Dietary Quality during Pregnancy and Congenital Heart Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Dietary Quality Evaluation
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
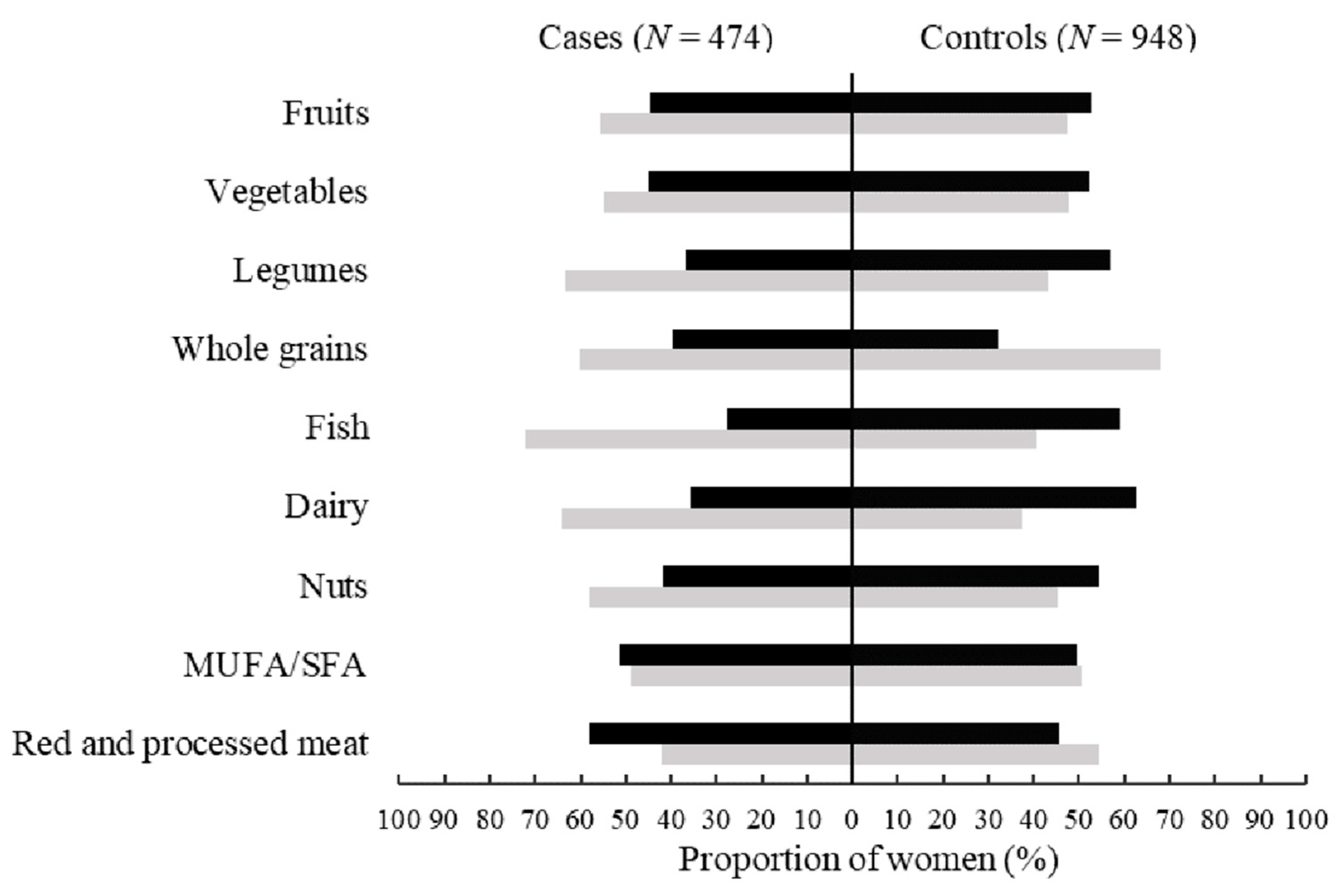
3.2. The Distribution of Food Components in GDQS and MDS among Cases and Controls
3.3. Associations of Maternal GDQS and MDS during Pregnancy with CHD
3.4. The Prediction Values for Maternal GDQS and MDS during Pregnancy on CHD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.M.; Liu, F.; Wu, L.; Ma, X.J.; Niu, C.; Huang, G.Y. Prevalence of Congenital Heart Disease at Live Birth in China. J. Pediatr. 2019, 204, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.S.; Smith, A.G.C.; Sable, C.A.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; Bhutta, Z.A.; Boucher, J.L.; et al. Global, regional, and national burden of congenital heart disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef]
- Nie, X.; Liu, X.; Wang, C.; Wu, Z.; Sun, Z.; Su, J.; Yan, R.; Peng, Y.; Yang, Y.; Wang, C.; et al. Assessment of evidence on reported non-genetic risk factors of congenital heart defects: The updated umbrella review. BMC Pregnancy Childbirth 2022, 22, 371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.N.; Wu, Q.J.; Liu, Y.S.; Lv, J.L.; Sun, H.; Chang, Q.; Liu, C.F.; Zhao, Y.H. Environmental Risk Factors and Congenital Heart Disease: An Umbrella Review of 165 Systematic Reviews and Meta-Analyses with More Than 120 Million Participants. Front. Cardiovasc. Med. 2021, 8, 640729. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kang, Y.; Chang, Q.; Zhang, B.; Liu, X.; Zeng, L.; Yan, H.; Dang, S. Maternal Zinc, Copper, and Selenium Intakes during Pregnancy and Congenital Heart Defects. Nutrients 2022, 14, 1055. [Google Scholar] [CrossRef]
- Zhang, R.; Guo, L.; Zhao, D.; Qu, P.; Dang, S.; Yan, H. Maternal B-vitamin intake and B-vitamin supplementation during pregnancy in relation to neonatal congenital heart defects: A case-control study with propensity score matching. Eur. J. Clin. Nutr. 2021, 75, 782–791. [Google Scholar] [CrossRef]
- Yang, J.; Kang, Y.; Cheng, Y.; Zeng, L.; Shen, Y.; Shi, G.; Liu, Y.; Qu, P.; Zhang, R.; Yan, H.; et al. Iron intake and iron status during pregnancy and risk of congenital heart defects: A case-control study. Int. J. Cardiol. 2020, 301, 74–79. [Google Scholar] [CrossRef]
