Abstract
This study attempted to learn the association between maternal betaine-homocysteine methyltransferase (BHMT) gene polymorphisms, maternal dietary habits, and their interactions with the risk of ventricular septal defects (VSD) in offspring. A total of 426 mothers of VSD children and 740 control mothers were included in the study. Logistic regression was used to evaluate the level of associations and interaction effects. Our study suggested that mothers reporting excessive intake of smoked foods (aOR = 2.44, 95%CI: 1.89–3.13), barbecued foods (aOR = 1.86, 95%CI: 1.39–2.48), fried foods (aOR = 1.93, 95%CI: 1.51–2.46), and pickled vegetables (aOR = 2.50, 95%CI: 1.92–3.25) were at a significantly higher risk of VSD in offspring, instead, mothers reporting regular intake of fresh fruits (aOR = 0.47, 95%CI: 0.36–0.62), fish and shrimp (aOR = 0.35, 95%CI: 0.28–0.44), fresh eggs, (aOR = 0.56, 95%CI: 0.45–0.71), beans (aOR = 0.68, 95%CI: 0.56–0.83), and milk products (aOR = 0.67, 95%CI: 0.56–0.80) were at a lower risk of VSD in offspring. In addition, maternal BHMT gene polymorphisms at rs1316753 (CG vs. CC: aOR = 2.01, 95%CI: 1.43–2.83) and rs1915706 (CT vs. TT: (aOR = 1.81, 95%CI: 1.33–2.46) were significantly associated with increased risk of VSD in offspring. Furthermore, a significant interaction between BHMT polymorphisms and maternal bean intake was identified in the study. In conclusion, Maternal BHMT polymorphisms at rs1316753 and rs1915706, dietary habits as well as their interaction were observed to be significantly associated with the risk of VSD in offspring.
1. Introduction
Congenital heart disease (CHD) is typically defined as a gross structural abnormality of the heart and/or great vessels that is present at birth [1,2]. It has been reported that the birth prevalence of CHD has increased significantly since the 1930s and reached a maximum of over 9 per 1000 live births since 1995 [1,3]. Ventricular septal defect (VSD) has been recognized as the most common congenital cardiac malformation and accounts for roughly 30–40% of all cardiac anomalies [1,4]. Over the past decades, considerable inherited causes and noninherited modifiable factors have been implicated in the development of CHD and its subgroups [5,6,7,8]. Recently, there has been a consensus that genetic factors and environmental factors interact in the etiology of most nonsyndromal forms of CHD [9,10], naturally including VSD.
A recent review showed strong evidence that oral prenatal fortification and supplementation dosing of folic acid (FA) can prevent the incidence of VSD and atrial septal defect (ASD) [11]. Women with a diverse diet during pregnancy (dietary diversity score, DDS ≥5) had lower risks of having fetuses with total CHD and VSD [12]. Furthermore, the dietary intake of vitamins and minerals was found to be associated with a reduced risk of CHD in offspring, including B-vitamin, vitamin D, zinc, and selenium [13,14,15]. Since different nutrients interact with one another in many metabolic pathways, it seems that the association would not remain constant when various nutrients coexist in the same food. In addition, the dietary pattern differs a lot owing to the discrepancy in economics, geographical environment, social culture, race, and so on. Therefore, the first concern we would care to discuss is the association between maternal dietary habits and VSD in offspring.
The human betaine-homocysteine methyltransferase (BHMT) gene maps to 5q13.1–q15, spans about 20 kilobases of DNA and contains eight exons and seven introns [16,17]. The enzyme it encodes, betaine-homocysteine methyltransferase, catalyzes the transfer of a methyl group from betaine to homocysteine (Hcy), forming dimethylglycine and methionine. Generally, the homeostasis of plasma homocysteine benefits from the transulfuration pathway involving cystathionine β synthase (CBS) and the remethylation pathway involving BHMT, BHMT2, and methionine synthase (MS) (Figure 1) [18]. In the latter pathway, the catalytic activity of BHMT2 is absolutely diet-dependent since its substrate, S-methylmethionine, can only be biosynthesized by various plants mainly belonging to the Brassicaceae family rather than mammals [19,20]. Experimental research conducted in mice suggested that BHMT is a predominant enzyme for the elimination of Hcy while the MS has little excess capacity to methylate the Hcy [18]. Therefore, the remethylation reaction catalyzed by BHMT seems to play a vital role in preventing the toxic accumulation of Hcy. In fact, BHMT catalyzes up to 50% of homocysteine metabolism in the human liver, where the enzyme is highly expressed [21,22]. The latest literature revealed that elevated Hcy concentrations acted as a risk factor for multiple congenital anomalies in human production, mainly comprising neural tube defects (NTD), orofacial clefts, and CHD [23,24,25]. The discovery has been generally accepted that the 677 C→T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene contributed to elevated tHcy and is a genetic risk factor for diseases associated with hyperhomocysteinaemia [26]. Moreover, this mutation has been applied to antenatal screening for pregnant women in China. The thought naturally emerged that polymorphisms of the BHMT gene exist that reduce BHMT activity and increase plasma Hcy levels and thus increase malformation risk. In fact, research has been dedicated to exploring the association between BHMT gene polymorphisms and CHD, but with fixed results and little involving subgroups of CHD [27,28,29,30]. In this study, we focused on the largest subcategory of CHD, namely, VSD, to detect its association with polymorphisms of the maternal BHMT gene.
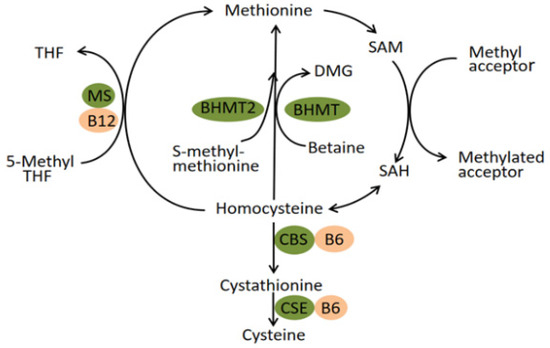
Figure 1.
