COVID-19 and Gestational Diabetes: The Role of Nutrition and Pharmacological Intervention in Preventing Adverse Outcomes
Abstract
:1. Introduction
2. Materials and Methods
3. Current Evidence on the Association between COVID-19 and Diabetes in Pregnancy
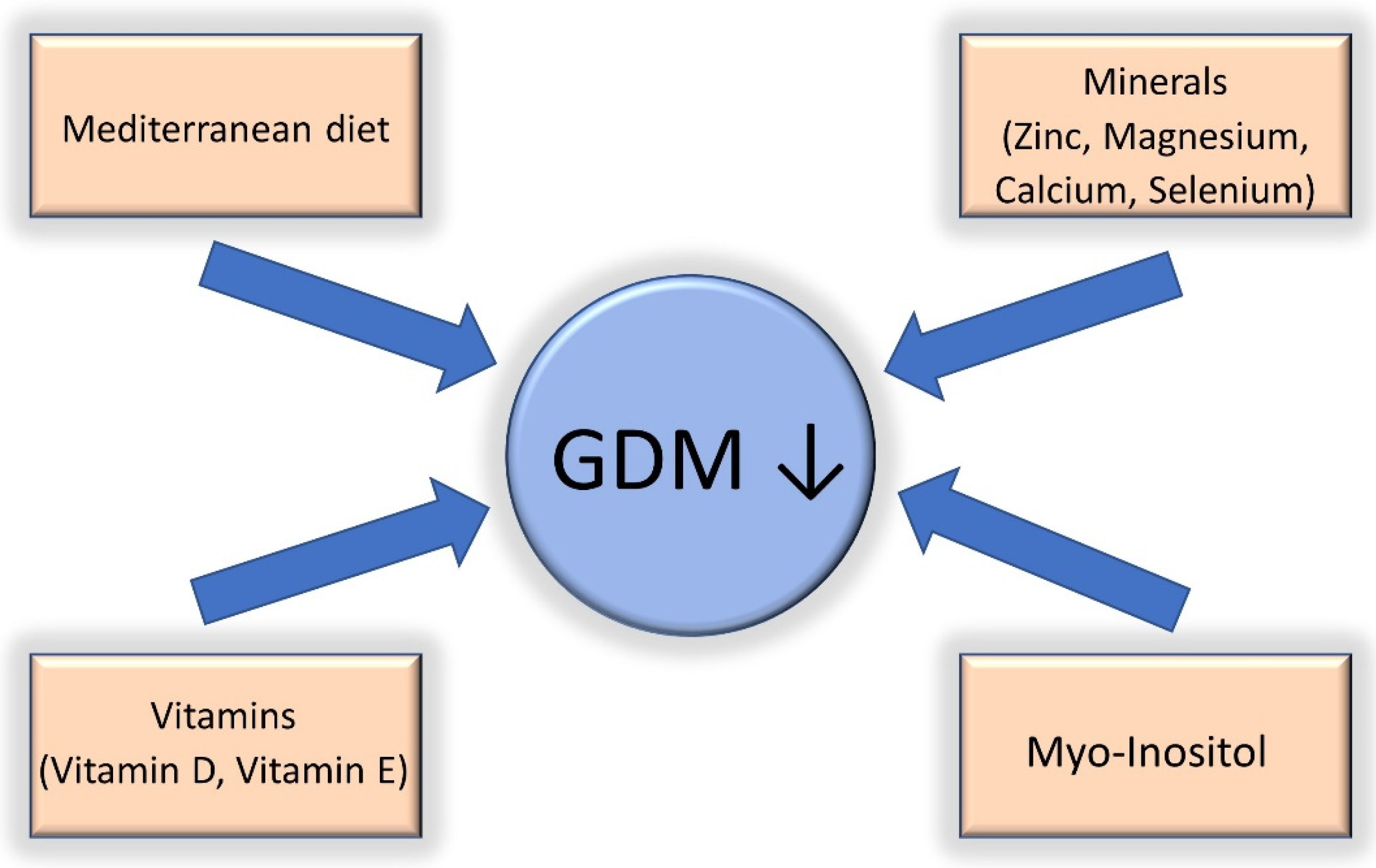
4. Gestational Diabetes Treated with Diet and COVID-19
5. Gestational Diabetes Treated with Insulin Therapy and COVID-19
6. Gestational Diabetes Treated with Metformin and COVID-19
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Shultz, J.M.; Perlin, A.; Saltzman, R.G.; Espinel, Z.; Galea, S. Pandemic March: 2019 Coronavirus Disease’s First Wave Circumnavigates the Globe. Disaster Med. Public Health Prep. 2020, 14, e28–e32. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection. JAMA Pediatr. 2021, 175, 817. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F.; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, 22 January–3 October 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef]
- Emami, A.; Javanmardi, F.; Pirbonyeh, N.; Akbari, A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2020, 8, e35. [Google Scholar]
- Hartmann-Boyce, J.; Morris, E.; Goyder, C.; Kinton, J.; Perring, J.; Nunan, D.; Mahtani, K.; Buse, J.B.; Del Prato, S.; Ji, L.; et al. Diabetes and COVID-19: Risks, Management, and Learnings From Other National Disasters. Diabetes Care 2020, 43, 1695–1703. [Google Scholar] [CrossRef]
- Turan, O.; Hakim, A.; Dashraath, P.; Jeslyn, W.J.L.; Wright, A.; Abdul-Kadir, R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: A systematic review. Int. J. Gynecol. Obstet. 2020, 151, 7–16. [Google Scholar] [CrossRef]
- Saccone, G.; Sen, C.; Di Mascio, D.; Galindo, A.; Grünebaum, A.; Yoshimatsu, J.; Stanojevic, M.; Kurjak, A.; Chervenak, F.; Suárez, M.J.R.; et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV2 infection. Ultrasound Obstet. Gynecol. 2021, 57, 232–241. [Google Scholar] [CrossRef]
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; van der Meulen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e759–e772. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.L.; Pham, N.M.; Binns, C.W.; Van Duong, D.; Lee, A.H. Prevalence of Gestational Diabetes Mellitus in Eastern and Southeastern Asia: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2018, 2018, 6536974. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, L.; Pettitt, D.J. Gestational Diabetes Mellitus. JAMA 2001, 286, 2516. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A. Increasing Prevalence of Gestational Diabetes Mellitus. Diabetes Care 2007, 30, S141–S146. [Google Scholar] [CrossRef]
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Tam, W.H.; Ma, R.C.W.; Yang, X.; Ko, G.T.C.; Tong, P.C.Y.; Cockram, C.S.; Sahota, D.S.; Rogers, M.S.; Chan, J.C.N. Glucose Intolerance and Cardiometabolic Risk in Children Exposed to Maternal Gestational Diabetes Mellitus in Utero. Pediatrics 2008, 122, 1229–1234. [Google Scholar] [CrossRef]