- Qu, Y.; Lin, S.; Zhuang, J.; Bloom, M.S.; Smith, M.; Nie, Z.; Mai, J.; Ou, Y.; Wu, Y.; Gao, X.; et al. First-Trimester Maternal Folic Acid Supplementation Reduced Risks of Severe and Most Congenital Heart Diseases in Offspring: A Large Case-Control Study. J. Am. Heart Assoc. 2020, 9, e015652. [Google Scholar] [CrossRef]
- Smedts, H.P.; Rakhshandehroo, M.; Verkleij-Hagoort, A.C.; de Vries, J.H.; Ottenkamp, J.; Steegers, E.A.; Steegers-Theunissen, R.P. Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects. Eur. J. Nutr. 2008, 47, 357–365. [Google Scholar] [CrossRef]
- Smedts, H.P.; de Vries, J.H.; Rakhshandehroo, M.; Wildhagen, M.F.; Verkleij-Hagoort, A.C.; Steegers, E.A.; Steegers-Theunissen, R.P. High maternal vitamin E intake by diet or supplements is associated with congenital heart defects in the offspring. BJOG 2009, 116, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, T.; Huang, P.; Zhang, S.; Song, X.; Sun, M.; Liu, Y.; Wei, J.; Shu, J.; Zhong, T.; et al. Association and Interaction Effect of BHMT Gene Polymorphisms and Maternal Dietary Habits with Ventricular Septal Defect in Offspring. Nutrients 2022, 14, 3094. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, T.; Huang, P.; Zhang, S.; Song, X.; Sun, M.; Liu, Y.; Wei, J.; Shu, J.; Zhong, T.; et al. Association of maternal dietary intakes and CBS gene polymorphisms with congenital heart disease in offspring. Int. J. Cardiol. 2021, 322, 121–128. [Google Scholar]
- Zhang, S.; Liu, X.; Yang, T.; Wang, T.; Chen, L.; Qin, J. Association of maternal dietary habits and ADIPOQ gene polymorphisms with the risk of congenital heart defects in offspring: A hospital-based case-control study. Eur. J. Clin. Nutr. 2022, 76, 373–381. [Google Scholar] [CrossRef]
- Yang, J.; Kang, Y.; Cheng, Y.; Zeng, L.; Yan, H.; Dang, S. Maternal Dietary Patterns during Pregnancy and Congenital Heart Defects: A Case-Control Study. Int. J. Environ. Res. Public Health. 2019, 16, 2957. [Google Scholar] [CrossRef]
- Sotres-Alvarez, D.; Siega-Riz, A.M.; Herring, A.H.; Carmichael, S.L.; Feldkamp, M.L.; Hobbs, C.A.; Olshan, A.F. Maternal dietary patterns are associated with risk of neural tube and congenital heart defects. Am. J. Epidemiol. 2013, 177, 1279–1288. [Google Scholar] [CrossRef]
- Obermann-Borst, S.A.; Vujkovic, M.; de Vries, J.H.; Wildhagen, M.F.; Looman, C.W.; de Jonge, R.; Steegers, E.A.; Steegers-Theunissen, R.P. A maternal dietary pattern characterised by fish and seafood in association with the risk of congenital heart defects in the offspring. BJOG 2011, 118, 1205–1215. [Google Scholar] [CrossRef]
