Dietary and Nutrient Intake, Eating Habits, and Its Association with Maternal Gestational Weight Gain and Offspring’s Birth Weight in Pregnant Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Dietary and Nutrient Intake, and Eating Habits
2.2. Anthropometric Data and Gestational Weight Gain
2.3. Neonatal Outcomes
2.4. Other Variables
2.5. Statistical Analyses
2.6. Ethical Aspects
3. Results
4. Discussion
4.1. Dietary and Nutrients Intake and Eating Habits
4.2. Gestational Weight Gain
4.3. Dietary and Nutrient Intake, Eating Habits, and GWG
4.4. Dietary Intake, Eating Habits, and Birth Weight
4.5. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Food Group | Recommendation | Real Intake * |
| Vegetables | >3 | 0 (0–1) |
| Fruits | 3–4 | 1.5 (0.5–3) |
| Grain and cereals | 8–11 | 8 (6–11) |
| Legumes | 2–2.5 | 0 (0–0) |
| Animal-source foods | 3.5–4 | 3 (2–4) |
| Fat and oils | 3–5 | 2 (1–3) |
| Milk and yogurt | 2–2.5 | 2 (1–2) |
| Sugar table | <5 | 1 (0–2) |
| Sugar sweetened beverages | 0 | 2 (0.5–11) |
| Academia Nacional de Medicina 2015. México (Fernández-Gaxiola et al. 2015). * Median (p25–75). | ||
Appendix B
| Gestational Weight Gain % | p-Value | Birth Weight | LGA, n = 20 | p-Value | ||
| Insufficient | Excessive | SGA, n = 108 | ||||
| Chronological age (years) | ||||||
| ≤15, n = 204 | 31.9 | 39.7 | 0.038 | 22.5 | 3.4 | 0.601 |
| ≥16, n = 326 | 42.9 | 32.5 | 19.0 | 4.0 | ||
| Beginning antenatal care | ||||||
| First, n = 89 | 37.1 | 33.7 | 0.762 | 15.7 | 3.4 | 0.134 |
| Second, n = 338 | 37.9 | 37.0 | 21.0 | 5.0 | ||
| Third, n = 103 | 42.7 | 31.1 | 22.3 | 0.0 | ||
| Marital status | ||||||
| Single, n = 323 | 37.8 | 37.2 | 0.525 | 20.4 | 4.3 | 0.694 |
| Cohabiting, n = 207 | 40.1 | 32.4 | 20.3 | 2.9 | ||
| Occupation | ||||||
| Student, n = 57 | 42.1 | 36.8 | 0.655 | 19.3 | 1.8 | 0.668 |
| Housewife, n = 473 | 38.3 | 35.1 | 20.5 | 4.0 | ||
| Socioeconomic level | ||||||
| Middle, n = 143 | 38.5 | 37.1 | 0.464 | 18.9 | 2.8 | 0.292 |
| Low, n = 213 | 34.7 | 37.1 | 17.8 | 3.3 | ||
| Very low, n = 174 | 43.7 | 31.6 | 24.7 | 5.1 | ||
| School dropout | ||||||
| No, n = 193 | 37.3 | 38.3 | 0.526 | 19.2 | 3.6 | 0.858 |
| Yes, n = 337 | 39.5 | 33.5 | 21.1 | 3.9 | ||
| Gestational age | ||||||
| Term >37, n = 473 | 40.0 | 34.7 | 0.215 | 19.9 | 3.6 | 0.550 |
| Preterm, n = 57 | 28.1 | 40.4 | 26.4 | 5.3 | ||
| Number of prenatal visits | ||||||
| ≤6, n = 437 | 41.6 | 33.9 | 0.459 | 19.7 | 3.4 | 0.877 |
| ≥7, n = 93 | 36.4 | 36.4 | 20.9 | 4.0 | ||
| Percentages estimated by rows. SGA: small for gestational age. LGA: large for gestational age. p-value determined by Pearson’s Chi-Square. | ||||||
References
- Riley, T.; Sully, E.; Ahmed, Z.; Biddlecom, A. Estimates of the Potential Impact of the COVID-19 Pandemic on Sexual and Reproductive Health In Low- and Middle-Income Countries. Int. Perspect. Sex. Reprod. Health 2020, 46, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Murro, R.; Guttmacher Institute; Chawla, R.; Pyne, S.; Venkatesh, S.; Sully, E. Adding It Up: Investing in the Sexual and Reproductive Health of Adolescents in India; Guttmacher Institute: New York, NY, USA, 2021. [Google Scholar]
- Annan, R.A.; Gyimah, L.A.; Apprey, C.; Asamoah-Boakye, O.; Aduku, L.N.E.; Azanu, W.; Luterodt, H.E.; Edusei, A.K. Predictors of Adverse Birth Outcomes among Pregnant Adolescents in Ashanti Region, Ghana. J. Nutr. Sci. 2021, 10, e67. [Google Scholar] [CrossRef] [PubMed]
- Loredo-Abdalá, A.; Vargas-Campuzano, E.; Casas-Muñoz, A.; González-Corona, J.; Gutiérrez-Leyva, C.J. Adolescent pregnancy: Its causes and repercussions in the dyad. Rev. Med. Inst. Mex. Seguro Soc. 2017, 55, 223–229. [Google Scholar] [PubMed]