- West, N.A.; Crume, T.L.; Maligie, M.A.; Dabelea, D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia 2011, 54, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, B.E. Long-term Outcomes in Mothers Diagnosed With Gestational Diabetes Mellitus and Their Offspring. Clin. Obstet. Gynecol. 2007, 50, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.; Gluckman, P.; Godfrey, K.; Harding, J.; Owens, J.; Robinson, J. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Nijs, H.; Benhalima, K. Gestational Diabetes Mellitus and the Long-Term Risk for Glucose Intolerance and Overweight in the Offspring: A Narrative Review. J. Clin. Med. 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Eberle, C.; Ament, C. Diabetic and Metabolic Programming: Mechanisms Altering the Intrauterine Milieu. ISRN Pediatr. 2012, 2012, 975685. [Google Scholar] [CrossRef] [PubMed]
- Eberle, C.; Merki, E.; Yamashita, T.; Johnson, S.; Armando, A.M.; Quehenberger, O.; Napoli, C.; Palinski, W. Maternal Immunization Affects In Utero Programming of Insulin Resistance and Type 2 Diabetes. PLoS ONE 2012, 7, e45361. [Google Scholar] [CrossRef]
- Yamashita, T.; Freigang, S.; Eberle, C.; Pattison, J.; Gupta, S.; Napoli, C.; Palinski, W. Maternal Immunization Programs Postnatal Immune Responses and Reduces Atherosclerosis in Offspring. Circ. Res. 2006, 99, e51–e64. [Google Scholar] [CrossRef]
- Collège National des Gynécologues et Obstétriciens Français. Société francophone du diabète [Gestational diabetes]. J. Gynecol. Obstet. Biol. Reprod. 2010, 39, S139–S342. [Google Scholar]
- Torlone, E.; Festa, C.; Formoso, G.; Scavini, M.; Sculli, M.A.; Succurro, E.; Sciacca, L.; Di Bartolo, P.; Purrello, F.; Lapolla, A. Italian recommendations for the diagnosis of gestational diabetes during COVID-19 pandemic: Position statement of the Italian Association of Clinical Diabetologists (AMD) and the Italian Diabetes Society (SID), diabetes, and pregnancy study group. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1418–1422. [Google Scholar] [CrossRef]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes. Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Society of Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: Pharmacological treatment of gestational diabetes. Am. J. Obstet. Gynecol. 2018, 218, B2–B4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-K.; Lin, S.-S.; Ji, X.-J.; Guo, L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010, 47, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Praissman, J.L.; Grant, O.C.; Cai, Y.; Xiao, T.; Rosenbalm, K.E.; Aoki, K.; Kellman, B.P.; Bridger, R.; Barouch, D.H.; et al. Virus-Receptor Interactions of Glycosylated SARS-CoV-2 Spike and Human ACE2 Receptor. Cell Host Microbe 2020, 28, 586–601.e6. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Amiel, S.A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R.H.; Mingrone, G.; Boehm, B.; Cooper, M.E.; Chai, Z.; et al. New-Onset Diabetes in COVID-19. N. Engl. J. Med. 2020, 383, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Accili, D. Can COVID-19 cause diabetes? Nat. Metab. 2021, 3, 123–125. [Google Scholar] [CrossRef]