- Botto, L.D.; Krikov, S.; Carmichael, S.L.; Munger, R.G.; Shaw, G.M.; Feldkamp, M.L. Lower rate of selected congenital heart defects with better maternal diet quality: A population-based study. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F43–F49. [Google Scholar] [CrossRef]
- Arimond, M.; Wiesmann, D.; Becquey, E.; Carriquiry, A.; Daniels, M.C.; Deitchler, M.; Fanou-Fogny, N.; Joseph, M.L.; Kennedy, G.; Martin-Prevel, Y.; et al. Simple food group diversity indicators predict micronutrient adequacy of women’s diets in 5 diverse, resource-poor settings. J. Nutr. 2010, 140, 2059s–2069s. [Google Scholar] [CrossRef]
- Madzorera, I.; Isanaka, S.; Wang, M.; Msamanga, G.I.; Urassa, W.; Hertzmark, E.; Duggan, C.; Fawzi, W.W. Maternal dietary diversity and dietary quality scores in relation to adverse birth outcomes in Tanzanian women. Am. J. Clin. Nutr. 2020, 112, 695–706. [Google Scholar] [CrossRef]
- Bromage, S.; Batis, C.; Bhupathiraju, S.N.; Fawzi, W.W.; Fung, T.T.; Li, Y.; Deitchler, M.; Angulo, E.; Birk, N.; Castellanos-Gutiérrez, A.; et al. Development and Validation of a Novel Food-Based Global Diet Quality Score (GDQS). J. Nutr. 2021, 151, 75s–92s. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Hassounah, S.; Swaka, A. The Impact of Mediterranean Dietary Patterns During Pregnancy on Maternal and Offspring Health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Deng, X.; Lin, S.; Han, F.; Chang, H.H.; Ou, Y.; Nie, Z.; Mai, J.; Wang, X.; Gao, X.; et al. Using Innovative Machine Learning Methods to Screen and Identify Predictors of Congenital Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 797002. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, X.; Hu, X.; Wen, B.; Wang, L.; Wang, C. A predictive model of offspring congenital heart disease based on maternal risk factors during pregnancy: A hospital based case-control study in Nanchong City. Int. J. Med. Sci. 2020, 17, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Z.; Guo, H.; Cao, H.; Song, C.; Guo, X.; Zhang, Y. Predicting congenital heart defects: A comparison of three data mining methods. PLoS ONE 2017, 12, e0177811. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, M.; Zheng, J.; Luo, J.; Zeng, R.; Feng, N.; Du, Q.; Fang, J. An artificial neural network prediction model of congenital heart disease based on risk factors: A hospital-based case-control study. Medicine 2017, 96, e6090. [Google Scholar] [CrossRef]