- Akseer, N.; Keats, E.C.; Thurairajah, P.; Cousens, S.; Bétran, A.P.; Oaks, B.M.; Osrin, D.; Piwoz, E.; Gomo, E.; Ahmed, F.; et al. Characteristics and Birth Outcomes of Pregnant Adolescents Compared to Older Women: An Analysis of Individual Level Data from 140,000 Mothers from 20 RCTs. EClinicalMedicine 2022, 45, 101309. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Chico-Barba, G.; Flores-Quijano, M.E.; Godínez-Martínez, E.; Martínez-Rojano, H.; Ortiz-Hernandez, L.; Nájera-Medina, O.; Hernández-Trejo, M.; Hurtado-Solache, C. Association of Pregestational BMI and Gestational Weight Gain with Maternal and Neonatal Outcomes in Adolescents and Adults from Mexico City. Int. J. Environ. Res. Public Health 2021, 19, 280. [Google Scholar] [CrossRef]
- Sundaram, A.; Puri, M.; Douglas-Hall, A.; Castle, K.; Wagle, K.; Weissman, E. Adding It Up: Costs and Benefits of Meeting the Contraceptive and Maternal and Newborn Health Needs of Women in Nepal; Guttmacher Institute: New York, NY, USA, 2019. [Google Scholar]
- Ronen, S.; Lee, J.; Patel, P.; Patel, P. A Comparison of Childbirth Costs for Adolescents and Adults From 2001 to 2010. J. Adolesc. Health 2018, 62, 59–62. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- De Amicis, R.; Mambrini, S.P.; Pellizzari, M.; Foppiani, A.; Bertoli, S.; Battezzati, A.; Leone, A. Ultra-Processed Foods and Obesity and Adiposity Parameters among Children and Adolescents: A Systematic Review. Eur. J. Nutr. 2022, 61, 2297–2311. [Google Scholar] [CrossRef]
- Harris, A.; Chilukuri, N.; West, M.; Henderson, J.; Lawson, S.; Polk, S.; Levine, D.; Bennett, W.L. Obesity-Related Dietary Behaviors among Racially and Ethnically Diverse Pregnant and Postpartum Women. J. Pregnancy 2016, 2016, 9832167. [Google Scholar] [CrossRef]
- Yang, J.; Wang, M.; Tobias, D.K.; Rich-Edwards, J.W.; Darling, A.M.; Abioye, A.I.; Pembe, A.B.; Madzorera, I.; Fawzi, W.W. Gestational Weight Gain during the Second and Third Trimesters and Adverse Pregnancy Outcomes, Results from a Prospective Pregnancy Cohort in Urban Tanzania. Reprod. Health 2022, 19, 140. [Google Scholar] [CrossRef]
- Christian, P.; Smith, E.R. Adolescent Undernutrition: Global Burden, Physiology, and Nutritional Risks. Ann. Nutr. Metab. 2018, 72, 316–328. [Google Scholar] [CrossRef]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.J.; Wong, Y.P.; Terry, N.; Abrams, B.; Bartholomew, A.; Bodnar, L.M.; Gernand, A.; Rasmussen, K.; et al. Dietary Patterns before and during Pregnancy and Gestational Age- and Sex-Specific Birth Weight: A Systematic Review; USDA Nutrition Evidence Systematic Review: Alexandria, VA, Egypt, 2022. [Google Scholar]
- Pobocik, R.S.; Benavente, J.C.; Boudreau, N.S.; Spore, C.L. Pregnant Adolescents in Guam Consume Diets Low in Calcium and Other Micronutrients. J. Am. Diet. Assoc. 2003, 103, 611–614. [Google Scholar] [CrossRef]
- Baker, P.N.; Wheeler, S.J.; Sanders, T.A.; Thomas, J.E.; Hutchinson, C.J.; Clarke, K.; Berry, J.L.; Jones, R.L.; Seed, P.T.; Poston, L. A Prospective Study of Micronutrient Status in Adolescent Pregnancy. Am. J. Clin. Nutr. 2009, 89, 1114–1124. [Google Scholar] [CrossRef]
- Pinho-Pompeu, M.; Paulino, D.S.M.; Surita, F.G. Influence of Breakfast and Meal Frequency in Calcium Intake among Pregnant Adolescents. Matern. Child Nutr. 2020, 16, e13034. [Google Scholar] [CrossRef]
- Sámano, R.; Morales, R.M.; Flores-García, A.; Lira, J.; Isoard, F.; de Santiago, S.; Casanueva, E. Las Adolescentes No Pierden Densidad Mineral ósea En El Posparto: Estudio Comparativo Con Adultas. Salud Pública México 2011, 53, 2–10. [Google Scholar] [CrossRef][Green Version]
- Bourassa, M.W.; Abrams, S.A.; Belizán, J.M.; Boy, E.; Cormick, G.; Quijano, C.D.; Gibson, S.; Gomes, F.; Hofmeyr, G.J.; Humphrey, J.; et al. Interventions to Improve Calcium Intake through Foods in Populations with Low Intake. Ann. N. Y. Acad. Sci. 2022, 1511, 40–58. [Google Scholar] [CrossRef]