Pathways of homocysteine metabolism. Abbreviation: BHMT Betaine-homocysteine S-methyltransferase; MS methionine synthase; CBS cystathionine β-synthase; CSE cystathionine-γ-lyase; THF tetrahydrofolate; DMG dimethylglycine; SAM S-adenosylmethionine; SAH S-adenosylhomocysteine.
In addition, betaine, the substrate of BHMT, can be either obtained from food resources or produced from choline endogenously [31]. Likewise, choline can also be produced endogenously via the hepatic phosphatidylethanolamine N-methyltransferase (PEMT) pathway. However, most people must consume this nutrient exogenously to prevent deficiency [32]. Therefore, the BHMT activity, to a certain degree, is diet dependent. Animal studies did observe that pane of nutrition or the supply of some nutrients, including choline and methionine, can alter BHMT activity [33,34,35]. In addition, it has been reported that women with a high intake of one-carbon cofactors had a lower risk of congenital anomalies in offspring, such as the neural tube defect (NTD) and perimembranous ventricular septal defect (VSDpm) [36,37]. Overall, these valuable clues were collected to put forward a reasonable hypothesis that BHMT gene polymorphisms may interact with maternal dietary habits on congenital anomalies.
In this study, we determined VSD, the most common subgroup in CHD, as the interested outcome variable, which is relatively more sensitive to maternal nutrient intake. A hospital-based case-control study was carried out in an attempt to learn the following questions: a. the association of maternal dietary habits with risk of VSD in offspring; b. the association of polymorphisms of maternal BHMT gene with risk of VSD in offspring; c. the interaction between BHMT genetic variants and maternal dietary habits on VSD.
2. Materials and Methods
2.1. Design and Participants
This is a hospital-based case-control study that started in February 2018 and was over in March 2020. The cases and controls came from different departments in the same hospital, Hunan children’s hospital, which is famous partly for its sophisticated diagnosis and treatment techniques for CHD within the province. Considering the characteristics of the relatively low incidence of VSD compared with other chronic diseases, a convenient sampling method was used in the recruitment of the cases. VSD children, verified by both doppler echocardiography and surgery, were consecutively recruited from the Department of Cardiothoracic Surgery. Children in the control, free of any congenital malformations, were randomly selected from the Department of Child Healthcare. It is worth noting that cases only included VSD children that may or may not be diagnosed with other congenital heart diseases; those coexisting with any other extra-cardiac malformations were excluded from the study. Additionally, informed consent was obtained from all of the participants, and the possible consequences of the study were explained. The exclusion criterions mainly included: minority mothers, mothers conceiving children through in vitro fertilization or other conception methods, adoptive mothers or stepmothers, and mothers suffering from mental disorders or any other physical diseases so that this did not hinder the provision of accurate exposure information and blood samples. Finally, a total of 426 mothers of VSD children and 740 control mothers were included in the study.
The protocol of this study was in accordance with the guidelines of the 1964 Helsinki Declaration, and the Ethics Committee of Xiangya School of Public Health, Central South University, officially approved this study in January 2018. (no. XYGW-2018-36).
2.2. Information Collection
The outcome we focused on in the study was VSD in offspring, which was diagnosed by professional physicians via both doppler echocardiography and surgery. The interested exposures were maternal dietary habits in early pregnancy, which were collected from a self-designed food frequency questionnaire. We consulted The Dietary Guidelines for Chinese Residents and went deep into the local food culture to develop the questionnaire. Eleven main categories were determined, involving smoked foods, barbecued foods, fried foods, pickled vegetables, fresh vegetables, fresh fruits, fresh meat, fish and shrimp, fresh eggs, beans, and milk products. Each category was provided with three choices: a. hardly (less than or equal to two times per week); b. sometimes (three to five times per week); c. often (more than or equal to six times per week). The questionnaire was pre-investigated using eligible mothers (test–retest reliability: r = 0.826; internal consistency: α = 0.769).
In addition, we also collected various pieces of maternal information that might influence the outcomes of their offspring, mainly including the child-bearing age (<35 years or ≥35 years), pre-pregnancy BMI (calculated with their pre-pregnancy height and weight, <18.5, 18.5–23.9, 24–26.9, or ≥27), education level (less than primary or primary, junior high school, high school or technical secondary school, college or above), consanguineous marriages (yes or no), gestational diabetes mellitus (yes or no), gestational hypertension (yes or no), abnormal pregnancy history before this pregnancy (yes or no), congenital malformations in family members (yes or no), exposure of environmental pollutants (yes or no), antibiotic use in early pregnancy (yes or no), tobacco exposure in early pregnancy (yes or no), alcohol exposure in early pregnancy (yes or no), and periconceptional folate use (yes or no).
An epidemiological survey was conducted by well-trained investigators when participants were waiting for their operation arrangements in the wards or medical check-ups in the Department of Child Health. In China, every expectant mother has a personal Maternal and Child Health Manual, which provides their sociodemographic information, the results of regular medical check-ups, and necessary exposure information. So, in the course of the investigation, we consulted the participants’ manual to further confirm the abovementioned information obtained from face-to-face interviews, which enabled us to reduce recall bias to a certain extent.
2.3. Sample Collection and Genotyping
Five milliliters of peripheral venous blood were collected from every single participant after the face-to-face interview. All of the obtained blood samples would be brought back to the laboratory at low temperatures (≤4 °C) within twelve hours and then divided into two layers using a high-speed centrifuge: the blood cell layer and the plasma layer. Both were stored in an ultra-low-temperature freezer until genotyping. The DNA was extracted from the blood cell samples with the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA). Genotyping was performed by matrix-assisted laser desorption and ionization time-of-flight mass spectrometry MassARRAY system (Agena iPLEX assay, San Diego, CA, USA). The laboratory technicians who performed SNP detection and recorded the genotype data were blind to whether each sample was from the cases or controls, thereby reducing selection bias to some extent.