- Crozier, S.R.; Robinson, S.M.; Godfrey, K.M.; Cooper, C.; Inskip, H.M. Women’s dietary patterns change little from before to during pregnancy. J. Nutr. 2009, 139, 1956–1963. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, Y.; Zeng, L.; Dang, S.; Yan, H. Maternal dietary diversity during pregnancy and congenital heart defects: A case-control study. Eur. J. Clin. Nutr. 2021, 75, 355–363. [Google Scholar] [CrossRef]
- Cheng, Y.; Yan, H.; Dibley, M.J.; Shen, Y.; Li, Q.; Zeng, L. Validity and reproducibility of a semi-quantitative food frequency questionnaire for use among pregnant women in rural China. Asia Pac. J. Clin. Nutr. 2008, 17, 166–177. [Google Scholar]
- Yang, J.; Dang, S.; Cheng, Y.; Qiu, H.; Mi, B.; Jiang, Y.; Qu, P.; Zeng, L.; Wang, Q.; Li, Q.; et al. Dietary intakes and dietary patterns among pregnant women in Northwest China. Public Health Nutr. 2017, 20, 282–293. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, Y.; Pei, L.; Jiang, Y.; Lei, F.; Zeng, L.; Wang, Q.; Li, Q.; Kang, Y.; Shen, Y.; et al. Maternal iron intake during pregnancy and birth outcomes: A cross-sectional study in Northwest China. Br. J. Nutr. 2017, 117, 862–871. [Google Scholar] [CrossRef]
- Institute of Nutrition and Food Safety, China Center for Disease Control. China Food Composition Book 2; Peking University Medical Press: Beijing, China, 2005. [Google Scholar]
- Institute of Nutrition and Food Safety, China Center for Disease Control. China Food Composition Book 1, 2nd ed.; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Mahmassani, H.A.; Switkowski, K.M.; Scott, T.M.; Johnson, E.J.; Rifas-Shiman, S.L.; Oken, E.; Jacques, P.F. Maternal diet quality during pregnancy and child cognition and behavior in a US cohort. Am. J. Clin. Nutr. 2022, 115, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Mickey, R.M.; Greenland, S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.L.; Yang, W.; Feldkamp, M.L.; Munger, R.G.; Siega-Riz, A.M.; Botto, L.D.; Shaw, G. Reduced risks of neural tube defects and orofacial clefts with higher diet quality. Arch. Pediatr. Adolesc. Med. 2012, 166, 121–126. [Google Scholar] [CrossRef]
- Feldkamp, M.L.; Krikov, S.; Botto, L.D.; Shaw, G.M.; Carmichael, S.L. Better diet quality before pregnancy is associated with reduced risk of gastroschisis in Hispanic women. J. Nutr. 2014, 144, 1781–1786. [Google Scholar]
