Vitamin D Status in Critically Ill Patients with SIRS and Its Relationship with Circulating Zn and Related Parameters during ICU Stay
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Nutritional Profile
2.3. Data Collection
2.4. Blood Sampling and Biochemical Parameters
2.4.1. Analytical Determination of Zn
2.4.2. Analytical Determination of Vitamin D
2.5. Statistical Analysis
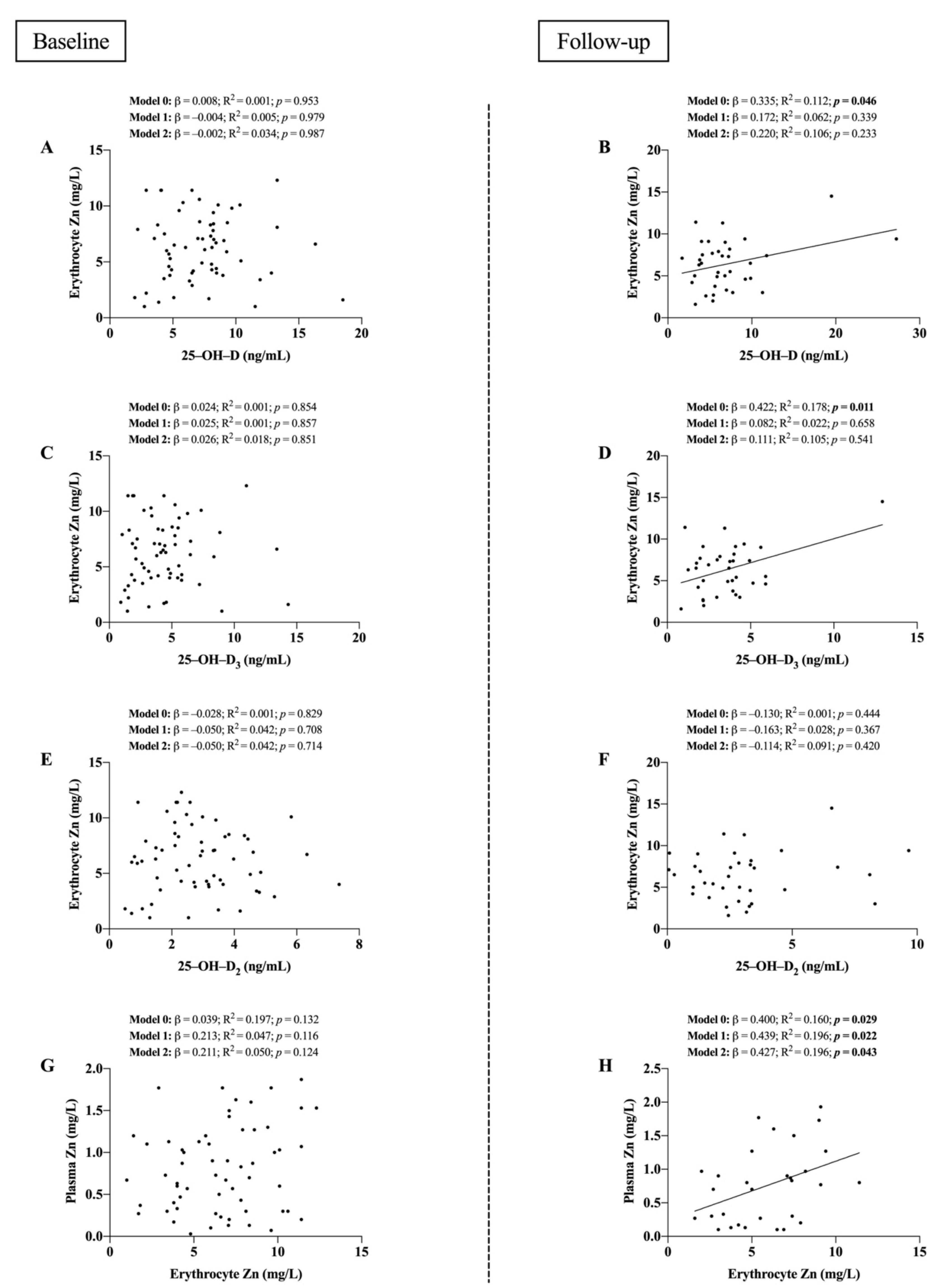
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Niekerk, G.; Engelbrecht, A.-M. Inflammation-Induced Metabolic Derangements or Adaptation: An Immunometabolic Perspective. Cytokine Growth Factor Rev. 2018, 43, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.K.; Burns, B. Systemic Inflammatory Response Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S.J. Quantitative Data on the Magnitude of the Systemic Inflammatory Response and Its Effect on Micronutrient Status Based on Plasma Measurements. Am. J. Clin. Nutr. 2012, 95, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D in Health and Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Current Understanding of the Function of the Nuclear Vitamin D Receptor in Response to Its Natural and Synthetic Ligands. Recent Results Cancer Res. Fortschritte Krebsforsch. Progres Dans Rech. Sur Cancer 2003, 164, 29–42. [Google Scholar] [CrossRef]
- Cornella, I.; Suárez, R.M.; Mouriño, A.; Sestelo, J.P.; Sarandeses, L.A. Stereoselective Convergent Synthesis of 24-Substituted Metabolites and Analogues of Vitamin D. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 19–23. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; Bergam, I.; Reiter, R.J.; Manucha, W. Metal Ion Homeostasis with Emphasis on Zinc and Copper: Potential Crucial Link to Explain the Non-Classical Antioxidative Properties of Vitamin D and Melatonin. Life Sci. 2021, 281, 119770. [Google Scholar] [CrossRef]
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Ishizawa, M.; Hirayu, A.; Makishima, M. Zinc Inhibits Cadherin 1 Expression Induced by 1α,25-Dihydroxyvitamin D3 in Colon Cancer Cells. Anticancer Res. 2021, 41, 5453–5459. [Google Scholar] [CrossRef]
- Islam, M.S.; Loots, D.T. Diabetes, Metallothionein, and Zinc Interactions: A Review. BioFactors 2007, 29, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc Requirements and the Risks and Benefits of Zinc Supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN Micronutrient Guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 Regulates the Zinc Transporter Zip14 in Liver and Contributes to the Hypozincemia of the Acute-Phase Response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kechrid, Z.; Hamdi, M.; Nazıroğlu, M.; Flores-Arce, M. Vitamin D Supplementation Modulates Blood and Tissue Zinc, Liver Glutathione and Blood Biochemical Parameters in Diabetic Rats on a Zinc-Deficient Diet. Biol. Trace Elem. Res. 2012, 148, 371–377. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K.; Genuis, S.J. Vitamin D, Essential Minerals, and Toxic Elements: Exploring Interactions between Nutrients and Toxicants in Clinical Medicine. Sci. World J. 2015, 2015, 318595. [Google Scholar] [CrossRef]
- Claro da Silva, T.; Hiller, C.; Gai, Z.; Kullak-Ublick, G.A. Vitamin D 3 Transactivates the Zinc and Manganese Transporter SLC30A10 via the Vitamin D Receptor. J. Steroid Biochem. Mol. Biol. 2016, 163, 77–87. [Google Scholar] [CrossRef]
- Litchfield, T.M.; Ishikawa, Y.; Wu, L.N.; Wuthier, R.E.; Sauer, G.R. Effect of Metal Ions on Calcifying Growth Plate Cartilage Chondrocytes. Calcif. Tissue Int. 1998, 62, 341–349. [Google Scholar] [CrossRef]
- Hie, M.; Tsukamoto, I. Administration of Zinc Inhibits Osteoclastogenesis through the Suppression of RANK Expression in Bone. Eur. J. Pharmacol. 2011, 668, 140–146. [Google Scholar] [CrossRef]
- Lutz, W.; Burritt, M.F.; Nixon, D.E.; Kao, P.C.; Kumar, R. Zinc Increases the Activity of Vitamin D-Dependent Promoters in Osteoblasts. Biochem. Biophys. Res. Commun. 2000, 271, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.A.; Benson, L.M.; Naylor, S.; Kumar, R. Modulation Effects of Zinc on the Formation of Vitamin D Receptor and Retinoid X Receptor α-DNA Transcription Complexes: Analysis by Microelectrospray Mass Spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Khan, S.; Naseem, I. Antioxidant Role of Vitamin D in Mice With Alloxan-Induced Diabetes. Can. J. Diabetes 2018, 42, 412–418. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Lorente, H.; Herrera-Quintana, L.; Molina-López, J.; Gamarra-Morales, Y.; López-González, B.; Miralles-Adell, C.; Planells, E. Response of Vitamin D after Magnesium Intervention in a Postmenopausal Population from the Province of Granada, Spain. Nutrients 2020, 12, 2283. [Google Scholar] [CrossRef]
- Herrera-Quintana, L.; Gamarra-Morales, Y.; Vázquez-Lorente, H.; Molina-López, J.; Castaño-Pérez, J.; Machado-Casas, J.F.; Coca-Zúñiga, R.; Pérez-Villares, J.M.; Planells, E. Bad Prognosis in Critical Ill Patients with COVID-19 during Short-Term ICU Stay Regarding Vitamin D Levels. Nutrients 2021, 13, 1988. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Majolo, F.; de Oliveira Paludo, F.J.; Ponzoni, A.; Graebin, P.; Dias, F.S.; Alho, C.S. Effect of 593C>T GPx1 SNP Alone and in Synergy with 47C>T SOD2 SNP on the Outcome of Critically Ill Patients. Cytokine 2015, 71, 312–317. [Google Scholar] [CrossRef]
