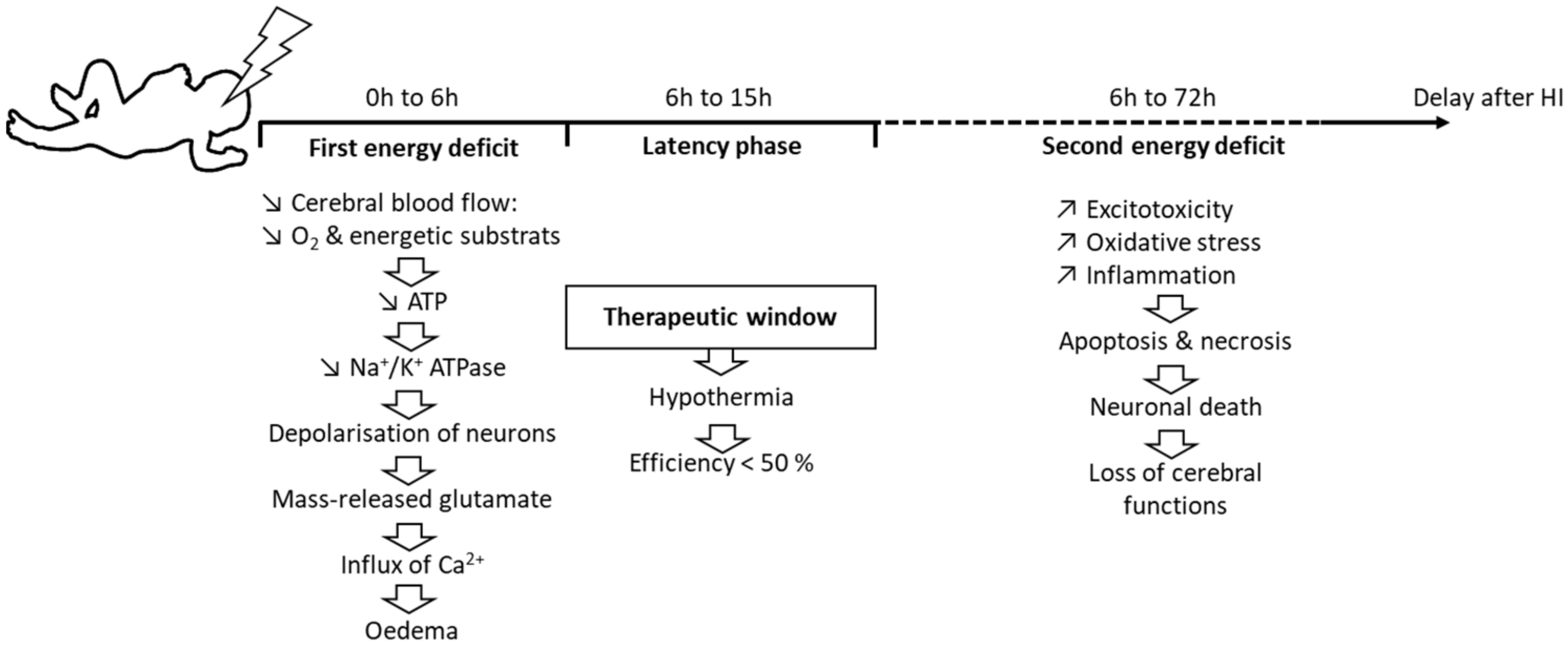
1. Introduction
2. Methods
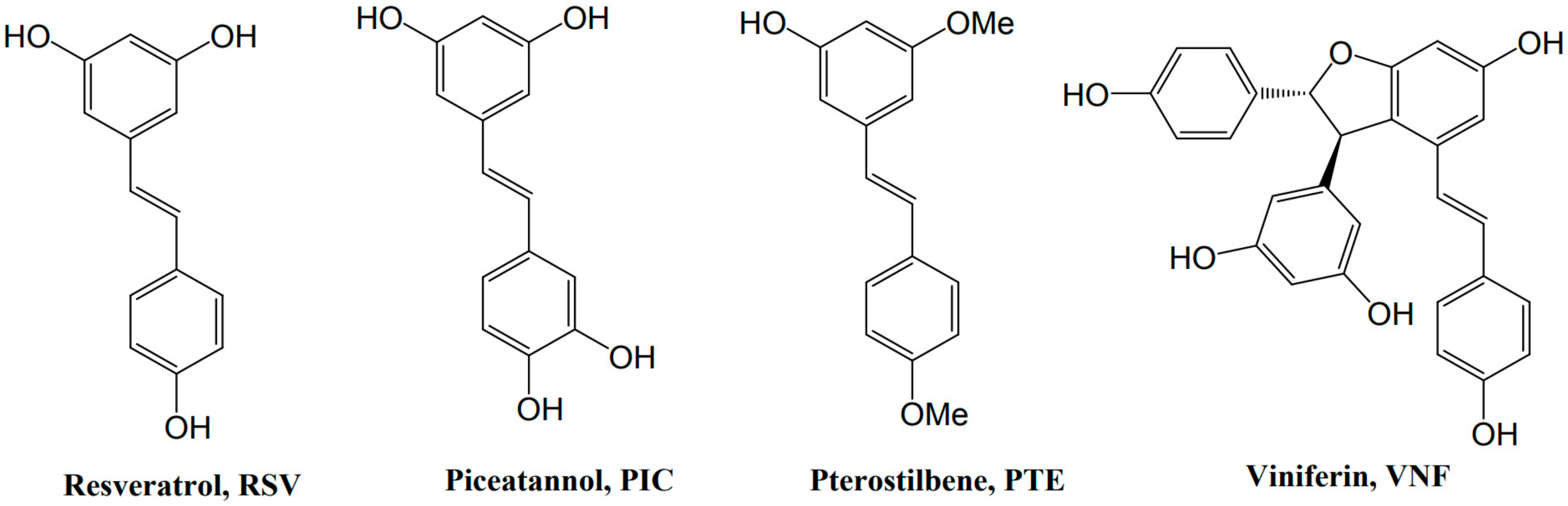
3. Resveratrol and Derivatives
4. Biological Activity
4.1. Anti-Apoptotic Properties
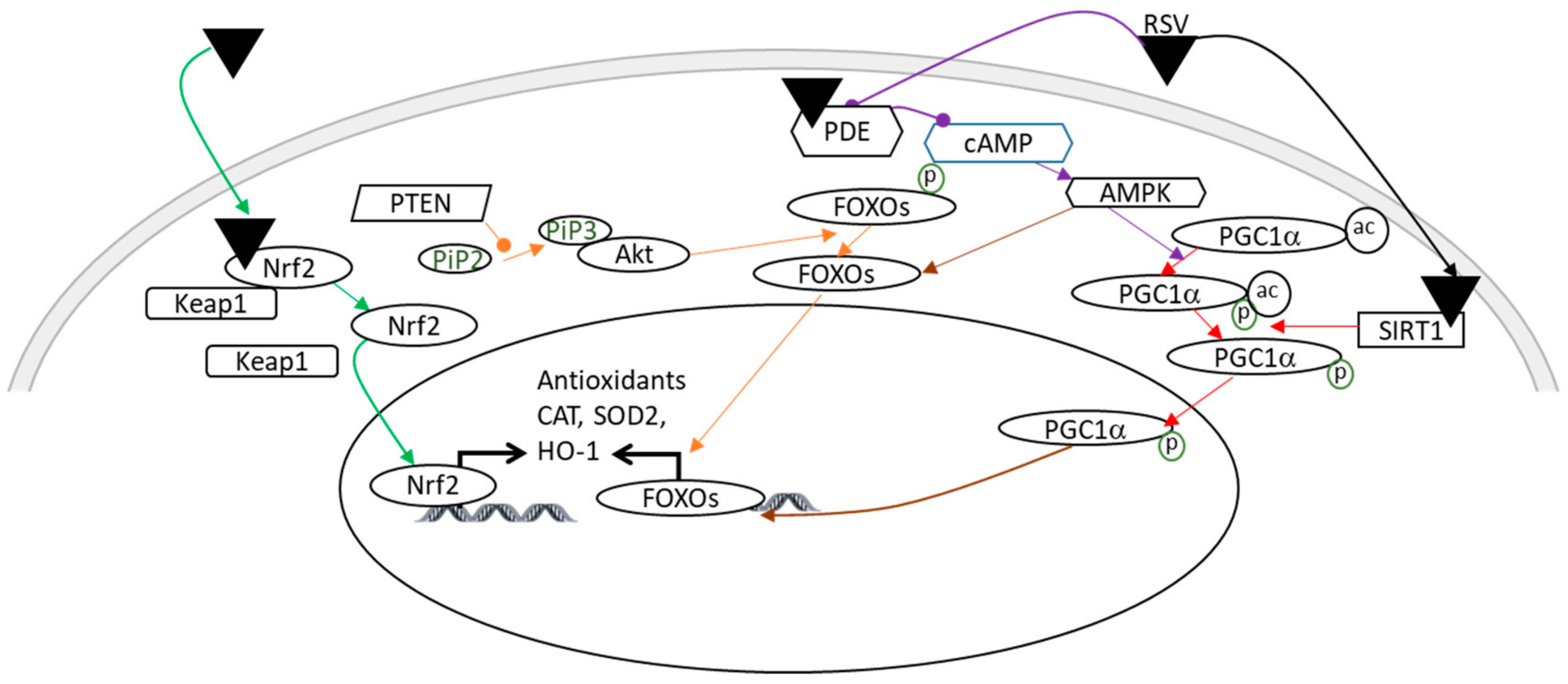
4.2. Antioxidant Properties
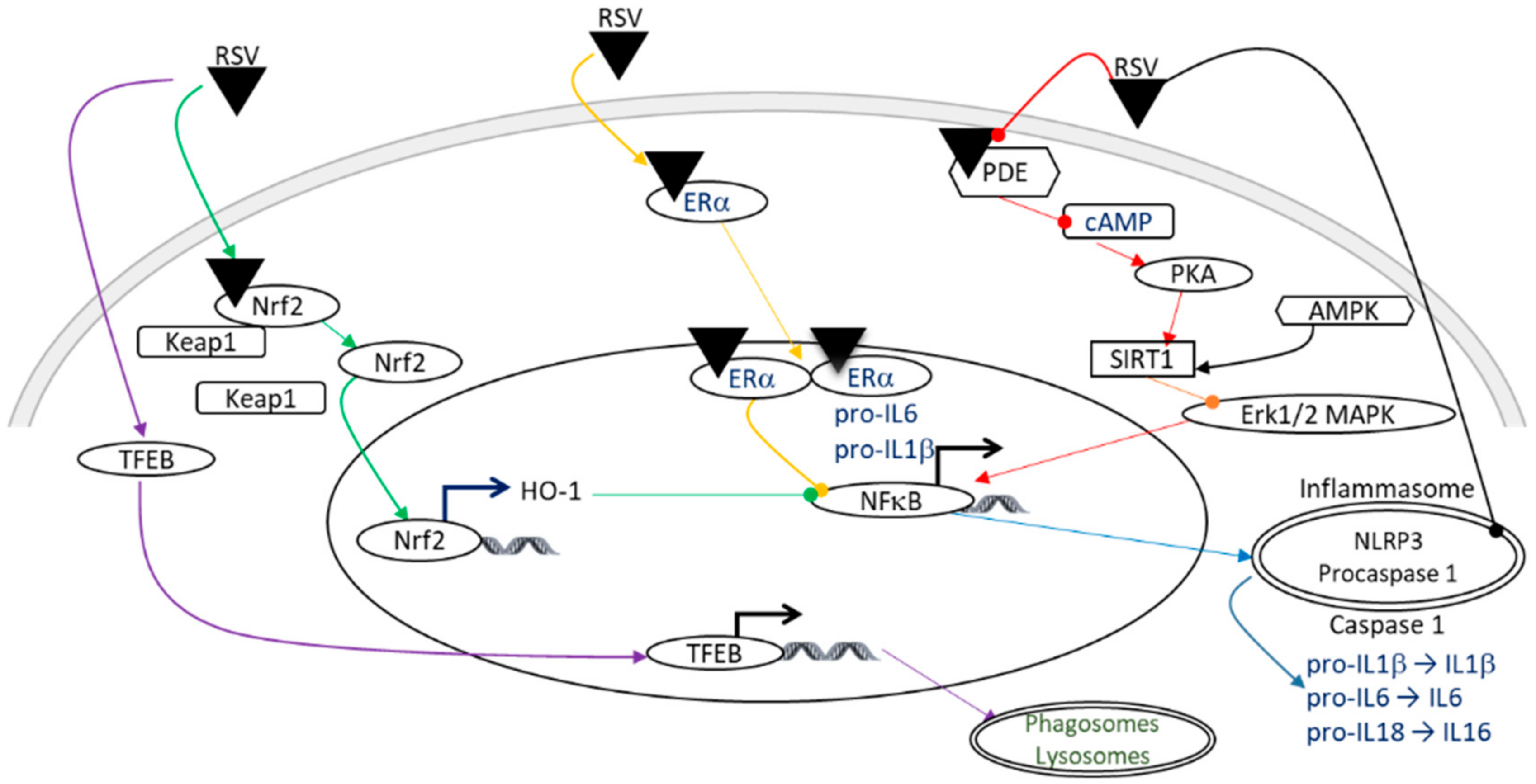
4.3. Anti-Inflammatory Properties
5. Direct RSV Administration for Neuroprotection
5.1. Preventive Strategies
5.2. Curative Strategies
6. Maternal Supplementation for Neuroprotection
6.1. Maternal Supplementation with RSV
6.2. Maternal Supplementation with PIC
6.3. Maternal Supplementation with Multiple Polyphenols
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanrahan, J.D.; Sargentoni, J.; Azzopardi, D.; Manji, K.; Cowan, F.M.; Rutherford, M.A.; Cox, I.J.; Bell, J.D.; Bryant, D.J.; Edwards, A.D. Cerebral metabolism within 18 h of birth asphyxia: A proton magnetic resonance spectroscopy study. Pediatr. Res. 1996, 39, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.V.; Ishida, A.; Ishida, W.N.; Matsushita, H.B.; Nishimura, A.; Tsuji, M. Plasticity and injury in the developing brain. Brain Dev. 2009, 31, 1–10. [Google Scholar] [CrossRef]
- Drury, P.P.; Bennet, L.; Gunn, A.J. Mechanisms of hypothermic neuroprotection. Clin. Perinatol. 2014, 41, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diaz, A.; Hilario, E.; De Cerio, F.G.; Valls-i-Soler, A.; Alvarez-Diaz, F.J. Hypoxic-ischemic injury in the immature brain—Key vascular and cellular players. Neonatology 2007, 92, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Iwata, O.; Iwata, S.; Thornton, J.S.; De Vita, E.; Bainbridge, A.; Herbert, L.; Scaravilli, F.; Peebles, D.; Wyatt, J.S.; Cady, E.B.; et al. “Therapeutic time window” duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007, 1154, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cotten, C.M.; Shankaran, S. Hypothermia for hypoxic-ischemic encephalopathy. Expert Rev. Obstet. Gynecol. 2010, 5, 227–239. [Google Scholar] [CrossRef]
- Buonocore, G.; Groenendaal, F. Anti-oxidant strategies. Semin. Fetal Neonatal Med. 2007, 12, 287–295. [Google Scholar] [CrossRef]
- Ferriero, D.M. Neonatal brain injury. N. Engl. J. Med. 2004, 351, 1985–1995. [Google Scholar] [CrossRef]
- Shalak, L.; Perlman, J.M. Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum. Dev. 2004, 80, 125–141. [Google Scholar] [CrossRef]
- Saliba, E.; Debillon, T. Hypothermia for hypoxic-ischemic encephalopathy in fullterm newborns. Arch. Pediatr. 2010, 17 (Suppl. 3), S67–S77. [Google Scholar] [CrossRef]
- Rogers, E.E.; Bonifacio, S.L.; Glass, H.C.; Juul, S.E.; Chang, T.; Mayock, D.E.; Durand, D.J.; Song, D.; Barkovich, A.J.; Ballard, R.A.; et al. Erythropoietin and hypothermia for hypoxic-ischemic encephalopathy. Pediatr. Neurol. 2014, 51, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Tokusoglu, O.; Unal, M.K.; Yemis, F. Determination of the phytoalexin resveratrol (3,5,4′-trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatography-mass spectrometry (GC-MS). J. Agric. Food Chem. 2005, 53, 5003–5009. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, A.; Wadhwa, N.; Tiwari, A. Therapeutic role of resveratrol and piceatannol in disease prevention. J. Blood Disord. Trans. 2014, 5, 9. [Google Scholar] [CrossRef]