- Morales, E.; García-Serna, A.M.; Larqué, E.; Sánchez-Campillo, M.; Serrano-Munera, A.; Martinez-Graciá, C.; Santaella-Pascual, M.; Suárez-Martínez, C.; Vioque, J.; Noguera-Velasco, J.A.; et al. Dietary Patterns in Pregnancy and Biomarkers of Oxidative Stress in Mothers and Offspring: The NELA Birth Cohort. Front. Nutr. 2022, 9, 869357. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Ma, C.; Feldkamp, M.L.; Munger, R.G.; Olney, R.S.; Botto, L.D.; Shaw, G.M.; Correa, A. Nutritional factors and hypospadias risks. Paediatr. Perinat. Epidemiol. 2012, 26, 353–360. [Google Scholar] [CrossRef]
- Fisher, S.A.; Burggren, W.W. Role of hypoxia in the evolution and development of the cardiovascular system. Antioxid. Redox Signal. 2007, 9, 1339–1352. [Google Scholar] [CrossRef]
- Liu, S.; Joseph, K.S.; Lisonkova, S.; Rouleau, J.; Van den Hof, M.; Sauve, R.; Kramer, M.S.; Canadian Perinatal Surveillance System (Public Health Agency of Canada). Association between maternal chronic conditions and congenital heart defects: A population-based cohort study. Circulation 2013, 128, 583–589. [Google Scholar] [CrossRef]
- Bunin, G.R.; Gyllstrom, M.E.; Brown, J.E.; Kahn, E.B.; Kushi, L.H. Recall of diet during a past pregnancy. Am. J. Epidemiol. 2001, 154, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Bosco, J.L.; Tseng, M.; Spector, L.G.; Olshan, A.F.; Bunin, G.R. Reproducibility of reported nutrient intake and supplement use during a past pregnancy: A report from the Children’s Oncology Group. Paediatr. Perinat. Epidemiol. 2010, 24, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yang, T.; Chen, L.; Wang, L.; Zhang, S.; Wang, T.; Zhao, L.; Ye, Z.; Chen, L.; Qin, J. Increased maternal Body Mass Index is associated with congenital heart defects: An updated meta-analysis of observational studies. Int. J. Cardiol. 2018, 273, 112–120. [Google Scholar] [CrossRef] [PubMed]


| Case (N = 474) | Control (N = 948) | p 1 | |
|---|---|---|---|
| Sociodemographic characteristics, % | |||
| Maternal age < 30 years | 66.5 | 65.8 | 0.812 |
| Maternal work, in employment | 50.6 | 78.7 | <0.001 |
| Maternal education, senior high school or above | 58.9 | 80.7 | <0.001 |
| Urban residence | 66.0 | 71.6 | 0.030 |
| Nulliparity | 57.8 | 80.3 | <0.001 |
| Maternal health-related factors in early pregnancy, % | |||
| Passive smoking | 33.5 | 9.3 | <0.001 |
| Anemia | 16.9 | 10.9 | 0.001 |
| Medication use | 41.6 | 30.4 | <0.001 |
| Folate/iron supplements use | 76.6 | 89.2 | <0.001 |
| Birth weight < 2500 g, % | 9.7 | 5.3 | 0.003 |