Before genotyping, we consulted the NCBI and HapMap databases to determine the major SNP sites of the BHMT gene and simultaneously excluded the SNPs whose minor allele frequencies (MAF) were less than 10%. Furthermore, we imposed a minimum SNP genotyping call rate at the level of 50%, which was applied to ensure the data integrity of the individual’s genotypes. Moreover, the success rates for the SNPlex assays were >94% for 2 SNPs, from 90 to 94% for 2 SNPs. Finally, these genetic loci (rs3733890, rs1316753, rs567754, and rs1915706) were selected as candidate loci for this study.
2.4. Statistical Analysis
The data for the qualitative variables were expressed as absolute numbers (percentages). The chi-square test was used to assess the differences in qualitative variables across groups. The Hardy–Weinberg equilibrium (HWE) test was used to compare the differences in genotype distribution frequency in the control group (significance level at p < 0.01). We utilized a logistic regression model to detect whether the association between maternal dietary habits in early pregnancy, BHMT gene polymorphisms, and VSD in offspring existed and the level of the association. Both univariate and multivariate logistic regressions were adopted; the crude odds ratio (cOR) and its 95% confidential intervals (CI) were calculated by the former one without any adjustment; the adjusted odds ratio (aOR) and its 95% confidential intervals (CI) were calculated by the latter one, which adjusted for the significant confounders found using the chi-square test. For the significant SNPs and maternal dietary habits, these originally ternary variables were converted into binary variables. We then introduced all of the potential confounders, genetic factors, environmental factors, and their multiplicative interaction term into the same logistic regression model to determine the presence or absence of gene–environment interaction and assess its significance. When it comes to multiple hypothesis testing, the false discovery rate (FDR) based on the Benjamini–Hochberg method was used to correct for bias. A false discovery rate P value (FDR_P) of <0.1 was considered to be statistically significant. The calculation of FDR_P was completed using R software (version 4.1.3, stats package). Basic analyses were performed using SPSS 26.0 software (SPSS Inc., Chicago, IL, USA). The statistically significant results were those with the two-sided p-value < 0.05, except where otherwise specified.
3. Results
3.1. Comparison of Maternal Baseline Characteristics
In the study, we recruited a total of 426 mothers of VSD children for cases and 740 mothers of non-congenital malformation children for controls. The selection of participants conformed strictly to the pre-made inclusion and exclusion criteria. The median (inter-quartile range) age of the children the in cases and controls was 8.4 (5.7) months and 7.8 (4.3) months, respectively. The comparisons of the maternal baseline characteristics between cases and controls are summarized in Table 1. There were statistically significant differences between the two groups in the following factors: pre-pregnancy BMI, education level, consanguineous marriages, gestational diabetes mellitus, gestational hypertension, abnormal pregnancy history before this pregnancy, congenital malformations in family members, exposure to environmental pollutants, antibiotic use in early pregnancy, tobacco exposure in early pregnancy, alcohol exposure in early pregnancy, and periconceptional folate use (all p values < 0.05). These abovementioned factors would be adjusted as confounders when evaluating the association of maternal dietary habits, SNPs of the BHMT gene, and their interactions with VSD in offspring.

Table 1.
Comparison of maternal baseline characteristics in cases and controls.
3.2. Maternal Dietary Habits and the Risk of VSD in Offspring
The association of maternal dietary intake in early pregnancy with the risk of VSD in offspring is shown in Table 2. Both univariate and multivariate logistic regression indicated that smoked foods, barbecued foods, fried foods, pickled vegetables, fresh fruits, fish and shrimp, fresh eggs, beans, and milk products were significantly associated with the risk of VSD in offspring. Specifically, children were predisposed to VSD when their mothers reported excessive intake of smoked foods (aOR = 2.44, 95%CI: 1.89–3.13), barbecued foods (aOR = 1.86, 95%CI: 1.39–2.48), fried foods (aOR = 1.93, 95%CI: 1.51–2.46), and pickled vegetables (aOR = 2.50, 95%CI: 1.92–3.25). Instead, a significantly decreased risk of VSD was observed in children whose mothers reported regular intake of fresh fruits (aOR = 0.47, 95%CI: 0.36–0.62), fish and shrimp (aOR = 0.35, 95%CI: 0.28–0.44), fresh eggs, (aOR = 0.56, 95%CI: 0.45–0.71), beans (aOR = 0.68, 95%CI: 0.56–0.83), and milk products (aOR = 0.67, 95%CI: 0.56–0.80).

Table 2.
Maternal dietary habits and the risk of VSD in offspring.
3.3. Maternal BHMT Gene Polymorphisms and the Risk of VSD in Offspring
Table 3 displays the genotypic distribution of four SNPs between two groups and the results of the HWE test in the controls. All of the SNPs were in accordance with the Hardy–Weinberg equilibrium (all of the p values were <0.05), indicating that the sample was qualified for good group representativeness.

Table 3.
Genotypic frequencies of maternal BHMT polymorphisms and P values of HWE test.
