Insulin-Lowering Diets in Metastatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
3. Review
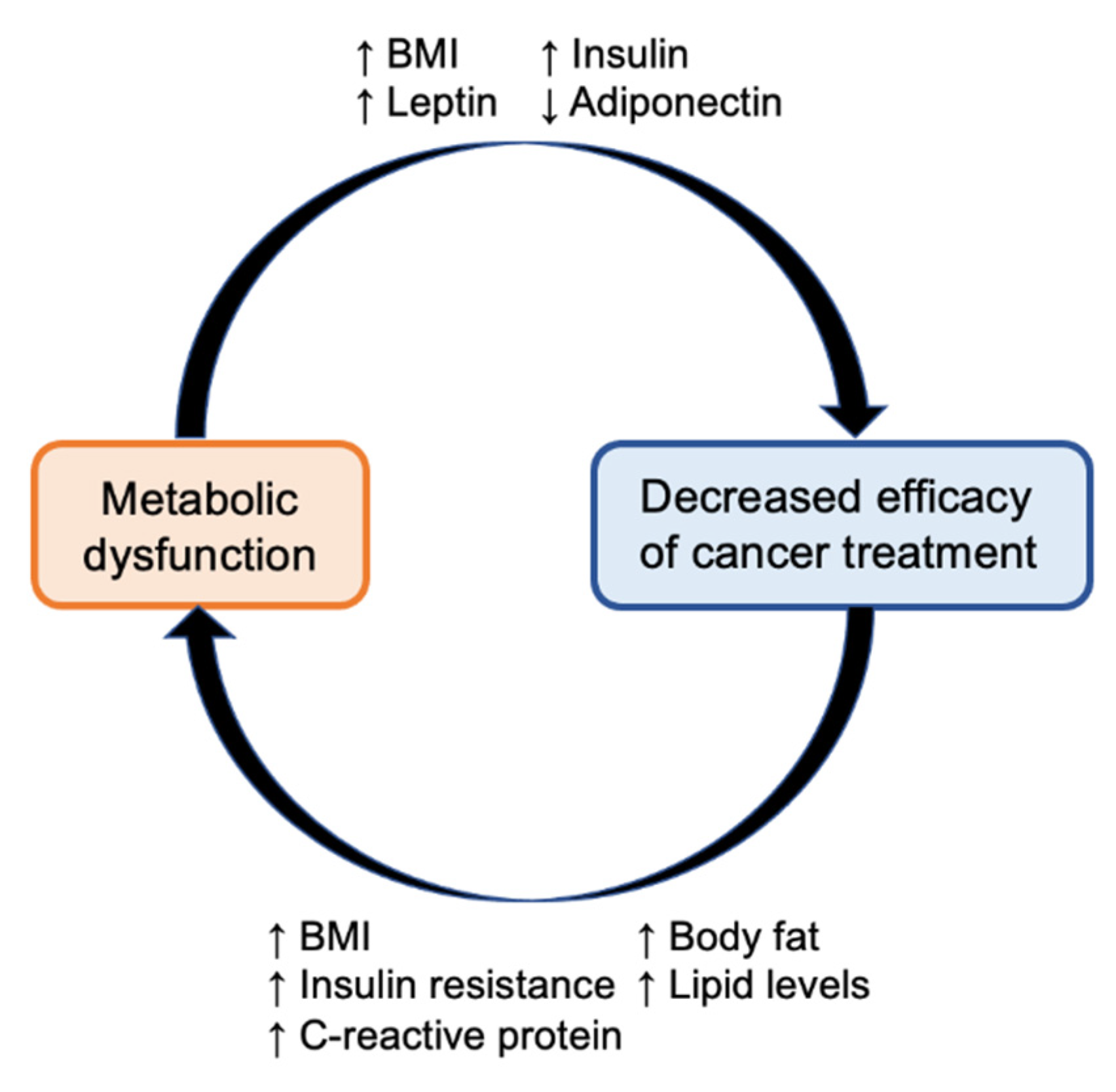
3.1. Insulin-Lowering Diets as an Anti-Cancer Therapeutic Strategy
3.2. Included Studies
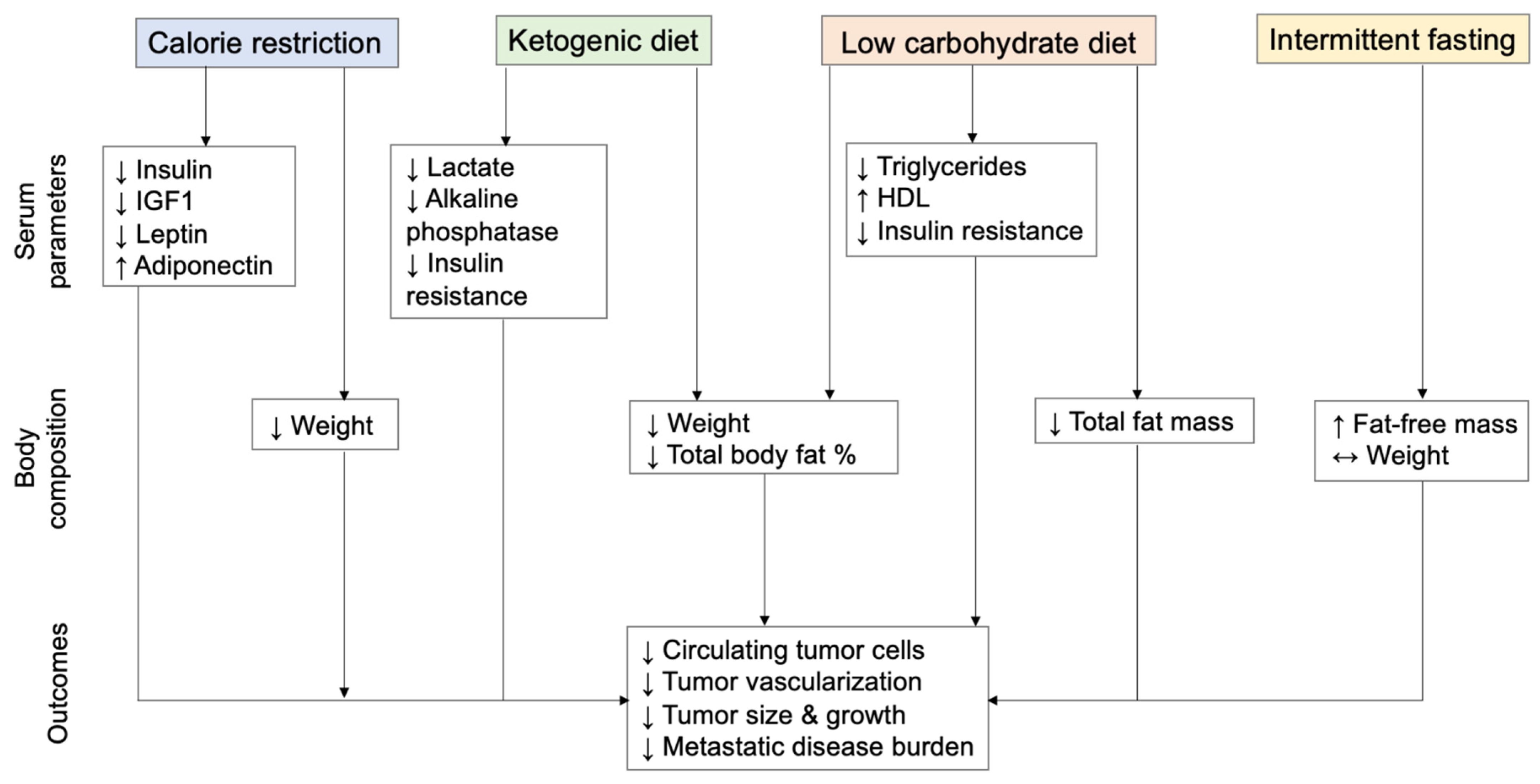
3.3. The Effects of Insulin-Lowering Diets on Metastasis in Animal Models
| Author | Year | Model | Intervention | Findings |
|---|---|---|---|---|
| De Lorenzo [42] | 2011 | Orthotopic 4T1 mammary cancer | 40% CR vs. no restriction | ↓metastatic tumor growth, ↓metastasis formation, ↓insulin, ↓IGF1, ↓leptin, ↑adiponectin |
| Phoenix [43] | 2010 | Orthotopic syngeneic 66cl4 triple-negative breast cancer | 30% CR vs. standard diet vs. diet with high levels of free sugar | ↓tumor growth, ↓number of metastatic lesions |
| Al-Wahab [44] | 2014 | Isogeneic ID8 ovarian cancer | 30% CR vs. regular diet vs. high-energy diet | ↓metastatic tumor burden, ↓size of tumor nodules, ↓ascites volume |
| Poff [45] | 2013 | Syngeneic VM-M3 metastatic cancer | KD vs. standard diet | ↓tumor growth, ↑survival |
| Otto [46] | 2008 | Xenograft 23132/87 gastric adenocarcinoma | KD vs. standard diet | Delayed tumor growth |
| Wang [47] | 2021 | Xenograft 4T1 breast cancer | KD vs. control diet | ↓circulating tumor cells, ↓metastatic disease burden |
| Poff [48] | 2014 | Syngeneic VM-M3 metastatic cancer | Standard diet supplemented with 1,3-butanediol or a ketone ester vs. standard diet alone | ↓proliferation and viability of cells in vitro, ↑survival |
| Ho [49] | 2014 | Transgenic prostate cancer (TRAMP) | 15% carbohydrate content of standard Western diet vs. standard diet | ↓incidence of metastasis formation |
| Chen [52] | 2012 | Xenograft A549 lung, HepG-2 liver, or SKOV-3 ovarian | 1-day fasting/6-day refeeding cycles × 4 weeks vs. control diet | ↑rate of complete tumor regression, ↑survival |
| Das [53] | 2021 | Orthotopic Py230 breast cancer | TRF diet vs. ad libitum-fed diet | ↓tumor cell proliferation, ↓tumor vascularization, ↓tumor growth, ↓lung metastases |
| Bonorden [54] | 2009 | Transgenic prostate cancer (TRAMP) | Intermittent CR (50% consumption × 2 weeks, ad libitum consumption × 2 weeks) vs. ad libitum diet vs. continuous CR | ↑latency period prior to tumor growth/detection, ↑survival |
| Kusuoka [55] | 2018 | Syngeneic CT26 colon cancer | Continuous CR vs. periodic 1-day fasting/6-day refeeding × 4 weeks vs. | ↓cancer stem cells in tumor/blood, ↓tumor weight/metastasis |