| Gestational age < 37 weeks, % | 6.1 | 5.1 | 0.407 |
| Daily nutrients intakes during pregnancy, median (25th percentile, 75th percentile) | |||
| Total energy, kcal | 1753.2 (1452.4, 2086.1) | 1907.1 (1563.3, 2415.9) | <0.001 |
| Protein, g | 44.5 (32.0, 60.5) | 56.9 (40.9, 78.9) | <0.001 |
| Fat, g | 30.9 (19.0, 47.8) | 41.7 (29.0, 59.5) | <0.001 |
| Monounsaturated fatty acid, g | 6.9 (3.9, 12.2) | 9.7 (6.6, 14.4) | <0.001 |
| Saturated fatty acid, g | 13.1 (8.1, 19.3) | 17.4 (12.6, 25.0) | <0.001 |
| Carbohydrate, g | 185.6 (142.0, 237.7) | 190.9 (142.5, 269.8) | 0.057 |
| Iron, mg | 17.5 (12.6, 23.3) | 20.4 (14.3, 28.9) | <0.001 |
| Zinc, mg | 4.7 (3.1, 6.8) | 6.4 (4.6, 9.1) | <0.001 |
| Selenium, μg | 22.7 (15.0, 32.5) | 30.8 (21.9, 43.7) | <0.001 |
| Calcium, mg | 457.4 (315.8, 643.8) | 500.9 (359.8, 707.0) | <0.001 |
| Niacin, mg | 9.6 (7.3, 13.2) | 12.5 (9.0, 17.3) | <0.001 |
| Folate, μg | 195.1 (161.3, 242.9) | 220.2 (181.5, 270.5) | <0.001 |
| Vitamin C, mg | 63.5 (42.1, 107.0) | 77.0 (51.8, 123.2) | <0.001 |
| Dietary quality scores, median (25th percentile, 75th percentile) | |||
| GDQS | 27.5 (23.7, 31.0) | 31.0 (27.3, 34.3) | <0.001 |
| MDS | 4.0 (2.0, 5.0) | 5.0 (3.0, 6.0) | <0.001 |
| Scoring Ranges 1 (Cutoffs, g/d) Low/Middle/High | GDQS Subscores | Case (N = 474) | Control (N = 948) | p 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low, % | Middle, % | High, % | Score 2 | Low, % | Middle, % | High, % | Score 2 | ||||
| Citrus fruits | <24/24–69/>69 | 0, 1, 2 | 69.6 | 17.9 | 12.4 | 0.0 (0.0, 1.0) | 62.6 | 26.1 | 11.4 | 0.0 (0.0, 1.0) | 0.032 |
| Deep orange fruits | <25/25–123/>123 | 0, 1, 2 | 96.8 | 3.2 | 0 | 0.0 (0.0, 0.0) | 93.4 | 6.6 | 0 | 0.0 (0.0, 0.0) | 0.007 |
| Other fruits | <27/27–107/>107 | 0, 1, 2 | 0.8 | 13.7 | 85.4 | 2.0 (2.0, 2.0) | 0.3 | 4.4 | 95.3 | 2.0 (2.0, 2.0) | <0.001 |
| Dark green leafy vegetables | <13/13–37/>37 | 0, 2, 4 | 6.8 | 42.4 | 50.8 | 4.0 (2.0, 4.0) | 6.5 | 39.7 | 53.8 | 4.0 (2.0, 4.0) | 0.321 |
| Cruciferous vegetables | <13/13–36/>36 | 0, 0.25, 0.5 | 7.0 | 43.7 | 49.4 | 0.25 (0.25, 0.5) | 2.7 | 37.8 | 59.5 | 0.5 (0.25, 0.5) | <0.001 |
| Deep orange vegetables | <9/9–45/>45 | 0, 0.25, 0.5 | 33.3 | 51.1 | 15.6 | 0.25 (0.0, 0.25) | 20.8 | 63.1 | 16.1 | 0.25 (0.25, 0.25) | <0.001 |
| Other vegetables | <23/23–114/>114 | 0, 0.25, 0.5 | 0.4 | 25.9 | 73.6 | 0.5 (0.25, 0.5) | 0.3 | 15.2 | 84.5 | 0.5 (0.5, 0.5) | <0.001 |