- Higgins, D.M.; Wischmeyer, P.E.; Queensland, K.M.; Sillau, S.H.; Sufit, A.J.; Heyland, D.K. Relationship of Vitamin D Deficiency to Clinical Outcomes in Critically Ill Patients. JPEN J. Parenter. Enteral Nutr. 2012, 36, 713–720. [Google Scholar] [CrossRef]
- Amrein, K.; Quraishi, S.A.; Litonjua, A.A.; Gibbons, F.K.; Pieber, T.R.; Camargo, C.A.; Giovannucci, E.; Christopher, K.B. Evidence for a U-Shaped Relationship between Prehospital Vitamin D Status and Mortality: A Cohort Study. J. Clin. Endocrinol. Metab. 2014, 99, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Ilia, S. Vitamin D Deficiency in Sepsis: “Body Humors” Imbalance or Sepsis “Epiphenomenon”? Crit. Care Med. 2017, 45, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Yasui, H.; Suzuki, K.; Saitou, T.; Yamamoto, Y.; Ishizaka, T.; Nishida, K.; Yoshihara, S.; Gohma, I.; Ogawa, Y. Analysis of the Predictive Factors for a Critical Illness of COVID-19 during Treatment—Relationship between Serum Zinc Level and Critical Illness of COVID-19−. Int. J. Infect. Dis. 2020, 100, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Pereira, C.G.M.; Santana, E.R.S.; Ramos, J.E.R.; da Silva, H.M.B.S.; Nunes, M.A.P.; Forbes, S.C.; Santos, H.O. Low Serum Zinc Levels and Associated Risk Factors in Hospitalized Patients Receiving Oral or Enteral Nutrition: A Case-Control Study. Clin. Ther. 2021, 43, e39–e55. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.A.; et al. Vegan Nutrition for Mothers and Children: Practical Tools for Healthcare Providers. Nutrients 2018, 11, 5. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wierzbicka, J.; Żmijewski, M.A. Vitamin D in the Skin Physiology and Pathology. Acta Biochim. Pol. 2016, 63, 17–29. [Google Scholar] [CrossRef]
- Mocayar Marón, F.J.; Ferder, L.; Reiter, R.J.; Manucha, W. Daily and Seasonal Mitochondrial Protection: Unraveling Common Possible Mechanisms Involving Vitamin D and Melatonin. J. Steroid Biochem. Mol. Biol. 2020, 199, 105595. [Google Scholar] [CrossRef]
- Chen, X.; Chu, C.; Doebis, C.; Xiong, Y.; Cao, Y.; Krämer, B.K.; von Baehr, V.; Hocher, B. Vitamin D Status and Its Association with Parathyroid Hormone in 23,134 Outpatients. J. Steroid Biochem. Mol. Biol. 2022, 220, 106101. [Google Scholar] [CrossRef]
- Goyal, R.; Jialal, I. Hyperphosphatemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ramasamy, I. Inherited Disorders of Calcium Homeostasis. Clin. Chim. Acta 2008, 394, 22–41. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and Non-Redox-Metal-Induced Formation of Free Radicals and Their Role in Human Disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Shiota, J.; Tagawa, H.; Izumi, N.; Higashikawa, S.; Kasahara, H. Effect of Zinc Supplementation on Bone Formation in Hemodialysis Patients with Normal or Low Turnover Bone. Ren. Fail. 2015, 37, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.K.; Puppa, M.