| Simone [56] | 2018 | Orthotopic 4T1 breast cancer | CR + chemotherapy vs. ad libitum diet + chemotherapy | ↓lung metastases, ↑survival |
| Zuo [57] | 2022 | Xenograft MCF7-ESR1 breast cancer | FMD + fulvestrant vs. control diet + fulvestrant | ↓metastatic disease burden, ↓visible liver metastases |
3.4. The Effects of Insulin-Lowering Diets in Patients with Metastatic Cancer
4. Discussion
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. Hyperinsulinaemia in cancer. Nat. Rev. Cancer 2020, 20, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch. Physiol. Biochem. 2008, 114, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Wolpin, B.; Giovannucci, E.; Liu, S.; Cochrane, B.; Manson, J.E.; Pollak, M.N.; Ma, J.; Fuchs, C.S. Prediagnostic plasma C-peptide and pancreatic cancer risk in men and women. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2007, 16, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Smith, G.D.; Ebrahim, S. Hyperinsulinaemia and increased risk of breast cancer: Findings from the British Women’s Heart and Health Study. Cancer Causes Control. CCC 2004, 15, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Kim, M.; Caan, B.J.; Chlebowski, R.T.; Gunter, M.J.; Ho, G.Y.; Rodriguez, B.L.; Shikany, J.M.; Strickler, H.D.; Vitolins, M.Z.; et al. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int. J. Cancer J. Int. Du Cancer 2009, 125, 2704–2710. [Google Scholar] [CrossRef]
- Yang, G.; Lu, G.; Jin, F.; Dai, Q.; Best, R.; Shu, X.O.; Chen, J.R.; Pan, X.Y.; Shrubsole, M.; Zheng, W. Population-based, case-control study of blood C-peptide level and breast cancer risk. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2001, 10, 1207–1211. [Google Scholar]
- Jenab, M.; Riboli, E.; Cleveland, R.J.; Norat, T.; Rinaldi, S.; Nieters, A.; Biessy, C.; Tjonneland, A.; Olsen, A.; Overvad, K.; et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer J. Int. Du Cancer 2007, 121, 368–376. [Google Scholar] [CrossRef]
- Ma, J.; Giovannucci, E.; Pollak, M.; Leavitt, A.; Tao, Y.; Gaziano, J.M.; Stampfer, M.J. A prospective study of plasma C-peptide and colorectal cancer risk in men. J. Natl. Cancer Inst. 2004, 96, 546–553. [Google Scholar] [CrossRef]
- Hammarsten, J.; Hogstedt, B. Hyperinsulinaemia: A prospective risk factor for lethal clinical prostate cancer. Eur. J. Cancer 2005, 41, 2887–2895. [Google Scholar] [CrossRef]