| Legumes | <9/9–42/>42 | 0, 2, 4 | 14.1 | 40.1 | 45.8 | 2.0 (2.0, 4.0) | 1.9 | 27.8 | 70.3 | 4.0 (2.0, 4.0) | <0.001 |
| Deep orange tubers | <12/12–63/>63 | 0, 0.25, 0.5 | 74.9 | 21.7 | 3.4 | 0.0 (0.0, 0.25) | 57.4 | 40.4 | 2.2 | 0.0 (0.0, 0.25) | <0.001 |
| Nuts and seeds | <7/7–13/>13 | 0, 2, 4 | 48.9 | 14.1 | 36.9 | 2.0 (0.0, 4.0) | 35.2 | 18.0 | 46.7 | 2.0 (0.0, 4.0) | <0.001 |
| Whole grains | <8/8–13/>13 | 0, 1, 2 | 29.1 | 11.0 | 59.9 | 2.0 (0.0, 2.0) | 32.0 | 15.5 | 52.5 | 2.0 (0.0, 2.0) | 0.082 |
| Liquid oils | <2/2–7.5/>7.5 | 0, 1, 2 | 0 | 33.1 | 66.9 | 2.0 (1.0, 2.0) | 0 | 27.4 | 72.6 | 2.0 (1.0, 2.0) | 0.026 |
| Fish and shellfish | <14/14–71/>71 | 0, 1, 2 | 69.0 | 26.4 | 4.6 | 0.0 (0.0, 1.0) | 38.1 | 49.3 | 12.7 | 1.0 (0.0, 1.0) | <0.001 |
| Poultry and game meat | <16/16–44/>44 | 0, 1, 2 | 93.2 | 5.1 | 1.7 | 0.0 (0.0, 0.0) | 81.6 | 16.6 | 1.8 | 0.0 (0.0, 0.0) | <0.001 |
| Low-fat dairy | <33/33–132/>132 | 0, 1, 2 | 100.0 | 0 | 0 | 0.0 (0.0, 0.0) | 100.0 | 0 | 0 | 0.0 (0.0, 0.0) | 1.000 |
| Eggs | <6/6–32/>32 | 0, 1, 2 | 27.2 | 35.0 | 37.8 | 1.0 (0.0, 2.0) | 8.4 | 34.9 | 56.6 | 2.0 (1.0, 2.0) | <0.001 |
| Processed meat | <9/9–30/>30 | 2, 1, 0 | 74.9 | 19.6 | 5.5 | 2.0 (1.0, 2.0) | 78.7 | 18.7 | 2.6 | 2.0 (2.0, 2.0) | 0.069 |
| Refined grains and baked goods | <7/7–33/>33 | 2, 1, 0 | 0 | 0.6 | 99.4 | 0.0 (0.0, 0.0) | 0.1 | 0.2 | 99.7 | 0.0 (0.0, 0.0) | 0.387 |
| Sweets and ice cream | <13/13–37/>37 | 2, 1, 0 | 93.9 | 3.8 | 2.3 | 2.0 (2.0, 2.0) | 94.2 | 4.2 | 1.6 | 2.0 (2.0, 2.0) | 0.790 |
| Sugar-sweetened beverages | <57/57–180/>180 | 2, 1, 0 | 98.3 | 0.8 | 0.8 | 2.0 (2.0, 2.0) | 99.4 | 0.6 | 0 | 2.0 (2.0, 2.0) | 0.056 |
| Juice | <36/36–144/>144 | 2, 1, 0 | 94.9 | 3.2 | 1.9 | 2.0 (2.0, 2.0) | 98.5 | 1.4 | 0.1 | 2.0 (2.0, 2.0) | <0.001 |
| White roots and tubers | <27/27–107/>107 | 2, 1, 0 | 55.9 | 39.9 | 4.2 | 2.0 (1.0, 2.0) | 53.9 | 44.7 | 1.4 | 2.0 (1.0, 2.0) | 0.814 |
| Purchased deep-fried foods | <9/9–45/>45 | 2, 1, 0 | 87.3 | 10.1 | 2.5 | 2.0 (2.0, 2.0) | 90.6 | 9.1 | 0.3 | 2.0 (2.0, 2.0) | 0.044 |
| High-fat dairy | <35/35–142/142–734/>734 | 0, 1, 2, 0 | 46.0 | 26.2/27.6 | 0.2 | 1.0 (0.0, 2.0) | 10.4 | 41.4/47.9 | 0.3 | 1.0 (1.0, 2.0) | <0.001 |
| Red meat | <9/9–46/>46 | 0, 1, 0 | 27.4 | 41.6 | 31.0 | 0.0 (0.0, 1.0) | 7.2 | 51.6 | 41.2 | 1.0 (0.0, 1.0) | <0.001 |
| Total Congenital Heart Defects (Ncases = 474) | Ventricular Septal Defects (Ncases = 222) | Atrial Septal Defects (Ncases = 218) | |||||
|---|---|---|---|---|---|---|---|