- Eskenazi, B.; Rauch, S.; Iurlaro, E.; Gunier, R.B.; Rego, A.; Gravett, M.G.; Cavoretto, P.I.; Deruelle, P.; García-May, P.K.; Mhatre, M.; et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: The INTERCOVID study. Am. J. Obstet. Gynecol. 2021, 227, 74.e1–74.e16. [Google Scholar] [CrossRef]
- Vousden, N.; Ramakrishnan, R.; Bunch, K.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Variant on the Severity of Maternal Infection and Perinatal Outcomes: Data From the UK Obstetric Surveillance System National Cohort. medRxiv 2021, 1–22. [Google Scholar] [CrossRef]
- Kleinwechter, H.J.; Weber, K.S.; Mingers, N.; Ramsauer, B.; Schaefer-Graf, U.M.; Groten, T.; Kuschel, B.; Backes, C.; Banz-Jansen, C.; Berghaeuser, M.A.; et al. Gestational diabetes mellitus and COVID-19: Results from the COVID-19–Related Obstetric and Neonatal Outcome Study (CRONOS). Am. J. Obstet. Gynecol. 2022. [Google Scholar] [CrossRef]
- Polcer, R.E.; Jones, E.; Pettersson, K. A Case Series on Critically Ill Pregnant or Newly Delivered Patients with COVID-19, Treated at Karolinska University Hospital, Stockholm. Case Rep. Obstet. Gynecol. 2021, 2021, 8868822. [Google Scholar] [CrossRef]
- Sitter, M.; Pecks, U.; Rüdiger, M.; Friedrich, S.; Fill Malfertheiner, S.; Hein, A.; Königbauer, J.T.; Becke-Jakob, K.; Zöllkau, J.; Ramsauer, B.; et al. Pregnant and Postpartum Women Requiring Intensive Care Treatment for COVID-19—First Data from the CRONOS-Registry. J. Clin. Med. 2022, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- Radan, A.-P.; Fluri, M.-M.; Nirgianakis, K.; Mosimann, B.; Schlatter, B.; Raio, L.; Surbek, D. Gestational diabetes is associated with SARS-CoV-2 infection during pregnancy: A case-control study. Diabetes Metab. 2022, 48, 101351. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, V.; Tortora, D.; Sandri, A.; Severino, L.; Mesirca, P.; Straface, G. COVID-19 pandemic: Impact on gestational diabetes mellitus prevalence. Diabetes Res. Clin. Pract. 2022, 183, 109149. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, S.; Fumagalli, S.; Guinea Montalvo, C.K.; Beretta, G.; Invernizzi, F.; Nespoli, A.; Vergani, P. Indirect impact of SARS-CoV-2 pandemic on pregnancy and childbirth outcomes: A nine-month long experience from a university center in Lombardy. Int. J. Gynecol. Obstet. 2022, 156, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Chelu, S.; Bernad, E.; Craina, M.; Neamtu, R.; Mocanu, A.G.; Vernic, C.; Chiriac, V.D.; Tomescu, L.; Borza, C. Prevalence of Gestational Diabetes in preCOVID-19 and COVID-19 Years and Its Impact on Pregnancy: A 5-Year Retrospective Study. Diagnostics 2022, 12, 1241. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1. [Google Scholar] [CrossRef]
- Danser, A.H.J.; Epstein, M.; Batlle, D. Renin-Angiotensin System Blockers and the COVID-19 Pandemic. Hypertension 2020, 75, 1382–1385. [Google Scholar] [CrossRef]