-A.; Wessels, I.; Rink, L. Vitamin D3 and Zinc Synergistically Induce Regulatory T Cells and Suppress Interferon-γ Production in Mixed Lymphocyte Culture. J. Nutr. Biochem. 2022, 102, 108942. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Lorente, H.; Herrera-Quintana, L.; Quintero-Osso, B.; Molina-López, J.; Planells, E. Current Trends in the Analytical Determination of Vitamin D. Nutr. Hosp. 2019, 36, 1418–1423. [Google Scholar] [CrossRef][Green Version]
| Parameters | Reference | Baseline Mean (SD) N = 65 | Follow-Up Mean (SD) N = 40 | Δ Change (%) | p-Value Men vs. Women | p-Value <62 years vs. >62 years | p-Value Baseline vs. Follow-Up |
|---|---|---|---|---|---|---|---|
| Age (years) | - | 60.1 (11.5) | - | - | 0.747 | - | - |
| BMI (kg/m2) | - | 26.7 (4.77) | - | - | 0.320 | 0.001 | - |
| Patient 7-day mortality (n/N, %) | - | 25/65 (38.5) | - | - | 0.474 | 0.070 | - |
| APACHE-II score | - | 17.2 (4.94) | - | - | 0.390 | 0.895 | - |
| SOFA score | - | 9.04 (3.39) | 5.12 (3.50) | −43.4 | 0.230 | 0.264 | <0.001 |
| Total Proteins (g/dL) | 6.60–8.30 | 5.27 (0.91) | 5.53 (0.76) | +4.93 | 0.138 | 0.658 | 0.115 |
| Albumin (g/dL) | 3.50–5.20 | 2.85 (0.58) | 2.63 (0.67) | −7.72 | 0.647 | 0.649 | 0.139 |
| Prealbumin (mg/dL) | 16.0–42.0 | 12.5 (4.65) | 16.3 (9.80) | +30.3 | 0.370 | 0.828 | 0.143 |
| Ferritin (ng/dL) | 20.0–275.0 | 401.6 (377.4) | 543.7 (482.9) | +35.4 | 0.861 | 0.493 | 0.024 |
| Transferrin (mg/dL) | 200.0–360.0 | 153.4 (63.1) | 146.9 (50.8) | −4.27 | 0.825 | 0.868 | 0.514 |
| Triglycerides (mg/dL) | 50.0–200.0 | 196.9 (144.3) | 197.8 (102.1) | +0.46 | 0.275 | 0.366 | 0.972 |
| Total Cholesterol (mg/dL) | 140.0–200.0 | 108.5 (38.2) | 134.8 (43.7) | +24.2 | 0.875 | 0.521 | 0.002 |
| CRP (mg/L) | 0.00–5.00 | 19.8 (11.8) | 10.7 (8.63) | −46.2 | 0.292 | 0.164 | 0.001 |
| GOT (U/L) | <37.0 | 104.0 (164.0) | 36.2 (23.2) | −65.2 | 0.290 | 0.260 | 0.045 |
| GPT (U/L) | <41.0 | 54.9 (78.8) | 28.5 (26.0) | −48.0 | 0.801 | 0.349 | 0.082 |
| GGT (U/L) | 11.0–50.0 | 56.6 (60.9) | 141.3 (98.4) | +149.7 | 0.932 | 0.473 | <0.001 |
| ALP (U/L) | 40.0–130.0 | 101.5 (76.3) | 126.1 (68.8) | +24.3 | 0.085 | 0.133 | 0.062 |
| Osteocalcin (ng/mL) | 15.0–46.0 | 2.97 (1.95) | 5.53 (3.98) | +86.2 | 0.385 | 0.163 | 0.002 |
| PTH (pg/mL) | 20.0–70.0 | 248.5 (151.0) | 133.5 (97.9) | −46.3 | 0.013 | 0.775 | <0.001 |
| Ca (mg/dL) | 8.80–10.6 | 7.53 (0.86) | 8.06 (0.71) | +7.04 | 0.930 | 0.812 | 0.001 |
| P (mg/dL) | 2.30–4.50 | 3.91 (1.87) | 3.65 (1.47) | −6.65 | 0.152 | 0.676 | 0.452 |
| Mg (mg/dL) | 1.60–2.60 | 2.14 (0.52) | 2.31 (0.46) | +7.94 | 0.564 | 0.320 | 0.213 |