- Marvin-Dowle, K.; Burley, V.J.; Soltani, H. Nutrient Intakes and Nutritional Biomarkers in Pregnant Adolescents: A Systematic Review of Studies in Developed Countries. BMC Pregnancy Childbirth 2016, 16, 268. [Google Scholar] [CrossRef]
- Gyimah, L.A.; Annan, R.A.; Apprey, C.; Edusei, A.; Aduku, L.N.E.; Asamoah-Boakye, O.; Azanu, W.; Lutterodt, H. Dietary Diversity and Its Correlates among Pregnant Adolescent Girls in Ghana. PLoS ONE 2021, 16, e0247979. [Google Scholar] [CrossRef]
- Guzmán-Mercado, E.; Vásquez-Garibay, E.M.; Troyo-Sanroman, R.; Romero-Velarde, E. Hábitos de Alimentación En Adolescentes Embarazadas de Acuerdo a Su Estado Civil. Nutr. Hosp. 2016, 33, 226–231. [Google Scholar] [CrossRef]
- Santander Ballestín, S.; Giménez Campos, M.I.; Ballestín Ballestín, J.; Luesma Bartolomé, M.J. Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review. Nutrients 2021, 13, 3134. [Google Scholar] [CrossRef]
- Cano-Ibáñez, N.; Martínez-Galiano, J.M.; Luque-Fernández, M.A.; Martín-Peláez, S.; Bueno-Cavanillas, A.; Delgado-Rodríguez, M. Maternal Dietary Patterns during Pregnancy and Their Association with Gestational Weight Gain and Nutrient Adequacy. Int. J. Environ. Res. Public Health 2020, 17, 7908. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.; Allen, L.H.; American Dietetic Association. Position of the American Dietetic Association: Nutrition and Lifestyle for a Healthy Pregnancy Outcome. J. Am. Diet. Assoc. 2008, 108, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Rugină, C.; Mărginean, C.O.; Meliţ, L.E.; Giga, D.V.; Modi, V.; Mărginean, C. Relationships between Excessive Gestational Weight Gain and Energy and Macronutrient Intake in Pregnant Women. J. Int. Med. Res. 2020, 48, 300060520933808. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.B.; Lobo, C.V.; Miranda, A.E.D.S.; Carvalho, B.D.C.; Santos, L.C.D. Dietary Patterns during Pregnancy and Gestational Weight Gain: A Systematic Review. Rev. Bras. Ginecol. Obstet. 2022, 44, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Tielemans, M.J.; Garcia, A.H.; Peralta Santos, A.; Bramer, W.M.; Luksa, N.; Luvizotto, M.J.; Moreira, E.; Topi, G.; de Jonge, E.A.L.; Visser, T.L.; et al. Macronutrient Composition and Gestational Weight Gain: A Systematic Review. Am. J. Clin. Nutr. 2016, 103, 83–99. [Google Scholar] [CrossRef]
- Zou, M.; Northstone, K.; Perry, R.; Johnson, L.; Leary, S. The Association between Later Eating Rhythm and Adiposity in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutr. Rev. 2022, 80, 1459–1479. [Google Scholar] [CrossRef]
- Cano-Ibáñez, N.; Martínez-Galiano, J.M.; Amezcua-Prieto, C.; Olmedo-Requena, R.; Bueno-Cavanillas, A.; Delgado-Rodríguez, M. Maternal Dietary Diversity and Risk of Small for Gestational Age Newborn: Findings from a Case-Control Study. Clin. Nutr. 2020, 39, 1943–1950. [Google Scholar] [CrossRef]
- Martínez-Galiano, J.M.; Amezcua-Prieto, C.; Salcedo-Bellido, I.; González-Mata, G.; Bueno-Cavanillas, A.; Delgado-Rodríguez, M. Maternal Dietary Consumption of Legumes, Vegetables and Fruit during Pregnancy, Does It Protect against Small for Gestational Age? BMC Pregnancy Childbirth 2018, 18, 486. [Google Scholar] [CrossRef]
- Martínez-Galiano, J.M.; Amezcua-Prieto, C.; Cano-Ibañez, N.; Olmedo-Requena, R.; Jiménez-Moleón, J.J.; Bueno-Cavanillas, A.; Delgado-Rodríguez, M. Diet as a Counteracting Agent of the Effect of Some Well-Known Risk Factors for Small for Gestational Age. Nutrition 2020, 72, 110665. [Google Scholar] [CrossRef]
- Mejía-Rodríguez, F.; Orjuela, M.A.; García-Guerra, A.; Quezada-Sanchez, A.D.; Neufeld, L.M. Validation of a Novel Method for Retrospectively Estimating Nutrient Intake during Pregnancy Using a Semi-Quantitative Food Frequency Questionnaire. Matern. Child Health J. 2012, 16, 1468–1483. [Google Scholar] [CrossRef]