- Lin, H.S.; Yue, B.D.; Ho, P.C. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2009, 23, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Caillaud, M.; Guillard, J.; Richard, D.; Milin, S.; Chassaing, D.; Paccalin, M.; Page, G.; Bilan, A.R. Trans epsilon viniferin decreases amyloid deposits and inflammation in a mouse transgenic Alzheimer model. PLoS ONE 2019, 14, e0212663. [Google Scholar] [CrossRef]
- Dumont, U.; Sanchez, S.; Repond, C.; Beauvieux, M.C.; Chateil, J.F.; Pellerin, L.; Bouzier-Sore, A.K.; Roumes, H. Neuroprotective Effect of Maternal Resveratrol Supplementation in a Rat Model of Neonatal Hypoxia-Ischemia. Front. Neurosci. 2020, 14, 616824. [Google Scholar] [CrossRef] [PubMed]
- Dumont, U.; Sanchez, S.; Olivier, B.; Chateil, J.F.; Pellerin, L.; Beauvieux, M.C.; Bouzier-Sore, A.K.; Roumes, H. Maternal consumption of piceatannol: A nutritional neuroprotective strategy against hypoxia-ischemia in rat neonates. Brain Res. 2019, 1717, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Roumes, H.; Sanchez, S.; Benkhaled, I.; Fernandez, V.; Goudeneche, P.; Perrin, F.; Pellerin, L.; Guillard, J.; Bouzier-Sore, A.K. Neuroprotective Effect of Eco-Sustainably Extracted Grape Polyphenols in Neonatal Hypoxia-Ischemia. Nutrients 2022, 14, 773. [Google Scholar] [CrossRef]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1beta in human chondrocytes. J. Orthop. Res. 2013, 31, 531–537. [Google Scholar] [CrossRef]
- He, D.S.; Hu, X.J.; Yan, Y.Q.; Liu, H. Underlying mechanism of Sirt1 on apoptosis and extracellular matrix degradation of osteoarthritis chondrocytes. Mol. Med. Rep. 2017, 16, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Shan, H.; Chen, T. Resveratrol ameliorates hypoxia/ischemia-induced brain injury in the neonatal rat via the miR-96/Bax axis. Childs Nerv. Syst. 2017, 33, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Li, S.; Hu, Y.; Zhang, H.; Liu, Y.; Jiang, H.; Fang, M.; Li, Z.; Xu, K.; Zhang, H.; et al. Resveratrol post-treatment protects against neonatal brain injury after hypoxia-ischemia. Oncotarget 2016, 7, 79247–79261. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Eaton, E.N.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- West, T.; Atzeva, M.; Holtzman, D.M. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev. Neurosci. 2007, 29, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, M.; Arteaga, O.; Alvarez, A.; Martinez-Ibargüen, A.; Hilario, E. Characterization of Gene Expression in the Rat Brainstem After Neonatal Hypoxic-Ischemic Injury and Antioxidant Treatment. Mol. Neurobiol. 2017, 54, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Siman, R.; Noszek, J.C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron 1988, 1, 279–287. [Google Scholar] [CrossRef]
- Bednarski, E.; Vanderklish, P.; Gall, C.; Saido, T.C.; Bahr, B.A.; Lynch, G. Translational suppression of calpain I reduces NMDA-induced spectrin proteolysis and pathophysiology in cultured hippocampal slices. Brain Res. 1995, 694, 147–157. [Google Scholar] [CrossRef]