| Parameters | Reference | Baseline Mean (SD) N = 65 | Follow-Up Mean (SD) N = 40 | Δ Change (%) | p-Value Baseline vs. Follow-Up |
|---|---|---|---|---|---|
| 25–OH–D (ng/mL) | 30.0–100.0 | 6.76 (2.96) | 6.51 (3.28) | −3.11 | 0.506 |
| 25–OH–D3 (ng/mL) | – | 3.90 (2.23) | 3.59 (2.10) | −7.18 | 0.035 |
| 25–OH–D2 (ng/mL) | – | 2.87 (1.60) | 2.93 (2.05) | +2.09 | 0.875 |
| Plasma Zn (mg/L) | 0.65–1.11 | 0.71 (0.51) | 0.74 (0.56) | +4.23 | 0.748 |
| Erythrocyte Zn (mg/L) | – | 6.22 (2.81) | 6.42 (2.90) | +3.12 | 0.708 |
| n/N (%) | n/N (%) | ||||
| 25–OH–D deficiency | <20 ng/mL | 65/65 (100) | 40/40 (100) | 0 | - |
| Hypozincemia (plasma Zn) | <0.7 mg/L | 28/65 (43) | 13/40 (32.5) | −24.4 | 0.001 |
| Characteristics | Δ 25–OH–D | Δ 25–OH–D3 | Δ 25–OH–D2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ß | R2 | p-Value | ß | R2 | p-Value | ß | R2 | p-Value | |
| Δ Osteocalcin (ng/mL) | 0.091 | 0.008 | 0.672 | −0.260 | 0.067 | 0.221 | 0.202 | 0.041 | 0.343 |
| Δ PTH (pg/mL) | 0.211 | 0.044 | 0.334 | 0.484 | 0.234 | 0.019 | −0.015 | 0.001 | 0.945 |
| Δ Ca (mg/dL) | −0.256 | 0.065 | 0.218 | 0.044 | 0.002 | 0.833 | −0.256 | 0.065 | 0.218 |
| Δ P (mg/dL) | 0.329 | 0.108 | 0.109 | 0.539 | 0.291 | 0.005 | 0.115 | 0.013 | 0.585 |
| Δ Mg (mg/dL) | −0.388 | 0.150 | 0.061 | 0.069 | 0.005 | 0.748 | −0.402 | 0.161 | 0.052 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Quintana, L.; Vázquez-Lorente, H.; Molina-López, J.; Gamarra-Morales, Y.; Martín-López, J.I.; Planells, E. Vitamin D Status in Critically Ill Patients with SIRS and Its Relationship with Circulating Zn and Related Parameters during ICU Stay. Nutrients 2022, 14, 3580. https://doi.org/10.3390/nu14173580
Herrera-Quintana L, Vázquez-Lorente H, Molina-López J, Gamarra-Morales Y, Martín-López JI, Planells E. Vitamin D Status in Critically Ill Patients with SIRS and Its Relationship with Circulating Zn and Related Parameters during ICU Stay. Nutrients. 2022; 14(17):3580. https://doi.org/10.3390/nu14173580
Chicago/Turabian StyleHerrera-Quintana, Lourdes, Héctor Vázquez-Lorente, Jorge Molina-López, Yenifer Gamarra-Morales, Javier Ignacio Martín-López, and Elena Planells. 2022. "Vitamin D Status in Critically Ill Patients with SIRS and Its Relationship with Circulating Zn and Related Parameters during ICU Stay" Nutrients 14, no. 17: 3580. https://doi.org/10.3390/nu14173580
APA StyleHerrera-Quintana, L., Vázquez-Lorente, H., Molina-López, J., Gamarra-Morales, Y., Martín-López, J. I., & Planells, E. (2022). Vitamin D Status in Critically Ill Patients with SIRS and Its Relationship with Circulating Zn and Related Parameters during ICU Stay. Nutrients, 14(17), 3580. https://doi.org/10.3390/nu14173580