- Cuschieri, S.; Grech, S. COVID-19 and diabetes: The why, the what and the how. J. Diabetes Complicat. 2020, 34, 107637. [Google Scholar] [CrossRef]
- Hill, M.A.; Mantzoros, C.; Sowers, J.R. Commentary: COVID-19 in patients with diabetes. Metabolism 2020, 107, 154217. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.J.; Meguer, J.-X.; Redman, J.; Baker, E.H. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003, 29, 2204–2210. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Vaccine Safety Datalink (VSD). Available online: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html (accessed on 22 February 2022).
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Pang, J.; Ji, P.; Zhong, Z.; Li, H.; Li, B.; Zhang, J. Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis. J. Med. Virol. 2021, 93, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Picu, A.; Petcu, L.; Cucu, N.; Chifiriuc, M.C. Gut Microbiota, Host Organism, and Diet Trialogue in Diabetes and Obesity. Front. Nutr. 2019, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; De Nigris, V.; Spiga, R.; Mancuso, E.; La Sala, L.; Antonicelli, R.; Testa, R.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res. Rev. 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Fedullo, A.L.; Schiattarella, A.; Morlando, M.; Raguzzini, A.; Toti, E.; De Franciscis, P.; Peluso, I. Mediterranean Diet for the Prevention of Gestational Diabetes in the COVID-19 Era: Implications of Il-6 In Diabesity. Int. J. Mol. Sci. 2021, 22, 1213. [Google Scholar] [CrossRef]
- Morisset, A.-S.; Dubé, M.-C.; Côté, J.A.; Robitaille, J.; Weisnagel, S.J.; Tchernof, A. Circulating interleukin-6 concentrations during and after gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2011, 90, 524–530. [Google Scholar] [CrossRef]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M.; et al. A Multicenter, Randomized Trial of Treatment for Mild Gestational Diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef]
- Franquesa, M.; Pujol-Busquets, G.; García-Fernández, E.; Rico, L.; Shamirian-Pulido, L.; Aguilar-Martínez, A.; Medina, F.; Serra-Majem, L.; Bach-Faig, A. Mediterranean Diet and Cardiodiabesity: A Systematic Review through Evidence-Based Answers to Key Clinical Questions. Nutrients 2019, 11, 655. [Google Scholar] [CrossRef]
- Silva-del Valle, M.A.; Sánchez-Villegas, A.; Serra-Majem, L. No TitleAssociation between the adherence to the Mediterranean diet and overweight and obesity in pregnant women in Gran Canaria. Nutr. Hosp. 2013, 28, 654–659. [Google Scholar] [PubMed]
- Assaf-Balut, C.; García de la Torre, N.; Fuentes, M.; Durán, A.; Bordiú, E.; del Valle, L.; Valerio, J.; Jiménez, I.; Herraiz, M.; Izquierdo, N.; et al. A High Adherence to Six Food Targets of the Mediterranean Diet in the Late First Trimester is Associated with a Reduction in the Risk of Materno-Foetal Outcomes: The St. Carlos Gestational Diabetes Mellitus Prevention Study. Nutrients 2018, 11, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmedo-Requena, R.; Gómez-Fernández, J.; Amezcua-Prieto, C.; Mozas-Moreno, J.; Khan, K.S.; Jiménez-Moleón, J.J. Pre-Pregnancy