- Blomgren, K.; Zhu, C.; Wang, X.; Karlsson, J.O.; Leverin, A.L.; Bahr, B.A.; Mallard, C.; Hagberg, H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: A mechanism of “pathological apoptosis”? J. Biol. Chem. 2001, 276, 10191–10198. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, F.; Zhang, J.; Wang, T.; Wei, X.; Wu, J.; Feng, Y.; Dai, Z.; Wu, Q. Neuroprotective effect of resveratrol on ischemia/reperfusion injury in rats through TRPC6/CREB pathways. J. Mol. Neurosci. 2013, 50, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, O.; Revuelta, M.; Urigüen, L.; Alvarez, A.; Montalvo, H.; Hilario, E. Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats. PLoS ONE 2015, 10, e0142424. [Google Scholar] [CrossRef]
- Lu, Q.; Wainwright, M.S.; Harris, V.A.; Aggarwal, S.; Hou, Y.; Rau, T.; Poulsen, D.J.; Black, S.M. Increased NADPH oxidase-derived superoxide is involved in the neuronal cell death induced by hypoxia-ischemia in neonatal hippocampal slice cultures. Free Radic. Biol. Med. 2012, 53, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bonilla, L.; Benakis, C.; Moore, J.; Iadecola, C.; Anrather, J. Immune mechanisms in cerebral ischemic tolerance. Front. Neurosci. 2014, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-E.; Kim, H.-S.; Park, E.-M. Acute resveratrol treatment modulates multiple signaling pathways in the ischemic brain. Neurochem. Res. 2012, 37, 2686–2696. [Google Scholar]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Gao, Y.; Fu, R.; Wang, J.; Yang, X.; Wen, L.; Feng, J. Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via Nrf2/HO-1 pathway. Pharm. Biol. 2018, 56, 440–449. [Google Scholar] [CrossRef]
- Kamesh, V.; Sumathi, T. Nephroprotective potential of Bacopa monniera on hypercholesterolemia induced nephropathy via the NO signaling pathway. Pharm. Biol. 2014, 52, 1327–1334. [Google Scholar] [CrossRef]
- Yousuf, S.; Atif, F.; Ahmad, M.; Hoda, N.; Ishrat, T.; Khan, B.; Islam, F. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 2009, 1250, 242–253. [Google Scholar] [CrossRef]
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Iuga, C.; Alvarez-Idaboy, J.R.; Russo, N. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: A quantum chemical and computational kinetics study. J. Org. Chem. 2012, 77, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Inglés, M.; Gambini, J.; Miguel, M.G.; Bonet-Costa, V.; Abdelaziz, K.M.; El Alami, M.; Viña, J.; Borrás, C. PTEN mediates the antioxidant effect of resveratrol at nutritionally relevant concentrations. Biomed. Res. Int. 2014, 2014, 580852. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qu, Y.; Mao, M.; Zhang, X.; Li, J.; Ferriero, D.; Mu, D. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Zhou, Y.; Wang, K.; Hou, Y.; Hou, R.; Ye, X. Resveratrol provides neuroprotection by inhibiting phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after stroke in rats. Brain Res. Bull. 2016, 121, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Gerszon, J.; Rodacka, A.; Puchala, M. Antioxidant Properties of Resveratrol and its Protective Effects in Neurodegenerative Diseases. Adv. Cell Biol. 2014, 4, 97–117. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Hu, M.M.; Xin, Y.F.; Gang, C. Resveratrol alleviates vascular inflammatory injury by inhibiting inflammasome activation in rats with hypercholesterolemia and vitamin D2 treatment. Inflamm. Res. 2015, 64, 321–332. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Fan, T.; Liu, J.; Su, X.; Fan, S.; Liu, Q.; et al. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int. J. Mol. Sci. 2013, 14, 14105–14118. [Google Scholar] [CrossRef]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Zhou, M.; Zhang, Y.; Liu, Y.; Hou, P.; Zeng, X.; Yi, L.; Mi, M. Resveratrol attenuates endothelial oxidative injury by inducing autophagy via the activation of transcription factor EB. Nutr. Metab. 2019, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, J.C.; Srinivasan, S.; Bruno, N.E.; Parent, A.A.; Hughes, T.; Pollock, J.A.; Gjyshi, O.; Cavett, V.; Nowak, J.; Garcia-Ordonez, R.D.; et al. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. eLife 2014, 3, e02057. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yao, W.; Xia, H.; Jin, Y.; Li, X.; Cai, J.; Hei, Z. Elevation of HO-1 Expression Mitigates Intestinal Ischemia-Reperfusion Injury and Restores Tight Junction Function in a Rat Liver Transplantation Model. Oxid. Med. Cell. Longev. 2015, 2015, 986075. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, H.J.; Ditelberg, J.S.; Chen, S.F.; Sarco, D.P.; Chan, P.H.; Epstein, C.J.; Ferriero, D.M. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann. Neurol. 1998, 44, 357–364. [Google Scholar] [CrossRef]
- Weis, S.N.; Schunck, R.V.; Pettenuzzo, L.F.; Krolow, R.; Matté, C.; Manfredini, V.; Peralba, M.D.C.R.; Vargas, C.R.; Dalmaz, C.; Wyse, A.T.; et al. Early biochemical effects after unilateral hypoxia-ischemia in the immature rat brain. Int. J. Dev. Neurosci. 2011, 29, 115–120. [Google Scholar] [CrossRef]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Silbereis, J.C.; Huang, E.; Back, S.A.; Rowitch, D.H. Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Dis. Model Mech. 2010, 3, 678–688. [Google Scholar] [CrossRef]
- Ham, P.B., 3rd; Raju, R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 2016, 157, 92–116. [Google Scholar] [CrossRef]
- Chang, C.-C. Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1 diabetic rats. Chin. J. Physiol. 2012, 55, 192–201. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Khoury, N.; Saul, I.; Loris, Z.B.; Cohan, C.H.; Stradecki-Cohan, H.M.; Dave, K.R.; Young, J.I.; Perez-Pinzon, M.A. Neuronal SIRT1 (Silent Information Regulator 2 Homologue 1) Regulates Glycolysis and Mediates Resveratrol-Induced Ischemic Tolerance. Stroke 2017, 48, 3117–3125. [Google Scholar] [CrossRef]
- Toader, A.-M.; Filip, A.; Decea, N.; Muresan, A. Neuroprotective strategy in an experimental newborn rat model of brain ischemia and hypoxia: Effects of Resveratrol and hypothermia. Clujul Med. 2013, 86, 203–207. [Google Scholar] [PubMed]