- Cust, A.E.; Allen, N.E.; Rinaldi, S.; Dossus, L.; Friedenreich, C.; Olsen, A.; Tjonneland, A.; Overvad, K.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; et al. Serum levels of C-peptide, IGFBP-1 and IGFBP-2 and endometrial cancer risk; results from the European prospective investigation into cancer and nutrition. Int. J. Cancer J. Int. Du Cancer 2007, 120, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Manson, J.E.; Li, J.; Harris, T.G.; Rohan, T.E.; Xue, X.; Ho, G.Y.; et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2008, 17, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lu, J.; Wu, S.; Bi, Y.; Mu, Y.; Zhao, J.; Liu, C.; Chen, L.; Shi, L.; Li, Q.; et al. Association of insulin resistance with breast, ovarian, endometrial and cervical cancers in non-diabetic women. Am. J. Cancer Res. 2016, 6, 2334–2344. [Google Scholar] [PubMed]
- Loftfield, E.; Freedman, N.D.; Lai, G.Y.; Weinstein, S.J.; McGlynn, K.A.; Taylor, P.R.; Mannisto, S.; Albanes, D.; Stolzenberg-Solomon, R.Z. Higher Glucose and Insulin Levels Are Associated with Risk of Liver Cancer and Chronic Liver Disease Mortality among Men without a History of Diabetes. Cancer Prev. Res. 2016, 9, 866–874. [Google Scholar] [CrossRef]
- Perseghin, G.; Calori, G.; Lattuada, G.; Ragogna, F.; Dugnani, E.; Garancini, M.P.; Crosignani, P.; Villa, M.; Bosi, E.; Ruotolo, G.; et al. Insulin resistance/hyperinsulinemia and cancer mortality: The Cremona study at the 15th year of follow-up. Acta Diabetol. 2012, 49, 421–428. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Kajio, H.; Sugiyama, T. Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: A population-based observational study. Int. J. Cancer J. Int. Du Cancer 2017, 141, 102–111. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Zhou, B.; Kontis, V.; Bentham, J.; Gunter, M.J.; Ezzati, M. Worldwide burden of cancer attributable to diabetes and high body-mass index: A comparative risk assessment. Lancet Diabetes Endocrinol. 2018, 6, 95–104. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Poloz, Y.; Stambolic, V. Obesity and cancer, a case for insulin signaling. Cell Death Dis. 2015, 6, e2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.F.; Zhang, G.; Zhao, L.J.; Qi, W.J.; Li, X.P.; Wang, J.L.; Wei, L.H. Overexpression of the insulin receptor isoform A promotes endometrial carcinoma cell growth. PLoS ONE 2013, 8, e69001. [Google Scholar] [CrossRef]
- Frisch, C.M.; Zimmermann, K.; Zillessen, P.; Pfeifer, A.; Racke, K.; Mayer, P. Non-small cell lung cancer cell survival crucially depends on functional insulin receptors. Endocr. Relat. Cancer 2015, 22, 609–621. [Google Scholar] [CrossRef]