Adherence to the Mediterranean Diet and Gestational Diabetes Mellitus: A Case-Control Study. Nutrients 2019, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, B.; Thanopoulou, A.; Anastasiou, E.; Assaad-Khalil, S.; Albache, N.; Bachaoui, M.; Slama, C.B.; El Ghomari, H.; Jotic, A.; Lalic, N.; et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2014, 68, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Melero, V.; Assaf-Balut, C.; de la Torre, N.G.; Jiménez, I.; Bordiú, E.; del Valle, L.; Valerio, J.; Familiar, C.; Durán, A.; Runkle, I.; et al. Benefits of Adhering to a Mediterranean Diet Supplemented with Extra Virgin Olive Oil and Pistachios in Pregnancy on the Health of Offspring at 2 Years of Age. Results of the San Carlos Gestational Diabetes Mellitus Prevention Study. J. Clin. Med. 2020, 9, 1454. [Google Scholar] [CrossRef] [PubMed]
- Renault, K.M.; Carlsen, E.M.; Nørgaard, K.; Nilas, L.; Pryds, O.; Secher, N.J.; Cortes, D.; Beck Jensen, J.-E.; Olsen, S.F.; Halldorsson, T.I. Intake of carbohydrates during pregnancy in obese women is associated with fat mass in the newborn offspring. Am. J. Clin. Nutr. 2015, 102, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Rifas-Shiman, S.L.; Georgiou, V.; Joung, K.E.; Koinaki, S.; Chalkiadaki, G.; Margioris, A.; Sarri, K.; Vassilaki, M.; Vafeiadi, M.; et al. Adherence to the Mediterranean diet during pregnancy and offspring adiposity and cardiometabolic traits in childhood. Pediatr. Obes. 2017, 12, 47–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.; Fu, W.; Liu, S.; Gong, C.; Dai, J. Mediterranean diet during pregnancy and childhood for asthma in children: A systematic review and meta-analysis of observational studies. Pediatr. Pulmonol. 2019, 54, 949–961. [Google Scholar] [CrossRef]
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain. Behav. Immun. 2020, 87, 53–54. [Google Scholar] [CrossRef]
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartín, A.; Sampedro-Nuñez, M.A.; Dávalos, A.; Marazuela, M. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Mellitus. Nutrients 2020, 12, 2327. [Google Scholar] [CrossRef]
- Munekawa, C.; Hosomi, Y.; Hashimoto, Y.; Okamura, T.; Takahashi, F.; Kawano, R.; Nakajima, H.; Osaka, T.; Okada, H.; Majima, S.; et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: A cross-section and retrospective cohort study. Endocr. J. 2021, 68, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Sankar, P.; Ahmed, W.N.; Mariam Koshy, V.; Jacob, R.; Sasidharan, S. Effects of COVID-19 lockdown on type 2 diabetes, lifestyle and psychosocial health: A hospital-based cross-sectional survey from South India. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Samman, S. Zinc and Redox Signaling: Perturbations Associated with Cardiovascular Disease and Diabetes Mellitus. Antioxid. Redox Signal. 2010, 13, 1549–1573. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.M.M.; Hoenderop, J.G.J.; Bindels, R.J.M.; de Baaij, J.H.F. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Jalali, M.-T.; Shahbazian, N.; Haghighizadeh, M.-H.; Azadegan, E. Comparison of Serum Levels of Vitamin D and Inflammatory Markers Between Women With Gestational Diabetes Mellitus and Healthy Pregnant Control. J. Fam. Reprod. Health 2016, 10, 1–8. [Google Scholar]