- Fernández-Gaxiola, A.C.; Arenas, A.B.; Belausteguigoitia, M.P.; Kaufer-Horwitz, M.; Pérez-Lizaur, A.B.; Dommarco, J.R. Guías Alimentarias y de Actividad Física: En Contexto de Sobrepeso y Obesidad en la Población Mexicana: Documento de Postura; Intersistemas: Mexico City, Mexico, 2015; ISBN 9786074435153. [Google Scholar]
- Moore, V.M.; Davies, M.J.; Willson, K.J.; Worsley, A.; Robinson, J.S. Dietary Composition of Pregnant Women Is Related to Size of the Baby at Birth. J. Nutr. 2004, 134, 1820–1826. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on the Definition of Dietary Fiber and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes Proposed Definition of Dietary Fiber; National Academies Press: Washington, DC, USA, 2001; ISBN -10. [Google Scholar]
- Institute of Medicine; Food and Nutrition Board; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Subcommittee on Upper Reference Levels of Nutrients; Panel on the Definition of Dietary Fiber; Panel on Macronutrients. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005; ISBN 9780309085250. [Google Scholar]
- Carrilho, T.R.B.; Rasmussen, K.; Farias, D.R.; Freitas Costa, N.C.; Araújo Batalha, M.; Reichenheim, M.E.; Ohuma, E.O.; Hutcheon, J.A.; Kac, G. Brazilian Maternal and Child Nutrition Consortium Agreement between Self-Reported Pre-Pregnancy Weight and Measured First-Trimester Weight in Brazilian Women. BMC Pregnancy Childbirth 2020, 20, 734. [Google Scholar] [CrossRef]
- Holland, E.; Moore Simas, T.A.; Doyle Curiale, D.K.; Liao, X.; Waring, M.E. Self-Reported Pre-Pregnancy Weight versus Weight Measured at First Prenatal Visit: Effects on Categorization of Pre-Pregnancy Body Mass Index. Matern. Child Health J. 2013, 17, 1872–1878. [Google Scholar] [CrossRef]
- Lohman, T.J.; Roache, A.F.; Martorell, R. Anthropometric Standardization Reference Manual. Med. Sci. Sport. Exerc. 1992, 24, 952. [Google Scholar] [CrossRef]
- Onis, M. Who Multicentre Growth Reference Study Group WHO Child Growth Standards Based on Length/height, Weight and Age. Acta Paediatr. 2007, 95, 76–85. [Google Scholar]
- Adu-Afarwuah, S.; Lartey, A.; Okronipa, H.; Ashorn, P.; Ashorn, U.; Zeilani, M.; Arimond, M.; Vosti, S.A.; Dewey, K.G. Maternal Supplementation with Small-Quantity Lipid-Based Nutrient Supplements Compared with Multiple Micronutrients, but Not with Iron and Folic Acid, Reduces the Prevalence of Low Gestational Weight Gain in Semi-Urban Ghana: A Randomized Controlled Trial. J. Nutr. 2017, 147, 697–705. [Google Scholar] [CrossRef]
- National Research Council; Institute of Medicine; Board on Children, Youth, and Families; Food and Nutrition Board; Committee to Reexamine. IOM Pregnancy Weight Guidelines Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009; ISBN 9780309149150. [Google Scholar]
- Beauchesne, A.R.; Cara, K.C.; Chen, J.; Yao, Q.; Penkert, L.P.; Yang, W.; Chung, M. Effectiveness of Multimodal Nutrition Interventions during Pregnancy to Achieve 2009 Institute of Medicine Gestational Weight Gain Guidelines: A Systematic Review and Meta-Analysis. Ann. Med. 2021, 53, 1179–1197. [Google Scholar] [CrossRef]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International Standards for Newborn Weight, Length, and Head Circumference by Gestational Age and Sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Available online: www.amai.org/NSE/NivelSocioeconomicoAMAI.pdf (accessed on 10 March 2022).
- Visintin, C.; Mugglestone, M.A.; Almerie, M.Q.; Nherera, L.M.; James, D.; Walkinshaw, S. Guideline Development Group Management of Hypertensive Disorders during Pregnancy: Summary of NICE Guidance. BMJ 2010, 341, c2207. [Google Scholar] [CrossRef]