- Li, H.; Li, X.; Liu, Z.; Wu, S.; Guo, J.; Shi, R.; Sun, Y.; Wang, Y.; Yin, H. Resveratrol reserved hypoxia-ischemia induced childhood hippocampal dysfunction and neurogenesis via improving mitochondrial dynamics. Neurosci. Res. 2020, 161, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, J.; Peng, J.; Jiang, H.; Le, K. Resveratrol Improves Synaptic Plasticity in Hypoxic-Ischemic Brain Injury in Neonatal Mice via Alleviating SIRT1/NF-kappaB Signaling-Mediated Neuroinflammation. J. Mol. Neurosci. 2022, 72, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Karalis, F.; Soubasi, V.; Georgiou, T.; Nakas, C.T.; Simeonidou, C.; Guiba-Tziampiri, O.; Spandou, E. Resveratrol ameliorates hypoxia/ischemia-induced behavioral deficits and brain injury in the neonatal rat brain. Brain Res. 2011, 1425, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Isac, S.; Panaitescu, A.M.; Spataru, A.; Iesanu, M.; Totan, A.; Udriste, A.; Cucu, N.; Peltecu, G.; Zagrean, L.; Zagrean, A.-M. Trans-resveratrol enriched maternal diet protects the immature hippocampus from perinatal asphyxia in rats. Neurosci. Lett. 2017, 653, 308–313. [Google Scholar] [CrossRef]
- Korfias, S.; Stranjalis, G.; Papadimitriou, A.; Psachoulia, C.; Daskalakis, G.; Antsaklis, A.; Sakas, D. Serum S-100B protein as a biochemical marker of brain injury: A review of current concepts. Curr. Med. Chem. 2006, 13, 3719–3731. [Google Scholar] [CrossRef]
- Roumes, H.; Dumont, U.; Sanchez, S.; Mazuel, L.; Blanc, J.; Raffard, G.; Chateil, J.-F.; Pellerin, L.; Bouzier-Sore, A.-K. Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. J. Cereb. Blood Flow Metab. 2021, 41, 342–358. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Dumont, U.; Sanchez, S.; Olivier, B.; Chateil, J.-F.; Deffieux, D.; Quideau, S.; Pellerin, L.; Beauvieux, M.-C.; Bouzier-Sore, A.-K.; Roumes, H. Maternal alcoholism and neonatal hypoxia-ischemia: Neuroprotection by stilbenoid polyphenols. Brain Res. 2020, 1738, 146798. [Google Scholar] [CrossRef] [PubMed]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Y.-M.; Fratkins, J.D.; LeBlanc, M.H. Grape seed extract suppresses lipid peroxidation and reduces hypoxic ischemic brain injury in neonatal rats. Brain Res. Bull. 2005, 66, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Y.-M.; Leblanc, M.H.; Bhatt, A.J.; Rhodes, P.G. Grape seed extract given three hours after injury suppresses lipid peroxidation and reduces hypoxic-ischemic brain injury in neonatal rats. Pediatr. Res. 2007, 61, 295–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.; Xu, J.; Rottinghaus, G.E.; Simonyi, A.; Lubahn, D.; Sun, G.Y.; Sun, A.Y. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002, 958, 439–447. [Google Scholar] [CrossRef]
- Williams, L.D.; Burdock, G.A.; Edwards, J.A.; Beck, M.; Bausch, J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 2009, 47, 2170–2182. [Google Scholar] [CrossRef]
- Malvasi, A.; Kosmas, I.; Mynbaev, O.A.; Sparic, R.; Gustapane, S.; Guido, M.; Tinelli, A. Can trans resveratrol plus d-chiro-inositol and myo-inositol improve maternal metabolic profile in overweight pregnant patients? Clin. 2017, 168, e240–e247. [Google Scholar]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults—A pilot study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef]
- Gadacha, W.; Ben-Attia, M.; Bonnefont-Rousselot, D.; Aouani, E.; Ghanem-Boughanmi, N.; Touitou, Y. Resveratrol opposite effects on rat tissue lipoperoxidation: Pro-oxidant during day-time and antioxidant at night. Redox Rep. 2009, 14, 154–158. [Google Scholar] [CrossRef]