The association of maternal BHMT gene polymorphisms with the risk of VSD in offspring based on logistic regression analysis was summarized in Table 4. After adjusting for potential confounders, statistically significant associations were found between the polymorphisms of the BHMT gene at rs1316753, rs1915706, and VSD in offspring. For rs1316753, mothers carrying the CG genotype (aOR = 2.01, 95%CI: 1.43–2.83) were at a significantly higher risk of VSD in offspring compared with those who had the CC genotype. In addition, the dominant model (aOR = 1.88, 95%CI: 1.36–2.61) and the additive model (aOR = 1.30, 95%CI: 1.06–1.60) of rs1316753 were also observed to be significantly associated with increased risk of VSD in offspring. For rs1915706, compared to the TT genotype, mothers with the CT genotype (aOR = 1.81, 95%CI: 1.33–2.46) were more likely to have VSD children. Additionally, the dominant model (aOR = 1.84, 95%CI: 1.37–2.48) and the additive model (aOR = 1.61, 95%CI: 127–2.05) of rs1915706 were significantly associated with an increased risk of VSD in offspring.

Table 4.
Polymorphisms of maternal BHMT gene associated with risk of VSD in offspring based on logistic regression analysis.
3.4. Interaction of the Polymorphisms of BHMT Gene and Maternal Dietary Habits on the Risk of VSD in Offspring
Figure 2 shows the level of association between genetic variants of the BHMT gene, maternal dietary intake, and VSD in offspring. The interaction of BHMT gene polymorphisms and maternal dietary habits in early pregnancy based on multivariate logistic regression analysis is displayed in Table 5. For rs1316753, statistically significant interactions were observed between the variant genotypes (CG + GG) and excessive intake of pickled vegetables (aOR = 0.48, 95%CI: 0.24–0.95) and beans (aOR = 0.40, 95%CI: 0.17–0.95). Nevertheless, this significance vanished from the multiple test corrections based on the Benjamini–Hochberg method (both FDR-p values > 0.1). As for rs1915706, there were significant interactions between the variant genotypes (CT + CC) and a regular intake of beans (aOR = 0.33, 95%CI: 0.15–0.73, FDR-p = 0.035).
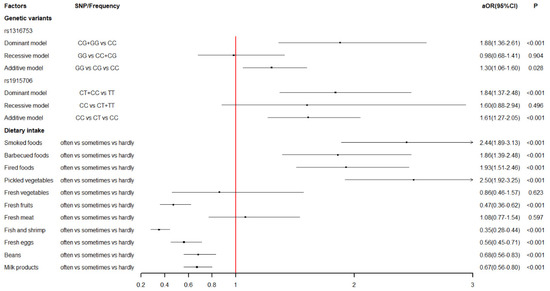
Figure 2.
The level of association between genetic variants of BHMT gene, maternal dietary intake and VSD in offspring.

Table 5.
Interactions of polymorphisms of BHMT gene and maternal dietary habits based on multivariate logistic regression.
The crossover analysis was conducted to further elucidate the interaction between BHMT gene polymorphisms at rs1915706 and maternal bean intake on the risk of VSD in offspring (Table 6 and Figure 3). Mothers who had the wild genotype (TT) and meanwhile reported regular intake of beans in early pregnancy were seen as the reference group. After adjustment for potential confounders detected in Table 1, mothers who had the variant genotypes (CT + CC) and meanwhile reported regular intake of beans (aOR = 1.52, 95%CI: 1.09–2.11) and a small intake of beans (aOR = 4.00, 95%CI: 2.17–7.40) were at a significantly higher risk of VSD in offspring compared with those who were in the reference group.

Table 6.
Interaction of rs1915706 and maternal beans intake based on crossover analysis.
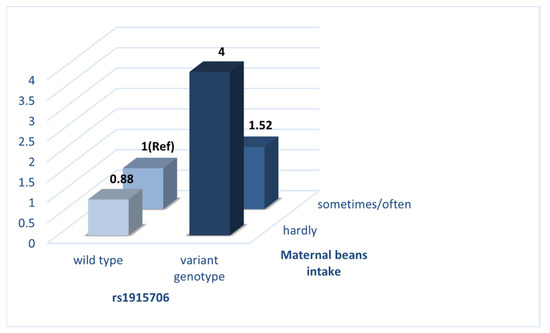
Figure 3.
Interaction of rs1915706 and maternal beans intake on the risk of VSD in offspring.
4. Discussion
Research on the causes of CHD has made great strides, and more than 400 genes and important environmental factors have been determined to have substantial evidence in relation to the risk of developing CHD and its subgroups [5,6,7,8]. However, the conclusion can be easily drawn from the other hand that a single genetic or environmental factor may impose minimal effects on CHD. Moreover, the interaction of the two factors cannot be overlooked in the occurrence and development of CHD and its subgroups. In this study, we attempted to achieve an insight into the etiology of VSD, the most common subtype of CHD. The main purpose was to decide whether the association and interaction effect of BHMT gene polymorphisms and maternal dietary habits with VSD exists, which is conducive to the achievement of molecular genetic diagnostics and provide diet instruction to expectant mothers in early pregnancy.