- Milazzo, G.; Giorgino, F.; Damante, G.; Sung, C.; Stampfer, M.R.; Vigneri, R.; Goldfine, I.D.; Belfiore, A. Insulin receptor expression and function in human breast cancer cell lines. Cancer Res. 1992, 52, 3924–3930. [Google Scholar]
- Osborne, C.K.; Monaco, M.E.; Lippman, M.E.; Kahn, C.R. Correlation among insulin binding, degradation, and biological activity in human breast cancer cells in long-term tissue culture. Cancer Res. 1978, 38, 94–102. [Google Scholar] [PubMed]
- Zhang, H.; Fagan, D.H.; Zeng, X.; Freeman, K.T.; Sachdev, D.; Yee, D. Inhibition of cancer cell proliferation and metastasis by insulin receptor downregulation. Oncogene 2010, 29, 2517–2527. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.V.; Jacobson, H.I.; Walf, A.A.; Frye, C.A. Estrogen action: A historic perspective on the implications of considering alternative approaches. Physiol. Behav. 2010, 99, 151–162. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.; Dorgan, J.F.; Longcope, C.; Stanczyk, F.Z.; Stephenson, H.E., Jr.; Falk, R.T.; Miller, R.; et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J. Natl. Cancer Inst. 2003, 95, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Goodwin, P.J. Obesity, insulin resistance and breast cancer outcomes. Breast 2015, 24 (Suppl. 2), S56–S59. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.D.; Maddocks, O.D. Engineered diets to improve cancer outcomes. Curr. Opin. Biotechnol. 2021, 70, 29–35. [Google Scholar] [CrossRef]
- Redman, L.M.; Martin, C.K.; Williamson, D.A.; Ravussin, E. Effect of caloric restriction in non-obese humans on physiological, psychological and behavioral outcomes. Physiol. Behav. 2008, 94, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. N. Engl. J. Med. 2013, 369, 145–154. [CrossRef]
- Alpers, D.H. CARBOHYDRATES|Digestion, Absorption, and Metabolism. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 881–887. [Google Scholar]
- Adam-Perrot, A.; Clifton, P.; Brouns, F. Low-carbohydrate diets: Nutritional and physiological aspects. Obes. Rev. 2006, 7, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Fanti, M.; Mishra, A.; Longo, V.D.; Brandhorst, S. Time-Restricted Eating, Intermittent Fasting, and Fasting-Mimicking Diets in Weight Loss. Curr. Obes. Rep. 2021, 10, 70–80. [Google Scholar] [CrossRef]
- de Oliveira Maranhao Pureza, I.R.; da Silva Junior, A.E.; Silva Praxedes, D.R.; Lessa Vasconcelos, L.G.; de Lima Macena, M.; Vieira de Melo, I.S.; de Menezes Toledo Florencio, T.M.; Bueno, N.B. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: A 12-month randomized clinical trial. Clin. Nutr. 2021, 40, 759–766. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L.; et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef]