- Guha, P.; Dey, A.; Chatterjee, A.; Chattopadhyay, S.; Bandyopadhyay, S. Pro-ulcer effects of resveratrol in mice with indomethacin-induced gastric ulcers are reversed by L-arginine. Br. J. Pharmacol. 2010, 159, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Rocha, K.; Souza, G.; Ebaid, G.; Seiva, F.; Cataneo, A.; Novelli, E. Resveratrol toxicity: Effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem. Toxicol. 2009, 47, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Guha, P.; Chattopadhyay, S.; Bandyopadhyay, S.K. Biphasic activity of resveratrol on indomethacin-induced gastric ulcers. Biochem. Biophys. Res. Commun. 2009, 381, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8, 478–500. [Google Scholar] [CrossRef]
- Prasad, K. Resveratrol, wine, and atherosclerosis. Int. J. Angiol. 2012, 21, 7–18. [Google Scholar] [CrossRef]
- Lee, S.S.; Lee, S.M.; Kim, M.; Chun, J.; Cheong, Y.K.; Lee, J. Analysis of trans-resveratrol in peanuts and peanut butters consumed in Korea. Food Res. Int. 2004, 37, 247–251. [Google Scholar] [CrossRef]
- Rege, S.; Egeetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef]
- Troian, S.A.; Vicenzi, K.; Alves, M.K. Content of resveratrol and total polyphenols in whole grape, reconstituted and sweetened juice sold in southern Brazil. Braz. J. Food Res. 2016, 7, 58–67. [Google Scholar] [CrossRef]
- Hurst, W.J.; Glinski, J.A.; Miller, K.B.; Apgar, J.; Davey, M.H.; Stuart, D.A. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J. Agric. Food Chem. 2008, 56, 8374–8378. [Google Scholar] [CrossRef]
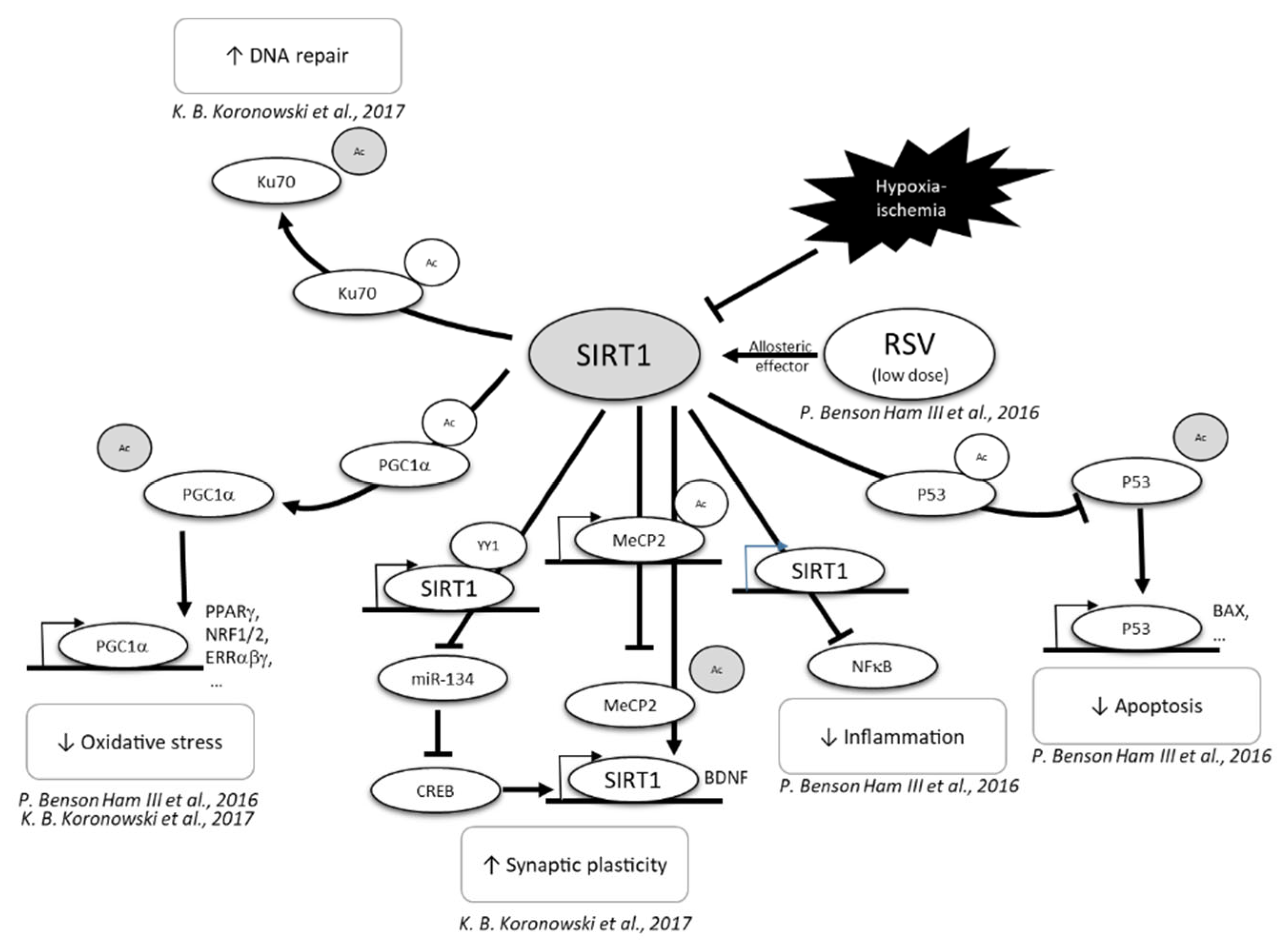
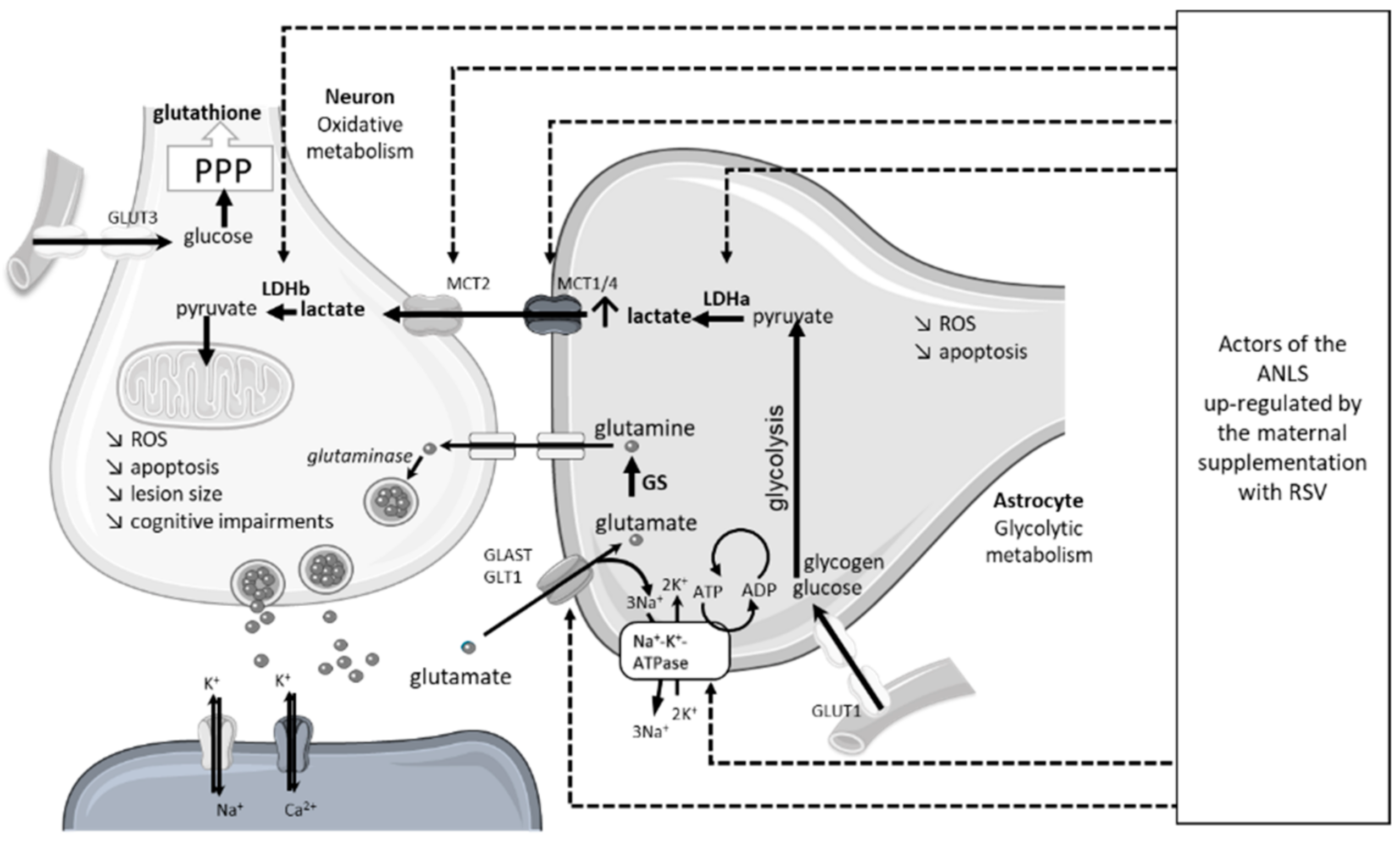
| Polyphenols | Species | Age of HI | Ischemia | Recovery | Hypoxia | Doses | Mode of Administration | Time of Administration | Main Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| RSV | Rat (n = 48) | P7 | Common carotid artery ligation | 1 h | 2.5 h 8% O2 92% N2 | 20 mg/kg/d 40 mg/kg/d | i.p. | During 7 d before HI | ↘ Cerebral edema | Gao et al., 2018 [38] |
| ↘ Brain lesion ↘ Lipid peroxidation | ||||||||||
| ↘ Inflammatory markers | ||||||||||
| ↗ Antioxidative status | ||||||||||
| ↗ HO-1 & Nrf2 | ||||||||||
| RSV | Rat (n = 33) | P7 | Cauterized common carotid | 1 h | 2 h 15 min 8% O2 92% N2 | 20 mg/kg | i.p. | 10 min before HI | ↘ Caspase 3 | Revuelta et al., 2017 [27] |
| ↘ TNFα | ||||||||||
| ↘ GFAP | ||||||||||
| RSV | Rat (n = 28) | P7 | Left common carotid artery ligation | 2 h | 2 h 15 min 8% O2 92% N2 | 20 mg/kg | i.p. | 10 min before HI | ↘ Brain lesion | Arteaga et al., 2015 [33] |
| Preservation of myelination | ||||||||||
| ↘ Astroglyosis | ||||||||||
| Maintenance of mitochondrial integrity | ||||||||||
| ↘ ROS production | ||||||||||
| ↗ Behavioral abilities | ||||||||||
| RSV | Rat (n = 80) | P7 | Clamp of the right commun carotid artery | 0 h | 1.5 h 9% O2 91% N2 | 20 mg/kg/d | i.p. | During the 7 days before HI | ↘ Oxydative stress | Toader et al., 2013 [62] |
| RSV | Mouse (n = 193) | P7 | Common carotid artery ligation | 2 h | 45 min 8% O2 92% N2 | 20 mg/kg 200 µg/kg | i.p. | 24 h or 10 min before HI | ↘ Caspase 3 ↘ Calpain | West et al., 2007 [26] |