Firstly, we explored the association between maternal dietary intake during early pregnancy and the risk of VSD in offspring. The results sent two messages. On the one hand, mothers who reported excessive intake of smoked foods (aOR = 2.44), barbecued foods (aOR = 1.86), fried foods (aOR = 1.93), and pickled vegetables (aOR = 2.50) were more likely to have a VSD-affected child. On the other hand, mothers with an excessive intake of fresh fruits (aOR = 0.47), fish and shrimp (aOR = 0.35), fresh eggs (aOR = 0.56), beans (aOR = 0.68), and milk products (aOR = 0.67) were less likely to have a VSD-affected child. Generally, various harmful chemicals can be generated from improper food processing, and most of them are teratogens and carcinogens, such as nitrites, acrylamide (ACR), polycyclic aromatic hydrocarbons (PAH), and so on. Pickled vegetables have a wide range of nitrite and nitrate contents. Gravidas, who have an excessive intake of pickles, may suffer hypoxemia because increased nitrite can react with hemoglobin, rendering it incapable of carrying oxygen [38]. A recent experimental study established a rodent model and reported that hypoxia was able to cause numerous abnormalities in cardiomyocyte gene expression, the electrophysiologic substrate of the heart, and contractile function, thus delaying cardiac maturation [39]. ACR, identified in food in 2002, is mainly formed via the Maillard reaction, whereby a carbonyl compound reacts with the amino group of asparagine processed at high temperatures (>120 °C, such as barbecuing, frying, and baking) [40,41]. Although no direct evidence manifested its relation to heart defects, a number of animal studies have shown strong neurotoxic, genotoxic, carcinogenic, mutagenic, and teratogenic effects [42]. Food is readily contaminated by PAH during the smoking process involving the combustion of fuel. A recent study reported that greater maternal levels of PAH exposure during pregnancy might be associated with an elevated prevalence of fetal CHD [43]. Moreover, prior experimental research provided strong evidence that PAH exposure can result in abnormal heart looping, an enlarged ventricle with a thinner ventricular wall, and even developmental cardiac defects [44,45]. The protective foods detected in the study, such as fruits, fish and shrimp, eggs, beans, and milk products, are common foods on tables. Furthermore, they are packed with numerous nutrients, such as high-quality protein, vitamins, minerals, and so on, which have been extensively accepted to play a vital role in maintaining the health of gravidas and fetuses.
Moreover, we determined the association between maternal BHMT gene polymorphisms and the risk of VSD in offspring. Four SNPs (rs3733890, rs1316753, rs567754, and rs1915706) were considered in this study, and two SNPs (rs1316753 and rs1915706) were for the first time found to have a statistically significant association with VSD. To date, the BHMT gene remained relatively little studied compared with other folate- and homocysteine-metabolizing genes. The results in our study were only partly in accordance with prior studies. The rs3733890 polymorphism is a well-studied exon of the BHMT gene and undergoes a G-to-A change at nucleotide 716, leading to an arginine-to-glutamine substitution at amino acid 239. Its association with congenital defects has been widely explored but with contradictory results [27,46,47,48]. In the present study, we did not detect its significance in the occurrence of VSD. Rs567754 is an intronic variant of the BHMT gene, and neither previous data nor our data revealed an association with CHD or VSD in offspring [27]. The interesting thing is that two other SNPs, rs1316753 and rs1915706, were observed to be statistically associated with a remarkably-increased risk of VSD in offspring. To the best of our knowledge, experimental or epidemiological research involving these two polymorphisms remains a nearly unworked area, meaning that their potential functional effects on BHMT are largely unknown. Qiping Feng et al. performed a genotype–phenotype correlation analysis on betaine-homocysteine methyltransferase and found that three introns (rs41272270, rs6875201, and rs7700790) and an intergenic variant (rs16876512) were significantly correlated with both BHMT activity and protein levels [22]. Although this convincing research did not cover the two significant SNPs in our study, the analogy seems to be reasonable that the two intergenic variants, rs1316753 and rs1915706, are also capable of producing potential effects on the expression of the BHMT gene and subsequently influencing plasma hcy concentrations. The correlation between maternal hyperhomocysteinemia and CHD has been extensively studied and reviewed [25,49,50]. Meanwhile, hcy-induced CHDs, such as the transposition of the great arteries (TGA), single ventricle defects (SVD), and VSD, have been found in embryos of different species (mice and chicken) [51]. Therefore, the statistical association between maternal BHMT polymorphisms and VSD in offspring might be explained by the pathway from BHMT activity to elevated hcy levels to multiple congenital anomalies. Nonetheless, more related research is encouraged to provide clearer evidence, thus elucidating the potential mechanism.
Lastly, we also analyzed the gene–environment interaction and observed a significant interaction between genetic variants of the BHMT gene at rs1915706 and maternal bean intake on the risk of VSD in offspring. The expectant mothers who had the variant genotypes (CT + CC) and meanwhile reported a small intake of beans were at a significantly higher risk of VSD in offspring (aOR = 4.00) compared with those with the wild genotype (TT) and reported having a regular intake of beans in early pregnancy. Beans are an excellent source of zinc, choline, and multiple B vitamins (such as folate, thiamin, niacin, riboflavin, and pyridoxine) [52,53]. Notably, BHMT is a zinc-dependent cytosolic enzyme, and its substrate, betaine, is partly derived from dietary choline [31,54]. In addition, a stronger risk reduction in CHD has been found in the maternal folate + B-vitamin supplementation group compared with the maternal folate supplementation group, both setting the non-users as the reference group [14]. Concerning whether a single nutrient can exist in various foods, we speculated that the deficiency of diverse nutrients coexisting in beans coincided with a genetic variant that contributes to the occurrence of VSD. This speculation seemed plausible since similar research had been conducted not long ago. Hartmut Cuny et al. demonstrated that when dietary undersupply during pregnancy was combined with a maternal heterozygous variant in Haao, a gene of the nicotinamide adenine dinucleotide (NAD) synthesis pathway, the incidence of embryo loss and malformations was significantly higher [55]. This is a classical experimental study forcibly indicating a gene-diet interaction. As Gibson G and Berger K commented, the discovery of such interaction suggests that the close monitoring of nutrition in at-risk carrier mothers would be the type of personalized and predictive intervention that advocates of genomic health call for [56]. Regardless, what we found in our study necessitates more convincing experimental research and crowd investigation to confirm it repeatedly.
Furthermore, several limitations in our study should be acknowledged. Firstly, although we perfected the study design and executed it strictly during the whole process as far as possible, the association found in this study, an observational case-control study, was relatively limited compared to a cohort study or an experimental study. So, in other words, well-designed prospective cohorts or reasonable experimental research are needed to validate our findings further. Secondly, the information on food frequency and related exposure in the questionnaire were obtained through retrospective investigation; we cannot rule out the possible limitation of recall bias. Thirdly, this is a hospital-based case-control study, and all of the cases came from the same department in a hospital; though its representativeness in the province for sophisticated diagnosis and treatment techniques, the selection bias still cannot be ignored. Fourthly, several potential confounders were determined and adjusted in the study, but there undoubtedly are other confounding covariates that might also influence the outcomes, such as common genetic polymorphisms and some nutritional biomarkers. The findings would be more convincing if taking these factors into consideration. Last but not least, maternal hcy concentration was not available in our research, which excludes the possibility of verifying the potential explanation that genetic variants of the BHMT gene may cause VSD by elevating maternal hcy levels.