- De Lorenzo, M.S.; Baljinnyam, E.; Vatner, D.E.; Abarzua, P.; Vatner, S.F.; Rabson, A.B. Caloric restriction reduces growth of mammary tumors and metastases. Carcinogenesis 2011, 32, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, K.N.; Vumbaca, F.; Fox, M.M.; Evans, R.; Claffey, K.P. Dietary energy availability affects primary and metastatic breast cancer and metformin efficacy. Breast Cancer Res. Treat. 2010, 123, 333–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Wahab, Z.; Tebbe, C.; Chhina, J.; Dar, S.A.; Morris, R.T.; Ali-Fehmi, R.; Giri, S.; Munkarah, A.R.; Rattan, R. Dietary energy balance modulates ovarian cancer progression and metastasis. Oncotarget 2014, 5, 6063–6075. [Google Scholar] [CrossRef]
- Poff, A.M.; Ari, C.; Seyfried, T.N.; D’Agostino, D.P. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS ONE 2013, 8, e65522. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Kaemmerer, U.; Illert, B.; Muehling, B.; Pfetzer, N.; Wittig, R.; Voelker, H.U.; Thiede, A.; Coy, J.F. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 2008, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Jia, Z.; Zhang, Y.; Wang, S.; Zhang, H. Evaluation of the Effects of Different Dietary Patterns on Breast Cancer: Monitoring Circulating Tumor Cells. Foods 2021, 10, 2223. [Google Scholar] [CrossRef]
- Poff, A.M.; Ari, C.; Arnold, P.; Seyfried, T.N.; D’Agostino, D.P. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int. J. Cancer J. Int. Du Cancer 2014, 135, 1711–1720. [Google Scholar] [CrossRef]
- Ho, V.W.; Hamilton, M.J.; Dang, N.H.; Hsu, B.E.; Adomat, H.H.; Guns, E.S.; Weljie, A.; Samudio, I.; Bennewith, K.L.; Krystal, G. A low carbohydrate, high protein diet combined with celecoxib markedly reduces metastasis. Carcinogenesis 2014, 35, 2291–2299. [Google Scholar] [CrossRef]
- Chi, J.T.; Lin, P.H.; Tolstikov, V.; Oyekunle, T.; Alvarado, G.C.G.; Ramirez-Torres, A.; Chen, E.Y.; Bussberg, V.; Chi, B.; Greenwood, B.; et al. The influence of low-carbohydrate diets on the metabolic response to androgen-deprivation therapy in prostate cancer. Prostate 2021, 81, 618–628. [Google Scholar] [CrossRef]