| Polyphenols | Species | Age of HI | Ischemia | Recovery | Hypoxia | Doses | Mode of Administration | Time of Administration | Main Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| RSV | Mouse (n = 24) | P7 | Right common carotid artery ligation | 2 h | 1 h 8% O2 92% N2 | 100 mg/kg | i.p. | 0 h, 24 h, 48 h | ↘ Hippocampal neuronal damage | Peng et al., 2022 [64] |
| ↗ Dendritic spine density | ||||||||||
| ↗ Synaptic proteins | ||||||||||
| Modulation of SIRT1/NF-κB axis | ||||||||||
| -Improvement of cognitive & memory deficit | ||||||||||
| ↘ cerebral edema | ||||||||||
| ↘ brain lesion | ||||||||||
| ↘ Lipid peroxidation | ||||||||||
| ↘ Inflammatory markers | ||||||||||
| ↗ Antioxidative status | ||||||||||
| ↗ HO-1 & Nrf2 | ||||||||||
| RSV | Mouse (n = 16–24) | P7 | Electro-coagulation of the right carotid artery | 1 h | 1 h 10% O2 90% N2 | 10 mg/kg 40 mg/kg | Oralgavage | P14 During 14 days | ↗ Proliferation of neuronal strem cells ↗ Neuronal differentia-tion in hippocampus | Li et al., 2020 [63] |
| Improvement | ||||||||||
| of spatial learning and memory | ||||||||||
| ↘ Depressive and anxiety-like mood | ||||||||||
| RSV | Rat | P7 | Left common carotid artery ligation | 2.5 h 8% O2 92% N2 | 100 mg/kg | i.p. | 0 h, 8 h, 18 h after HI | ↗ miR-96 | Bian et al., 2017 [23] | |
| ↘ Bax | ||||||||||
| ↘ Brain lesion | ||||||||||
| RSV | Rat (n = 67) | P7 | Left common carotid artery ligation | 1.5 h | 2.5 h 8% O2 92% N2 | 100 mg/kg | i.p. | 0 h, 8 h, 18 h, 48 h, 72 h after HI | Inhibition of microglia activation | Pan et al., 2016 [24] |
| Anti-apoptotic effect | ||||||||||
| ↘ Bax, Bcl-2 and caspase 3 | ||||||||||
| RSV | Rat (n = 38) | P7 | Left common carotid artery ligation | 2–3 h | 1 h 8% O2 92% N2 | 90 mg/kg | i.p. | 0 h after HI | ↘ Brain lesion | Karalis et al., 2011 [65] |
| Improvement of behavioral abilities |
| Polyphenols | Species | Age of HI | Ischemia | Recovery | Hypoxia | Doses | Mode of Administration | Time of Administration | Main Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| RSV | Rat (n = 126) | P7 | Left common carotid artery ligation | 0.5 h | 2 h 8% O2 92% N2 | 0.15 mg/kg/d | Maternal supplemen-tation | Last week of gesta-tion + first week of lactation | Anti-oxydativeAnti-apoptotic Stimulation of brain energy metabolism | Dumont et al., 2021 [18] |
| RSV | Rat (n = 24) | P6 | Ø | 1.5 h 9% O2 20% CO2 71% N2 | 50 mg/kg/d | Maternal supplemen-tation | P30 of the dams until pus were7 d old | ↘ Neuroinflam-mation | Isac et al., 2017 [66] | |
| ↘ Neuronal injury |
| Polyphenols | Species | Age of HI | Ischemia | Recovery | Hypoxia | Doses | Mode of Administration | Time of Administration | Main Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| RSV or PIC | Rat (n = 78) | P7 | Left common carotid artery ligation | 0.5 h | 2 h 8% O2 92% N2 | 0.15 mg/kg/d0.15 mg/kg/d | Maternal supplementation | Last week of gesta-tion + first week of lactation | ↘ Brain lesion ↗ Sensitivo-motor & cognitive abilitiesNeuroportection of PIC better than RSV | Dumont et al., 2020 [71] |