- Goyal, A.; Gupta, Y.; Singla, R.; Kalra, S.; Tandon, N. American Diabetes Association “Standards of Medical Care-2020 for Gestational Diabetes Mellitus”: A Critical Appraisal. Diabetes Ther. 2020, 11, 1639–1644. [Google Scholar] [CrossRef]
- Aburto, T.C.; Batis, C.; Pedroza-Tobías, A.; Pedraza, L.S.; Ramírez-Silva, I.; Rivera, J.A. Dietary Intake of the Mexican Population: Comparing Food Group Contribution to Recommendations, 2012–2016. Salud Publica Mex. 2022, 64, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Slater, K.; Rollo, M.E.; Szewczyk, Z.; Ashton, L.; Schumacher, T.; Collins, C. Do the Dietary Intakes of Pregnant Women Attending Public Hospital Antenatal Clinics Align with Australian Guide to Healthy Eating Recommendations? Nutrients 2020, 12, 2438. [Google Scholar] [CrossRef] [PubMed]
- Appiah, P.K.; Naa Korklu, A.R.; Bonchel, D.A.; Fenu, G.A.; Wadga-Mieza Yankey, F. Nutritional Knowledge and Dietary Intake Habits among Pregnant Adolescents Attending Antenatal Care Clinics in Urban Community in Ghana. J. Nutr. Metab. 2021, 2021, 8835704. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramírez, S.; Gaona-Pineda, E.B.; Martínez-Tapia, B.; Arango-Angarita, A.; Kim-Herrera, E.Y.; Valdez-Sánchez, A.; Medina-Zacarías, M.C.; Shamah-Levy, T.; Ramírez-Silva, I. Consumo de Grupos de Alimentos Y Su Asociación Con Características Sociodemográficas En Población Mexicana. Ensanut 2018–2019. Salud Pública México 2020, 62, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, C.; Escalante, A.; Huerta, J.; Villarreal, M.E. Efectos de La Frecuencia de Consumo de Alimentos Ultraprocesados Y Su Asociación Con Los Indicadores Del Estado Nutricional de Una Población Económicamente Activa En México. Revista Chilena Nutrición 2021, 48, 852–861. [Google Scholar] [CrossRef]
- Quezada-Pinedo, H.G.; Cassel, F.; Duijts, L.; Muckenthaler, M.U.; Gassmann, M.; Jaddoe, V.W.V.; Reiss, I.K.M.; Vermeulen, M.J. Maternal Iron Status in Pregnancy and Child Health Outcomes after Birth: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2221. [Google Scholar] [CrossRef]
- O’Brien, W.; Issartel, J.; Belton, S. Relationship between Physical Activity, Screen Time and Weight Status among Young Adolescents. Sports 2018, 6, 57. [Google Scholar] [CrossRef]
- Gamble, A.; Beech, B.M.; Blackshear, C.; Herring, S.J.; Welsch, M.A.; Moore, J.B. Changes in Physical Activity and Television Viewing From Pre-Pregnancy Through Postpartum Among a Socioeconomically Disadvantaged Perinatal Adolescent Population. J. Pediatr. Adolesc. Gynecol. 2021, 34, 832–838. [Google Scholar] [CrossRef]
- Santos, S.F.M.D.; Costa, A.C.C.D.; Araújo, R.G.P.D.S.; Silva, L.A.T.; Gama, S.G.N.D.; Fonseca, V.D.M. Factors associated with the adequacy of gestational weight gain among Brazilian teenagers. Ciência Saúde Coletiva 2022, 27, 2629–2642. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The Obesity Transition: Stages of the Global Epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational Weight Gain across Continents and Ethnicity: Systematic Review and Meta-Analysis of Maternal and Infant Outcomes in More than One Million Women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef]
- Wrottesley, S.V.; Pisa, P.T.; Norris, S.A. The Influence of Maternal Dietary Patterns on Body Mass Index and Gestational Weight Gain in Urban Black South African Women. Nutrients 2017, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-H.; Hsieh, T.-T. Pregestational Body Mass Index, Gestational Weight Gain, and Risks for Adverse Pregnancy Outcomes among Taiwanese Women: A Retrospective Cohort Study. Taiwan. J. Obstet. Gynecol. 2016, 55, 575–581. [Google Scholar] [CrossRef]