5. Conclusions
In this hospital-based case-control study, statistically significant associations were found between the polymorphisms of the BHMT gene at rs1316753, rs1915706, and VSD in offspring. Maternal dietary habits were also observed to have a significant impact on the occurrence and development of VSD. A significant interaction between BHMT polymorphisms and maternal bean intake was identified in the study. Concerning the limitations of our study, more convincing crowd investigation and experimental research are necessary to repeatedly verify the findings and further elucidate the potential mechanism.
Author Contributions
Conceptualization, T.W., P.Z. and J.Q.; Data curation, M.L.; Formal analysis, M.L.; Funding acquisition, J.Q.; Investigation, X.S., M.S., Y.L., J.W., T.Z. and Q.C.; Methodology, M.L.; Project administration, J.Q.; Resources, P.H.; Software, S.Z. and J.S.; Supervision, T.W.; Visualization, M.L.; Writing—original draft, M.L.; Writing—review & editing, M.L. and T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Project Funded by National Key Research and Development Project (2018YFE0114500), National Natural Science Foundation Program of China (82073653 and 81803313), China Postdoctoral Science Foundation (2020M682644), Hunan Provincial Science and Technology Talent Support Project (2020TJ-N07), Hunan Provincial Key Research and Development Program (2018SK2063 and 2018SK2064), Natural Science Foundation of Hunan Province (2018JJ2551), Open Project from NHC Key Laboratory of Birth Defect for Research and Prevention (KF2020006), and Science and Technology Planning Project of Guangdong Province (2020A1414010152), Hunan Outstanding Youth Fund Project (2022JJ10087), Natural Science Foundation of Hunan Province of China (2022JJ40207); Changsha Municipal Natural Science Foundation (kq2202470), Postgraduate Scientific Research Innovation Project of Central South University(1053320215987).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee for Clinical Research of Xiangya School of Public Health of Central South University (no. XYGW-2018-36).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The department of cardiothoracic surgery in Hunan Children’s Hospital lent full support to the research. The staff in the department helped us collect data and blood samples of cases and their busy work provided support for our epidemiological investigation. We want to express our sincere appreciation to all of them.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970-2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar]
- Mitchell, S.C.; Korones, S.B.; Berendes, H.W. Congenital heart disease in 56, 109 births. Incidence and natural history. Circulation 1971, 43, 323–332. [Google Scholar]
- Van Der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Penny, D.J.; Vick, G.W., 3rd. Ventricular septal defect. Lancet 2011, 377, 1103–1112. [Google Scholar] [CrossRef]
- Gelb, B.D.; Chung, W.K. Complex genetics and the etiology of human congenital heart disease. Cold Spring Harb. Perspect. Med. 2014, 4, a013953. [Google Scholar] [CrossRef]
- Zhang, T.N.; Wu, Q.J.; Liu, Y.S.; Lv, J.L.; Sun, H.; Chang, Q.; Liu, C.F.; Zhao, Y.H. Environmental Risk Factors and Congenital Heart Disease: An Umbrella Review of 165 Systematic Reviews and Meta-Analyses with More than 120 Million Participants. Front. Cardiovasc Med. 2021, 8, 640729. [Google Scholar] [CrossRef]
- Morton, S.U.; Quiat, D.; Seidman, J.G.; Seidman, C.E. Genomic frontiers in congenital heart disease. Nat. Rev. Cardiol. 2022, 19, 26–42. [Google Scholar]
- Boyd, R.; McMullen, H.; Beqaj, H.; Kalfa, D. Environmental Exposures and Congenital Heart Disease. Pediatrics 2022, 149, e2021052151. [Google Scholar]
- van der Bom, T.; Zomer, A.C.; Zwinderman, A.H.; Meijboom, F.J.; Bouma, B.J.; Mulder, B.J. The changing epidemiology of congenital heart disease. Nat. Rev. Cardiol. 2011, 8, 50–60. [Google Scholar] [CrossRef]
- Cao, J.; Wu, Q.; Huang, Y.; Wang, L.; Su, Z.; Ye, H. The role of DNA methylation in syndromic and non-syndromic congenital heart disease. Clin. Epigenetics. 2021, 13, 93. [Google Scholar] [CrossRef]
- Wilson, R.D.; O’Connor, D.L. Maternal folic acid and multivitamin supplementation: International clinical evidence with considerations for the prevention of folate-sensitive birth defects. Prev. Med. Rep. 2021, 24, 101617. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, Y.; Zeng, L.; Dang, S.; Yan, H. Maternal dietary diversity during pregnancy and congenital heart defects: A case-control study. Eur. J. Clin. Nutr. 2021, 75, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kang, Y.; Chang, Q.; Zhang, B.; Liu, X.; Zeng, L.; Yan, H.; Dang, S. Maternal Zinc, Copper, and Selenium Intakes during Pregnancy and Congenital Heart Defects. Nutrients 2022, 14, 1055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Guo, L.; Zhao, D.; Qu, P.; Dang, S.; Yan, H. Maternal B-vitamin intake and B-vitamin supplementation during pregnancy in relation to neonatal congenital heart defects: A case-control study with propensity score matching. Eur. J. Clin. Nutr. 2021, 75, 782–791. [Google Scholar] [CrossRef]