| PIC | Rat (n = 52) | P7 | Left common carotid artery ligation | 0.5 h | 2 h 8% O2 92% N2 | 0.15 mg/kg/d | Maternal supplementation | Last week of gesta-tion + first week of lactation | ↘ Brain lesion | Dumont et al., 2019 [19] |
| ↗ Sensitivo-motor & cognitive abilities |
| Polyphenols | Species | Age of HI | Ischemia | Recovery | Hypoxia | Doses | Mode of Administration | Time of Administration | Main Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| RSV+VNF+PTE | Rat (n = 58) | P7 | Left common carotid artery ligation | 0.5 h | 2 h 8% O2 92% N2 | 0.15 mg/kg/d 0.30 mg/kg/d 0.15 mg/kg/d | Maternal supplemen-tation | Last week of gesta-tion + first week of lactation | ↘ Brain lesion | Roumes et al., 2022 [20] |
| Preservation of sensori-motor & cognitive function High striatal neuroprotec-tion | ||||||||||
| Grape seed extract | Rat (n = 27) | P7 | Right common carotid artery ligation | 2–3 h | 2.5 h 8% O2 92% N2 | 50 mg/kg | i.p. | 5 min to 5 h post-HI + 3 doses in 26 h post-HI | ↘ Brain lesion | Feng et al., 2007 [74] |
| ↘ Lipid | ||||||||||
| peroxidation | ||||||||||
| Grape seed extract | Rat (n = 123) | P7 | Right common carotidartery ligation | 2–3 h | 2.5 h 8% O2 92% N2 | 25 or 50 mg/kg | i.p. | 5 min before HI 4 h after HI Twice daily for 1 d | ↘ Brain lesion | Feng et al., 2005 [73] |
| ↘ Lipid | ||||||||||
| peroxidation |
| Product | Dose Used in Preclinical Studies | Food | Daily Food Intake Necessary for a Pregnant Woman |
|---|---|---|---|
| RSV | 50 mg/kg/d (maternal supplementation) [66] | Fresh grape [84,85] | 1–21 kg |
| Fresh peanuts [86] | 12–9 kg | ||
| Cranberry juice [84] | 17,500 L | ||
| Grape juice [87,88] | 241–7778 L | ||
| Black chocolate [89] | 10 kg | ||
| Milk chocolate [89] | 35 kg | ||
| 10 mg/kg/d (oral gavage of the pups) [63] | Fresh grape | 200 g–4 kg | |
| Fresh peanuts | 3–13 kg | ||
| Grape juice | 48–1555 L | ||
| Cramberry juice | 3500 L | ||
| Black chocolate | 2 kg | ||
| Milk chocolate | 7 kg | ||
| 0.15 mg/kg/d (maternal supplementation) [18,19,20,71] | Fresh grape | 3–66 g | |
| Fresh peanuts | 35–117 g | ||
| Grape juice | 4–23 L | ||
| Cranberry juice | 53 L | ||
| Black chocolate | 30 g | ||
| Milk chocolate | 100 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumes, H.; Goudeneche, P.; Pellerin, L.; Bouzier-Sore, A.-K. Resveratrol and Some of Its Derivatives as Promising Prophylactic Treatments for Neonatal Hypoxia-Ischemia. Nutrients 2022, 14, 3793. https://doi.org/10.3390/nu14183793
Roumes H, Goudeneche P, Pellerin L, Bouzier-Sore A-K. Resveratrol and Some of Its Derivatives as Promising Prophylactic Treatments for Neonatal Hypoxia-Ischemia. Nutrients. 2022; 14(18):3793. https://doi.org/10.3390/nu14183793
Chicago/Turabian StyleRoumes, Hélène, Pierre Goudeneche, Luc Pellerin, and Anne-Karine Bouzier-Sore. 2022. "Resveratrol and Some of Its Derivatives as Promising Prophylactic Treatments for Neonatal Hypoxia-Ischemia" Nutrients 14, no. 18: 3793. https://doi.org/10.3390/nu14183793
APA StyleRoumes, H., Goudeneche, P., Pellerin, L., & Bouzier-Sore, A.-K. (2022). Resveratrol and Some of Its Derivatives as Promising Prophylactic Treatments for Neonatal Hypoxia-Ischemia. Nutrients, 14(18), 3793. https://doi.org/10.3390/nu14183793