- Sisson, S.B.; Shay, C.M.; Broyles, S.T.; Leyva, M. Television-Viewing Time and Dietary Quality among U.S. Children and Adults. Am. J. Prev. Med. 2012, 43, 196–200. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Zhao, Y.; Zhang, Q.; Liu, Z.; Li, J.; Chen, X.; Yang, Z.; Yu, C.; Xiao, X. Severe maternal hyperglycemia exacerbates the development of insulin resistance and fatty liver in the offspring on high fat diet. Exp. Diabetes Res. 2012, 2012, 254976. [Google Scholar] [CrossRef]
- da Mota Santana, J.; de Oliveira Queiroz, V.A.; Pereira, M.; Paixão, E.S.; Brito, S.M.; Dos Santos, D.B.; Oliveira, A.M. Associations between Maternal Dietary Patterns and Infant Birth Weight in the NISAMI Cohort: A Structural Equation Modeling Analysis. Nutrients 2021, 13, 4054. [Google Scholar] [CrossRef]
- Mayer-Davis, E.; Leidy, H.; Mattes, R.; Naimi, T.; Novotny, R.; Schneeman, B.; Kingshipp, B.J.; Spill, M.; Cole, N.C.; Bahnfleth, C.L.; et al. Beverage Consumption During Pregnancy and Birth Weight: A Systematic Review [Internet]; USDA Nutrition Evidence Systematic Review: Alexandria, Egypt, 2020. [Google Scholar]
- Scholl, T.O.; Hediger, M.L. A review of the epidemiology of nutrition and adolescent pregnancy: Maternal growth during pregnancy and its effect on the fetus. J. Am. Coll. Nutr. 1993, 12, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Chia, A.-R.; Chen, L.-W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F.-F. Maternal Dietary Pat-terns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 685–695. [Google Scholar] [CrossRef]
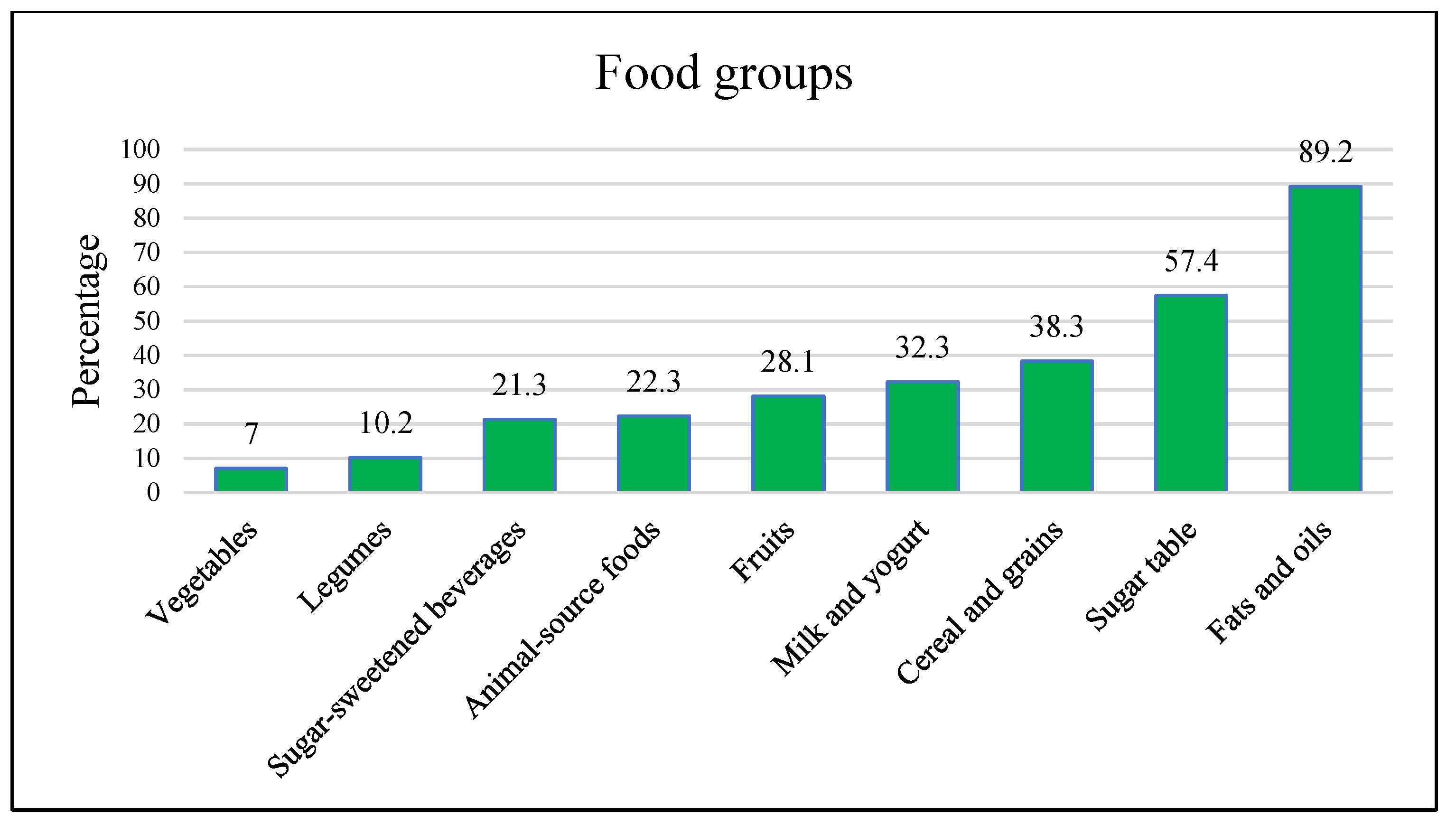
| Food Group | Intake | Gestational Weight Gain (%) | Birth Weight (%) | ||||
|---|---|---|---|---|---|---|---|
| Insufficient, n = 205 | Excessive, n = 187 | p-Value | SGA, n = 108 | LGA, n = 20 | p-Value | ||
| Vegetables | Adequate, n = 37 | 32.4 | 37.8 | 0.785 | 27.0 | 5.4 | 0.472 |
| Insufficient, n = 493 | 37.7 | 36.5 | 19.9 | 3.7 | |||
| Fruits | Adequate, n = 149 | 33.6 | 36.2 | 0.335 | 21.5 | 3.4 | 0.891 |
| Insufficient, n = 381 | 38.8 | 36.7 | 19.9 | 3.9 | |||
| Legumes | Adequate, n = 54 | 42.6 | 20.4 | 0.023 | 16.7 | 1.9 | 0.536 |
| Insufficient, n = 476 | 36.8 | 38.4 | 20.8 | 4.0 | |||
| Cereal and grains | Adequate, 7–11 n = 203 | 33.5 | 36.9 | ≤0.001 | 23.6 | 3.0 | 0.256 |
| Insufficient, <7 n = 211 | 53.1 | 20.4 | 20.4 | 3.3 | |||
| Excessive ≥12 n = 116 | 15.5 | 65.5 | 14.7 | 6.0 | |||
| Animal- | Adequate, n = 118 | 31.4 | 39.0 | ≤0.001 | 22.0 | 3.4 | 0.941 |
| source | Insufficient, n = 368 | 41.3 | 32.1 | 20.1 | 4.1 | ||
| foods | Excessive, n = 44 | 20.5 | 68.2 | 18.2 | 2.3 | ||
| Fats and | Adequate, n = 151 | 33.1 | 43.0 | 0.275 | 17.9 | 3.3 | 0.795 |
| oils | Insufficient, n = 361 | 39.1 | 33.5 | 21.1 | 3.9 | ||
| Excessive, n = 18 | 38.9 | 44.4 | 27.8 | 5.6 | |||
| Milk and | Adequate, n = 171 | 38.6 | 40.9 | 0.270 | 20.5 | 3.5 | 0.822 |
| yogurt | Insufficient, n = 257 | 38.1 | 33.5 | 20.2 | 4.7 | ||
| Excessive, n = 102 | 33.5 | 37.3 | 20.6 | 2.0 | |||
| Sugar table | Adequate, n = 226 | 39.8 | 33.6 | 0.442 | 18.6 | 4.0 | 0.671 |
| Excessive, n = 304 | 35.5 | 38.8 | 21.7 | 3.6 | |||
| Sugar-sweetened | Adequate, n = 171 | 33.3 | 33.3 | 0.030 | 14.6 | 4.7 | 0.066 |
| beverage | Excessive, n = 359 | 39.3 | 38.2 | 23.1 | 3.3 | ||
| Number of | ≥4, n = 106 | 29.2 | 40.6 | 0.151 | 18.9 | 2.8 | 0.754 |
| food groups | ≤3, n = 424 | 39.4 | 35.6 | 20.8 | 4.0 | ||
| Nutrient Intake | Gestational Weight Gain (%) | Birth Weight (%) | p-Value | |||
|---|---|---|---|---|---|---|
| Insufficient, n = 205 | Excessive, n = 187 | p-Value | SGA, n = 108 | LGA, n = 20 | ||
| Adequacy energy | ||||||
| Adequate (80–120%) n = 248 | 35.9 | 38.3 | 0.577 | 18.5 | 3.6 | 0.736 |
| Low (<80%), n = 147 | 37.4 | 32.7 | 19.7 | 4.1 | ||
| Excessive (>120), n = 135 | 40.7 | 37.8 | 24.4 | 3.7 | ||
| Carbohydrates | ||||||
| Adequate (45–55%),n = 226 | 36.7 | 37.2 | 0.727 | 19.0 | 4.9 | 0.698 |
| Low (<45%), n = 91 | 31.9 | 39.6 | 18.7 | 3.3 | ||