- Koster, M.P.; van Duijn, L.; Krul-Poel, Y.H.; Laven, J.S.; Helbing, W.A.; Simsek, S.; Steegers-Theunissen, R.P. A compromised maternal vitamin D status is associated with congenital heart defects in offspring. Early Hum. Dev. 2018, 117, 50–56. [Google Scholar] [CrossRef]
- Sunden, S.L.; Renduchintala, M.S.; Park, E.I.; Miklasz, S.D.; Garrow, T.A. Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch. Biochem. Biophys. 1997, 345, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Park, E.I.; Garrow, T.A. Interaction between dietary methionine and methyl donor intake on rat liver betaine-homocysteine methyltransferase gene expression and organization of the human gene. J. Biol. Chem. 1999, 274, 7816–7824. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.W.; Cerdena, I.; Zeisel, S.H. Homocysteinemia in mice with genetic betaine homocysteine S-methyltransferase deficiency is independent of dietary folate intake. J. Nutr. 2012, 142, 1964–1967. [Google Scholar] [CrossRef]
- Szegedi, S.S.; Castro, C.C.; Koutmos, M.; Garrow, T.A. Betaine-homocysteine S-methyltransferase-2 is an S-methylmethionine-homocysteine methyltransferase. J. Biol. Chem. 2008, 283, 8939–8945. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, H.R.; Shim, S.M. Determination of S-methyl-L-methionine (SMM) from Brassicaceae Family Vegetables and Characterization of the Intestinal Transport of SMM by Caco-2 Cells. J. Food Sci. 2017, 82, 36–43. [Google Scholar] [CrossRef]
- Pérez-Miguelsanz, J.; Vallecillo, N.; Garrido, F.; Reytor, E.; Pérez-Sala, D.; Pajares, M.A. Betaine homocysteine S-methyltransferase emerges as a new player of the nuclear methionine cycle. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1165–1182. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Kalari, K.; Fridley, B.L.; Jenkins, G.; Ji, Y.; Abo, R.; Hebbring, S.; Zhang, J.; Nye, M.D.; Leeder, J.S.; et al. Betaine-homocysteine methyltransferase: Human liver genotype-phenotype correlation. Mol. Genet. Metab. 2011, 102, 126–133. [Google Scholar] [CrossRef]
- Blanco, R.; Colombo, A.; Pardo, R.; Suazo, J. Maternal biomarkers of methylation status and non-syndromic orofacial cleft risk: A meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 1323–1332. [Google Scholar] [CrossRef]
- Kucha, W.; Seifu, D.; Tirsit, A.; Yigeremu, M.; Abebe, M.; Hailu, D.; Tsehay, D.; Genet, S. Folate, Vitamin B12, and Homocysteine Levels in Women With Neural Tube Defect-Affected Pregnancy in Addis Ababa, Ethiopia. Front. Nutr. 2022, 9, 873900. [Google Scholar] [CrossRef]
- Dilli, D.; Doğan, N.N.; Örün, U.A.; Koç, M.; Zenciroğlu, A.; Karademir, S.; Akduman, H. Maternal neonatal micronutrient levels in newborns with, C.H.D. Cardiol. Young 2018, 28, 523–529. [Google Scholar] [CrossRef]
- Nelen, W.L. Hyperhomocysteinaemia and human reproduction. Clin. Chem. Lab. Med. 2001, 39, 758–763. [Google Scholar] [CrossRef]
- Shaw, G.M.; Lu, W.; Zhu, H.; Yang, W.; Briggs, F.; Carmichael, S.L.; Barcellos, L.F.; Lammer, E.J.; Finnell, R.H. 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med. Genet. 2009, 10, 49. [Google Scholar] [CrossRef]
- Hobbs, C.A.; Cleves, M.A.; MacLeod, S.L.; Erickson, S.W.; Tang, X.; Li, J.; Li, M.; Nick, T.; Malik, S.; National Birth Defects Prevention Study. Conotruncal heart defects and common variants in maternal and fetal genes in folate, homocysteine, and transsulfuration pathways. Birth Defects Res. A Clin. Mol. Teratol. 2014, 100, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Nembhard, W.N.; Tang, X.; Hu, Z.; MacLeod, S.; Stowe, Z.; Webber, D. Maternal and infant genetic variants, maternal periconceptional use of selective serotonin reuptake inhibitors, and risk of congenital heart defects in offspring: Population based study. Bmj 2017, 356, j832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, X.; Cleves, M.A.; Nick, T.G.; Li, M.; MacLeod, S.L.; Erickson, S.W.; Li, J.; Shaw, G.M.; Mosley, B.S.; National Birth Defects Prevention Study. Obstructive heart defects associated with candidate genes, maternal obesity, and folic acid supplementation. Am. J. Med. Genet. A 2015, 167, 1231–1242. [Google Scholar] [CrossRef]
- Ganu, R.S.; Ishida, Y.; Koutmos, M.; Kolokotronis, S.O.; Roca, A.L.; Garrow, T.A.; Schook, L.B. Evolutionary Analyses and Natural Selection of Betaine-Homocysteine S-Methyltransferase (BHMT) and BHMT2 Genes. PLoS ONE 2015, 10, e0134084. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Zeisel, S.H. Choline: The Neurocognitive Essential Nutrient of Interest to Obstetricians and Gynecologists. J. Diet Suppl. 2020, 17, 733–752. [Google Scholar] [CrossRef] [PubMed]
- Vailati-Riboni, M.; Crookenden, M.; Kay, J.K.; Meier, S.; Mitchell, M.D.; Heiser, A.; Roche, J.R.; Loor, J.J. Hepatic one-carbon metabolism enzyme activities and intermediate metabolites are altered by prepartum body condition score and plane of nutrition in grazing Holstein dairy cows. J. Dairy Sci. 2020, 103, 2662–2676. [Google Scholar] [CrossRef] [PubMed]