| Ncases/Ncontrols | Unadjusted OR (95%CI) | Adjusted OR (95%CI) 1 | Unadjusted OR (95%CI) | Adjusted OR (95%CI) 1 | Unadjusted OR (95%CI) | Adjusted OR (95%CI) 1 | |
| GDQS | |||||||
| Quartile 1 | 218/218 | 1 | 1 | 1 | 1 | 1 | 1 |
| Quartile 2 | 131/241 | 0.54 (0.41, 0.72) | 0.59 (0.42, 0.82) | 0.51 (0.35, 0.74) | 0.51 (0.32, 0.79) | 0.57 (0.39, 0.83) | 0.51 (0.34, 0.79) |
| Quartile 3 | 75/237 | 0.32 (0.23, 0.44) | 0.36 (0.24, 0.53) | 0.35 (0.23, 0.52) | 0.38 (0.23, 0.63) | 0.39 (0.26, 0.59) | 0.35 (0.21, 0.59) |
| Quartile 4 | 50/252 | 0.20 (0.14, 0.28) | 0.26 (0.16, 0.42) | 0.18 (0.11, 0.29) | 0.23 (0.12, 0.44) | 0.21 (0.13, 0.34) | 0.21 (0.11, 0.41) |
| p for trend | 474/948 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Per 1 higher score | 474/948 | 0.87 (0.85, 0.89) | 0.88 (0.85, 0.91) | 0.87 (0.84, 0.90) | 0.88 (0.84, 0.92) | 0.88 (0.85, 0.91) | 0.87 (0.84, 0.91) |
| MDS | |||||||
| Quartile 1 | 142/164 | 1 | 1 | 1 | 1 | 1 | 1 |
| Quartile 2 | 159/271 | 0.67 (0.50, 0.91) | 0.76 (0.54, 1.08) | 0.68 (0.46, 1.01) | 0.87 (0.55, 1.38) | 0.80 (0.53, 1.19) | 0.83 (0.53, 1.31) |
| Quartile 3 | 75/162 | 0.53 (0.37, 0.76) | 0.70 (0.46, 1.08) | 0.62 (0.39, 0.98) | 0.81 (0.52, 1.26) | 0.69 (0.43, 1.11) | 0.80 (0.47, 1.36) |
| Quartile 4 | 98/351 | 0.32 (0.23, 0.44) | 0.53 (0.34, 0.83) | 0.34 (0.22, 0.52) | 0.57 (0.34, 0.96) | 0.44 (0.28, 0.67) | 0.61 (0.38, 0.97) |
| p for trend | 474/948 | <0.001 | 0.007 | <0.001 | 0.010 | <0.001 | 0.016 |
| Per 1 higher score | 474/948 | 0.79 (0.75, 0.84) | 0.88 (0.80, 0.95) | 0.80 (0.74, 0.87) | 0.89 (0.81, 0.98) | 0.85 (0.78, 0.91) | 0.90 (0.83, 0.98) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Chang, Q.; Dang, S.; Liu, X.; Zeng, L.; Yan, H. Dietary Quality during Pregnancy and Congenital Heart Defects. Nutrients 2022, 14, 3654. https://doi.org/10.3390/nu14173654
Yang J, Chang Q, Dang S, Liu X, Zeng L, Yan H. Dietary Quality during Pregnancy and Congenital Heart Defects. Nutrients. 2022; 14(17):3654. https://doi.org/10.3390/nu14173654
Chicago/Turabian StyleYang, Jiaomei, Qianqian Chang, Shaonong Dang, Xin Liu, Lingxia Zeng, and Hong Yan. 2022. "Dietary Quality during Pregnancy and Congenital Heart Defects" Nutrients 14, no. 17: 3654. https://doi.org/10.3390/nu14173654
APA StyleYang, J., Chang, Q., Dang, S., Liu, X., Zeng, L., & Yan, H. (2022). Dietary Quality during Pregnancy and Congenital Heart Defects. Nutrients, 14(17), 3654. https://doi.org/10.3390/nu14173654