| Excessive (>55), n = 213 | 40.4 | 34.7 | 22.5 | 2.8 | ||
| Lipids | ||||||
| Adequate (25–30%), n = 253 | 38.3 | 36.4 | 0.590 | 24.1 | 4.0 | 0.245 |
| Low (<25%), n = 125 | 41.6 | 33.6 | 18.4 | 2.4 | ||
| Excessive (>30%), n = 152 | 32.2 | 39.5 | 15.8 | 4.6 | ||
| Proteins | ||||||
| Adequate (15–20%), n = 227 | 38.3 | 35.2 | 0.881 | 24.2 | 3.5 | 0.379 |
| Low (<15%), n = 225 | 35.6 | 39.1 | 17.3 | 4.4 | ||
| Excessive (>21%), n = 78 | 39.7 | 33.3 | 17.9 | 2.6 | ||
| Eating Habits | Gestational Weight Gain (%) | Birth Weight (%) | ||||
|---|---|---|---|---|---|---|
| Insufficient, n = 205 | Excessive, n = 187 | p-Value | SGA, n = 108 | LGA, n = 20 | p-Value | |
| Number of meals | ||||||
| ≥3, n = 121 | 35.5 | 34.7 | 0.695 | 23.1 | 3.3 | 0.855 |
| 3, n = 300 | 38.7 | 35.7 | 20.3 | 3.7 | ||
| ≤2, n = 109 | 35.8 | 41.3 | 17.4 | 4.6 | ||
| Having breakfast | ||||||
| Yes, n = 505 | 36.6 | 36.8 | 0.263 | 19.6 | 4.0 | 0.099 |
| No, n = 25 | 52 | 32.0 | 36.0 | 0.0 | ||
| Having lunch | ||||||
| Yes, n = 524 | 37.8 | 36.6 | 0.047 | 20.6 | 3.8 | 0.381 |
| No, n = 6 | 0.0 | 33.3 | 0.0 | 0.0 | ||
| Having dinner-super | ||||||
| Yes, n = 467 | 36.8 | 37.3 | 0.591 | 21.2 | 3.9 | 0.408 |
| No, n = 63 | 42.9 | 31.7 | 14.3 | 3.2 | ||
| Skipping meals | ||||||
| Never, n = 245 | 36.3 | 37.6 | 0.157 | 20.8 | 3.3 | 0.819 |
| 1–3 times/week, n = 222 | 40.1 | 32.0 | 21.2 | 4.5 | ||
| 4–5 times/week, n = 63 | 31.7 | 49.2 | 15.9 | 3.2 | ||
| Eating out of home | ||||||
| Yes, n = 73 | 32.9 | 34.2 | 0.349 | 20.6 | 3.7 | 0.954 |
| No, n = 457 | 38.1 | 37.0 | 19.2 | 4.1 | ||
| Eating alone | ||||||
| Yes, n = 107 | 35.5 | 43.0 | 0.262 | 19.6 | 5.6 | 0.535 |
| No, n = 423 | 37.0 | 35.0 | 20.6 | 3.3 | ||
| Activities during the meals | ||||||
| None, n = 205 | 36.6 | 34.6 | 0.793 | 22.4 | 1.5 | 0.190 |
| Watching TV or using a cellphone, n = 270 | 37.4 | 38.5 | 18.5 | 5.6 | ||
| Doing household chores, n = 55 | 40.0 | 34.5 | 21.8 | 3.6 | ||
| Modify their feeding | ||||||
| Was better, n = 353 | 36.3 | 36.5 | 0.544 | 19.8 | 3.4 | 0.644 |
| Was worse, n = 103 | 38.8 | 40.8 | 23.3 | 5.8 | ||
| No change, n = 74 | 40.5 | 31.1 | 18.9 | 5.8 | ||
| Gestational Weight Gain | Birth Weight | |||||||
|---|---|---|---|---|---|---|---|---|
| Insufficient | Excessive | SGA | LGA | |||||
| PR | 95% CI | PR | 95% CI | PR | 95% CI | PR | 95% CI | |
| Legumes | ||||||||
| M1 | 1.16 | 0.75–1.79 | 1.89 | 1.03–3.47 | – | – | – | – |
| M2 | 0.80 | 0.51–1.28 | 1.95 | 1.05–3.60 | – | – | – | – |
| M3 | 0.82 | 0.52–1.28 | 1.86 | 1.00–3.44 | – | – | – | – |
| Cereal and grains | ||||||||
| <7 servings | ||||||||
| M1 | 1.59 | 1.17–2.14 | 0.55 | 0.38–0.80 | – | – | – | – |
| M2 | 1.61 | 1.19–2.18 | 0.55 | 0.38–0.80 | – | – | – | – |
| M3 | 1.56 | 1.14–2.12 | 0.57 | 0.39–0.83 | – | – | – | – |
| >12 Excessive | ||||||||
| M1 | 0.46 | 0.28–0.78 | 1.77 | 1.29–2.44 | – | – | – | – |
| M2 | 0.47 | 0.28–0.79 | 1.77 | 1.29–2.44 | – | – | – | – |
| M3 | 0.49 | 0.29–0.82 | 1.65 | 1.18–2.29 | – | – | – | – |
| Animal–source foods | ||||||||
| Insufficient | ||||||||
| M1 | 1.32 | 0.92–1.89 | 0.82 | 0.59–1.16 | – | – | – | – |
| M2 | 1.35 | 0.94–1.94 | 0.81 | 0.57–1.14 | – | – | – | – |
| M3 | 1.43 | 0.99–2.05 | 0.72 | 0.51–1.02 | – | – | – | – |
| Excessive | ||||||||
| M1 | 0.65 | 0.32–1.35 | 1.75 | 1.04–2.77 | – | – | – | – |
| M2 | 0.70 | 0.34–1.46 | 1.65 | 1.03–2.65 | – | – | – | – |
| M3 | 0.80 | 0.38–1.68 | 1.33 | 0.82–2.17 | – | – | – | – |
| Consume sweetened beverages | ||||||||
| M1 | 1.18 | 0.87–1.60 | 1.15 | 0.84–1.56 | 1.58 | 1.01–2.47 | 0.71 | 0.29–1.75 |
| M2 | 1.16 | 0.85–1.59 | 1.14 | 0.84–1.56 | 1.58 | 1.00–2.47 | 0.77 | 0.31–1.94 |
| M3 | 1.19 | 0.87–1.62 | 1.14 | 0.84–1.56 | 1.58 | 1.01–2.49 | 0.78 | 0.31–1.98 |
| ≤3 Food groups | ||||||||