- Jacometo, C.B.; Zhou, Z.; Luchini, D.; Corrêa, M.N.; Loor, J.J. Maternal supplementation with rumen-protected methionine increases prepartal plasma methionine concentration and alters hepatic mRNA abundance of 1-carbon, methionine, and transsulfuration pathways in neonatal Holstein calves. J. Dairy Sci. 2017, 100, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.N.; Vailati-Riboni, M.; Elolimy, A.A.; Cardoso, F.C.; Rodriguez-Zas, S.L.; Miura, M.; Pan, Y.X.; Loor, J.J. Hepatic betaine-homocysteine methyltransferase and methionine synthase activity and intermediates of the methionine cycle are altered by choline supply during negative energy balance in Holstein cows. J. Dairy Sci. 2019, 102, 8305–8318. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.M.; Parker, S.E.; Crider, K.S.; Tinker, S.C.; Mitchell, A.A.; Werler, M.M. One-Carbon Cofactor Intake and Risk of Neural Tube Defects Among Women Who Meet Folic Acid Recommendations: A Multicenter Case-Control Study. Am. J. Epidemiol. 2019, 188, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Stingone, J.A.; Luben, T.J.; Carmichael, S.L.; Aylsworth, A.S.; Botto, L.D.; Correa, A.; Gilboa, S.M.; Langlois, P.H.; Nembhard, W.N.; Richmond-Bryant, J.; et al. Maternal Exposure to Nitrogen Dioxide, Intake of Methyl Nutrients, and Congenital Heart Defects in Offspring. Am. J. Epidemiol. 2017, 186, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Bedale, W.; Sindelar, J.J.; Milkowski, A.L. Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions. Meat Sci. 2016, 120, 85–92. [Google Scholar] [CrossRef]
- Romanowicz, J.; Guerrelli, D.; Dhari, Z.; Mulvany, C.; Reilly, M.; Swift, L.; Vasandani, N.; Ramadan, M.; Leatherbury, L.; Ishibashi, N.; et al. Chronic perinatal hypoxia delays cardiac maturation in a mouse model for cyanotic congenital heart disease. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1873–H1886. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Bušová, M.; Bencko, V.; Veszelits Laktičová, K.; Holcátová, I.; Vargová, M. Risk of exposure to acrylamide. Cent. Eur. J. Public Health 2020, 28, S43–S46. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Li, N.; Liu, Z.; Qiu, J.; Deng, Y.; Li, X.; Chen, M.; Yu, J.; Zhu, J.; Yu, P.; et al. Risk of congenital heart diseases associated with NAT2 genetic polymorphisms and maternal polycyclic aromatic hydrocarbons exposure. Prenat. Diagn. 2019, 39, 968–975. [Google Scholar] [CrossRef]
- Sarmah, S.; Marrs, J.A. Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function. Int. J. Mol. Sci. 2016, 17, 2123. [Google Scholar] [CrossRef]
- Huang, L.; Wang, C.; Zhang, Y.; Li, J.; Zhong, Y.; Zhou, Y.; Chen, Y.; Zuo, Z. Benzo[a]pyrene exposure influences the cardiac development and the expression of cardiovascular relative genes in zebrafish (Danio rerio) embryos. Chemosphere 2012, 87, 369–375. [Google Scholar] [CrossRef]
- Hobbs, C.A.; Cleves, M.A.; Karim, M.A.; Zhao, W.; MacLeod, S.L. Maternal folate-related gene environment interactions and congenital heart defects. Obstet Gynecol. 2010, 116 Pt 1, 16–22. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Y.; Zhang, R.; Dong, L.; Cui, H.; Fang, Y.; Zhao, L.; Shi, O.; Cai, C. Association of neural tube defects with gene polymorphisms in one-carbon metabolic pathway. Childs Nerv. Syst. 2018, 34, 277–284. [Google Scholar] [CrossRef]
- Imani, M.M.; Lopez-Jornet, P.; López, E.P.; Ghanbari, F.; Sadeghi, M. Association of Betaine-Homocysteine S-Methyl Transferase (rs3797546 and rs3733890) polymorphisms with non-syndromic cleft lip/palate: A meta-analysis. Int. Orthod. 2019, 17, 643–651. [Google Scholar] [CrossRef]
- Kalisch-Smith, J.I.; Ved, N.; Sparrow, D.B. Environmental Risk Factors for Congenital Heart Disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a037234. [Google Scholar] [CrossRef]
- Malik, R.A.; Lone, M.R.; Ahmed, A.; Koul, K.A.; Malla, R.R. Maternal hyperhomocysteinemia and congenital heart defects: A prospective case control study in Indian population. Indian Heart J. 2017, 69, 17–19. [Google Scholar] [CrossRef]
- Mei, X.; Qi, D.; Zhang, T.; Zhao, Y.; Jin, L.; Hou, J.; Wang, J.; Lin, Y.; Xue, Y.; Zhu, P.; et al. Inhibiting MARSs reduces hyperhomocysteinemia-associated neural tube and congenital heart defects. EMBO Mol. Med. 2020, 12, e9469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, G. Biofortification of pulses and legumes to enhance nutrition. Heliyon 2020, 6, e03682. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef]
- Millian, N.S.; Garrow, T.A. Human betaine-homocysteine methyltransferase is a zinc metalloenzyme. Arch. Biochem. Biophys. 1998, 356, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Cuny, H.; Rapadas, M.; Gereis, J.; Martin, E.M.; Kirk, R.B.; Shi, H.; Dunwoodie, S.L. NAD deficiency due to environmental factors or gene-environment interactions causes congenital malformations and miscarriage in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 3738–3747. [Google Scholar] [CrossRef]
- Gibson, G.; Berger, K. Dietary modification, penetrance, and the origins of congenital malformation. Proc. Natl. Acad. Sci. USA 2020, 117, 5097–5099. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).