- Grissa, O.; Atègbo, J.-M.; Yessoufou, A.; Tabka, Z.; Miled, A.; Jerbi, M.; Dramane, K.L.; Moutairou, K.; Prost, J.; Hichami, A.; et al. Antioxidant status and circulating lipids are altered in human gestational diabetes and macrosomia. Transl. Res. 2007, 150, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, Z.; Pan, Z.; Yang, Y.; Zhang, J. The effects of vitamin and mineral supplementation on women with gestational diabetes mellitus. BMC Endocr. Disord. 2021, 21, 106. [Google Scholar] [CrossRef]
- D’Anna, R.; Scilipoti, A.; Giordano, D.; Caruso, C.; Cannata, M.L.; Interdonato, M.L.; Corrado, F.; Di Benedetto, A. myo -Inositol Supplementation and Onset of Gestational Diabetes Mellitus in Pregnant Women With a Family History of Type 2 Diabetes. Diabetes Care 2013, 36, 854–857. [Google Scholar] [CrossRef]
- D’Anna, R.; Di Benedetto, A.; Scilipoti, A.; Santamaria, A.; Interdonato, M.L.; Petrella, E.; Neri, I.; Pintaudi, B.; Corrado, F.; Facchinetti, F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women. Obstet. Gynecol. 2015, 126, 310–315. [Google Scholar] [CrossRef]
- Crawford, T.J.; Crowther, C.A.; Alsweiler, J.; Brown, J. Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database Syst. Rev. 2015, 2015, CD011507. [Google Scholar] [CrossRef]
- Bizzarri, M.; Carlomagno, G. Inositol: History of an effective therapy for Polycystic Ovary Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1896–1903. [Google Scholar] [PubMed]
- Cabrera-Cruz, H.; Oróstica, L.; Plaza-Parrochia, F.; Torres-Pinto, I.; Romero, C.; Vega, M. The insulin-sensitizing mechanism of myo-inositol is associated with AMPK activation and GLUT-4 expression in human endometrial cells exposed to a PCOS environment. Am. J. Physiol. Metab. 2020, 318, E237–E248. [Google Scholar] [CrossRef] [PubMed]
- Mierzyński, R.; Poniedziałek-Czajkowska, E.; Sotowski, M.; Szydełko-Gorzkowicz, M. Nutrition as Prevention Factor of Gestational Diabetes Mellitus: A Narrative Review. Nutrients 2021, 13, 3787. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Bagheri, N.; Mohammad-Alizadeh-Charandabi, S.; Kashani, N.; Mobaraki-Asl, N.; Mirghafurvand, M.; Asgharian, H.; Ansari, F.; Pourjafar, H. Prevention of Gestational Diabetes Mellitus (GDM) and Probiotics: Mechanism of Action: A Review. Curr. Diabetes Rev. 2020, 16, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Rogozińska, E.; Chamillard, M.; Hitman, G.A.; Khan, K.S.; Thangaratinam, S. Nutritional Manipulation for the Primary Prevention of Gestational Diabetes Mellitus: A Meta-Analysis of Randomised Studies. PLoS ONE 2015, 10, e0115526. [Google Scholar] [CrossRef]