| M1 | 1.40 | 0.95–2.07 | 0.88 | 0.63–1.23 | – | – | – | – |
| M2 | 1.31 | 0.89–1.93 | 0.90 | 0.64–1.27 | – | – | – | – |
| M3 | 1.34 | 0.91–1.98 | 0.86 | 0.61–1.21 | – | – | – | – |
| Lipids | Gestational Gain, % | Birth Weight | ||||||
|---|---|---|---|---|---|---|---|---|
| Insufficient | Excessive | Small | Large | |||||
| PR | 95% CI | PR | 95% CI | PR | 95% CI | PR | 95% CI | |
| Adequate REF | ||||||||
| Insufficient | ||||||||
| M1 | – | – | – | – | 0.70 | 0.46–1.08 | 0.55 | 0.14–2.21 |
| M2 | – | – | – | – | 0.74 | 0.43–1.26 | 0.35 | 0.08–1.48 |
| M3 | – | – | – | – | 0.74 | 0.44–1.27 | 0.35 | 0.08–1.52 |
| Excessive | ||||||||
| M1 | – | – | – | – | 0.71 | 0.42–1.20 | 0.95 | 0.35–2.56 |
| M2 | – | – | – | – | 0.73 | 0.47–1.26 | 0.86 | 0.31–1.49 |
| M3 | – | – | – | – | 0.75 | 0.49–1.27 | 0.85 | 0.31–2.34 |
| Skipping meals | ||||||||
| None REF | ||||||||
| 1–3 time/week | ||||||||
| M1 | 1.10 * | 0.82–1.48 | 0.85 | 0.63–1.16 | – | – | – | – |
| M2 | 1.14 | 0.85–1.53 | 0.85 | 0.62–1.16 | – | – | – | – |
| M3 | 1.15 | 0.85–1.54 | 0.82 | 0.60–1.12 | – | – | – | – |
| 4–5 time/week | ||||||||
| M1 | 0.87 | 0.54–1.42 | 1.31 | 0.87–1.97 | – | – | – | – |
| M2 | 0.87 | 0.54–1.42 | 1.30 | 0.87–1.97 | – | – | – | – |
| M3 | 0.92 | 0.57–1.51 | 1.17 | 0.77–1.77 | – | – | – | – |
| Number of meals | ||||||||
| >3 REF | ||||||||
| 3 | ||||||||
| M1 | 1.01 | 0.69–1.47 | 1.09 | 0.73–1.62 | – | – | – | – |
| M2 | 1.03 | 0.70–1.52 | 1.12 | 0.75–1.69 | – | – | – | – |
| M3 | 1.03 | 0.70–1.52 | 1.08 | 0.72–1.64 | – | – | – | – |
| <2 | ||||||||
| M1 | 1.01 | 0.66–1.54 | 1.22 | 0.79–1.88 | – | – | – | – |
| M2 | 1.04 | 0.68–1.59 | 1.23 | 0.80–1.90 | – | – | – | – |
| M3 | 1.08 | 0.70–1.65 | 1.09 | 0.70–1.70 | – | – | – | – |
| Activities during the meals | ||||||||
| Seeing TV | ||||||||
| M1 | – | – | – | – | 0.83 | 0.55–1.23 | 3.80 | 1.10–13.11 |
| M2 | – | – | – | – | 0.84 | 0.56–1.26 | 3.92 | 1.11–13.84 |
| M3 | – | – | – | – | 0.87 | 0.58–1.30 | 3.76 | 1.06–13.36 |
| Doing household chores | ||||||||
| M1 | – | – | – | – | 0.97 | 0.52–1.84 | 2.49 | 0.42–14.87 |
| M2 | – | – | – | – | 0.98 | 0.52–1.87 | 2.67 | 0.44–16.45 |
| M3 | – | – | – | – | 0.98 | 0.52–1.86 | 2.89 | 0.46–18.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sámano, R.; Martínez-Rojano, H.; Ortiz-Hernández, L.; Nájera-Medina, O.; Chico-Barba, G.; Godínez-Martínez, E.; Gamboa, R.; Aguirre-Minutti, E. Dietary and Nutrient Intake, Eating Habits, and Its Association with Maternal Gestational Weight Gain and Offspring’s Birth Weight in Pregnant Adolescents. Nutrients 2022, 14, 4545. https://doi.org/10.3390/nu14214545
Sámano R, Martínez-Rojano H, Ortiz-Hernández L, Nájera-Medina O, Chico-Barba G, Godínez-Martínez E, Gamboa R, Aguirre-Minutti E. Dietary and Nutrient Intake, Eating Habits, and Its Association with Maternal Gestational Weight Gain and Offspring’s Birth Weight in Pregnant Adolescents. Nutrients. 2022; 14(21):4545. https://doi.org/10.3390/nu14214545
Chicago/Turabian StyleSámano, Reyna, Hugo Martínez-Rojano, Luis Ortiz-Hernández, Oralia Nájera-Medina, Gabriela Chico-Barba, Estela Godínez-Martínez, Ricardo Gamboa, and Estefanía Aguirre-Minutti. 2022. "Dietary and Nutrient Intake, Eating Habits, and Its Association with Maternal Gestational Weight Gain and Offspring’s Birth Weight in Pregnant Adolescents" Nutrients 14, no. 21: 4545. https://doi.org/10.3390/nu14214545
APA StyleSámano, R., Martínez-Rojano, H., Ortiz-Hernández, L., Nájera-Medina, O., Chico-Barba, G., Godínez-Martínez, E., Gamboa, R., & Aguirre-Minutti, E. (2022). Dietary and Nutrient Intake, Eating Habits, and Its Association with Maternal Gestational Weight Gain and Offspring’s Birth Weight in Pregnant Adolescents. Nutrients, 14(21), 4545. https://doi.org/10.3390/nu14214545