- Bo, S.; Rosato, R.; Ciccone, G.; Canil, S.; Gambino, R.; Poala, C.B.; Leone, F.; Valla, A.; Grassi, G.; Ghigo, E.; et al. Simple lifestyle recommendations and the outcomes of gestational diabetes. A 2×2 factorial randomized trial. Diabetes Obes. Metab. 2014, 16, 1032–1035. [Google Scholar] [CrossRef]
- Padayachee, C. Exercise guidelines for gestational diabetes mellitus. World J. Diabetes 2015, 6, 1033. [Google Scholar] [CrossRef]
- Mitanchez, D.; Ciangura, C.; Jacqueminet, S. How Can Maternal Lifestyle Interventions Modify the Effects of Gestational Diabetes in the Neonate and the Offspring? A Systematic Review of Meta-Analyses. Nutrients 2020, 12, 353. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Sardone, R.; Sila, A.; Giagulli, V.A.; Triggiani, V.; Cincione, R.I.; Giannelli, G.; De Pergola, G. Preliminary Trajectories in Dietary Behaviors during the COVID-19 Pandemic: A Public Health Call to Action to Face Obesity. Int. J. Environ. Res. Public Health 2020, 17, 7073. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Sciomer, S.; Cocchi, C.; Maffei, S.; Gallina, S. Quarantine during COVID-19 outbreak: Changes in diet and physical activity increase the risk of cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ferran, M.; de la Guía-Galipienso, F.; Sanchis-Gomar, F.; Pareja-Galeano, H. Metabolic Impacts of Confinement during the COVID-19 Pandemic Due to Modified Diet and Physical Activity Habits. Nutrients 2020, 12, 1549. [Google Scholar] [CrossRef] [PubMed]
- Ghesquière, L.; Garabedian, C.; Drumez, E.; Lemaître, M.; Cazaubiel, M.; Bengler, C.; Vambergue, A. Effects of COVID-19 pandemic lockdown on gestational diabetes mellitus: A retrospective study. Diabetes Metab. 2021, 47, 101201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef] [PubMed]
- McGurnaghan, S.J.; Weir, A.; Bishop, J.; Kennedy, S.; Blackbourn, L.A.K.; McAllister, D.A.; Hutchinson, S.; Caparrotta, T.M.; Mellor, J.; Jeyam, A.; et al. Risks of and risk factors for COVID-19 disease in people with diabetes: A cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021, 9, 82–93. [Google Scholar] [CrossRef]
- Chun, S.-Y.; Kim, D.W.; Lee, S.A.; Lee, S.J.; Chang, J.H.; Choi, Y.J.; Kim, S.W.; Song, S.O. Does Diabetes Increase the Risk of Contracting COVID-19? A Population-Based Study in Korea. Diabetes Metab. J. 2020, 44, 897–907. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Zhang, J. Insulin Treatment May Increase Adverse Outcomes in Patients With COVID-19 and Diabetes: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 696087. [Google Scholar] [CrossRef]
- Brundage, S.I.; Kirilcuk, N.N.; Lam, J.C.; Spain, D.A.; Zautke, N.A. Insulin Increases the Release of Proinflammatory Mediators. J. Trauma Inj. Infect. Crit. Care 2008, 65, 367–372. [Google Scholar] [CrossRef]
- Filgueiras, L.R.; Capelozzi, V.L.; Martins, J.O.; Jancar, S. Sepsis-induced lung inflammation is modulated by insulin. BMC Pulm. Med. 2014, 14, 177. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence (NICE). Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. Available online: https://www.nice.org.uk/guidance/ng3 (accessed on 25 February 2022).
- American College of Obstetricians and Gynecologists (ACOG). Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet. Gynecol. 2013, 122, 406–416. [Google Scholar] [CrossRef]
- American Diabetes Association (ADA). Standards of Medical Care in Diabetes—2017: Summary of Revisions. Diabetes Care 2017, 40, S4–S5. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Hague, W.M.; Gao, W.; Battin, M.R.; Moore, M.P. Metformin versus Insulin for the Treatment of Gestational Diabetes. N. Engl. J. Med. 2008, 358, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-F.; Chen, X.-Y.; Ding, T.; Wang, X.-F.; Zhu, Z.-N.; Su, S.-W. Comparative Efficacy and Safety of OADs in Management of GDM: Network Meta-analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2015, 100, 2071–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butalia, S.; Gutierrez, L.; Lodha, A.; Aitken, E.; Zakariasen, A.; Donovan, L. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: A systematic review and meta-analysis. Diabet. Med. 2017, 34, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, J.; Martin, M.; He, M.; Gongol, B.; Marin, T.L.; Chen, L.; Shi, X.; Yin, Y.; Shang, F.; et al. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Ciaffi, J.; Landini, M.P.; Meliconi, R. COVID-19 and diabetes: Is metformin a friend or foe? Diabetes Res. Clin. Pract. 2020, 164, 108167. [Google Scholar] [CrossRef] [PubMed]
- Matsiukevich, D.; Piraino, G.; Lahni, P.; Hake, P.W.; Wolfe, V.; O’Connor, M.; James, J.; Zingarelli, B. Metformin ameliorates gender-and age-dependent hemodynamic instability and myocardial injury in murine hemorrhagic shock. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 2680–2691. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, J.H.; Park, Y.H.; Park, S.J.; Cheon, J.H.; Kim, W.H.; Kim, T. Il Sex-dependent difference in the effect of metformin on colorectal cancer-specific mortality of diabetic colorectal cancer patients. World J. Gastroenterol. 2017, 23, 5196. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Cheng, B.; Chen, J.; Peng, A.; Yang, C.; Liu, C.; Xiong, M.; Deng, A.; Zhang, Y.; et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care 2020, 43, 1399–1407. [Google Scholar] [CrossRef]
- Kow, C.S.; Hasan, S.S. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis. J. Med. Virol. 2021, 93, 695–697. [Google Scholar] [CrossRef]
- Lukito, A.A.; Pranata, R.; Henrina, J.; Lim, M.A.; Lawrensia, S.; Suastika, K. The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Yan, P.; Sun, T.; Zeng, Z.; Li, S. Metformin in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 704666. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Rubino, F.; Khunti, K.; Mingrone, G.; Hopkins, D.; Birkenfeld, A.L.; Boehm, B.; Amiel, S.; Holt, R.I.; Skyler, J.S.; et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 546–550. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez Zegarra, R.; Dall’Asta, A.; Revelli, A.; Ghi, T. COVID-19 and Gestational Diabetes: The Role of Nutrition and Pharmacological Intervention in Preventing Adverse Outcomes. Nutrients 2022, 14, 3562. https://doi.org/10.3390/nu14173562
Ramirez Zegarra R, Dall’Asta A, Revelli A, Ghi T. COVID-19 and Gestational Diabetes: The Role of Nutrition and Pharmacological Intervention in Preventing Adverse Outcomes. Nutrients. 2022; 14(17):3562. https://doi.org/10.3390/nu14173562
Chicago/Turabian StyleRamirez Zegarra, Ruben, Andrea Dall’Asta, Alberto Revelli, and Tullio Ghi. 2022. "COVID-19 and Gestational Diabetes: The Role of Nutrition and Pharmacological Intervention in Preventing Adverse Outcomes" Nutrients 14, no. 17: 3562. https://doi.org/10.3390/nu14173562
APA StyleRamirez Zegarra, R., Dall’Asta, A., Revelli, A., & Ghi, T. (2022). COVID-19 and Gestational Diabetes: The Role of Nutrition and Pharmacological Intervention in Preventing Adverse Outcomes. Nutrients, 14(17), 3562. https://doi.org/10.3390/nu14173562