- Freedland, S.J.; Howard, L.; Allen, J.; Smith, J.; Stout, J.; Aronson, W.; Inman, B.A.; Armstrong, A.J.; George, D.; Westman, E.; et al. A lifestyle intervention of weight loss via a low-carbohydrate diet plus walking to reduce metabolic disturbances caused by androgen deprivation therapy among prostate cancer patients: Carbohydrate and prostate study 1 (CAPS1) randomized controlled trial. Prostate Cancer Prostatic Dis. 2019, 22, 428–437. [Google Scholar] [CrossRef]
- Chen, X.; Lin, X.; Li, M. Comprehensive modulation of tumor progression and regression with periodic fasting and refeeding circles via boosting IGFBP-3 loops and NK responses. Endocrinology 2012, 153, 4622–4632. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Ellies, L.G.; Kumar, D.; Sauceda, C.; Oberg, A.; Gross, E.; Mandt, T.; Newton, I.G.; Kaur, M.; Sears, D.D.; et al. Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models. Nat. Commun. 2021, 12, 565. [Google Scholar] [CrossRef]
- Bonorden, M.J.; Rogozina, O.P.; Kluczny, C.M.; Grossmann, M.E.; Grambsch, P.L.; Grande, J.P.; Perkins, S.; Lokshin, A.; Cleary, M.P. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr. Cancer 2009, 61, 265–275. [Google Scholar] [CrossRef]
- Kusuoka, O.; Fujiwara-Tani, R.; Nakashima, C.; Fujii, K.; Ohmori, H.; Mori, T.; Kishi, S.; Miyagawa, Y.; Goto, K.; Kawahara, I.; et al. Intermittent calorie restriction enhances epithelial-mesenchymal transition through the alteration of energy metabolism in a mouse tumor model. Int. J. Oncol. 2018, 52, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Simone, B.A.; Palagani, A.; Strickland, K.; Ko, K.; Jin, L.; Lim, M.K.; Dan, T.D.; Sarich, M.; Monti, D.A.; Cristofanilli, M.; et al. Caloric restriction counteracts chemotherapy-induced inflammation and increases response to therapy in a triple negative breast cancer model. Cell Cycle 2018, 17, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Mogol, A.N.; Liu, Y.J.; Santaliz Casiano, A.; Chien, C.; Drnevich, J.; Imir, O.B.; Kulkoyluoglu-Cotul, E.; Park, N.H.; Shapiro, D.J.; et al. Targeting metabolic adaptations in the breast cancer-liver metastatic niche using dietary approaches to improve endocrine therapy efficacy. Mol. Cancer Res. 2022, 20, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, A.; Akbari, M.E.; Mirzaei, H.R.; Seyfried, T.N.; Kalamian, M.; Davoodi, S.H. Effects of Ketogenic metabolic therapy on patients with breast cancer: A randomized controlled clinical trial. Clin. Nutr. 2021, 40, 751–758. [Google Scholar] [CrossRef]
- Khodabakhshi, A.; Akbari, M.E.; Mirzaei, H.R.; Mehrad-Majd, H.; Kalamian, M.; Davoodi, S.H. Feasibility, Safety, and Beneficial Effects of MCT-Based Ketogenic Diet for Breast Cancer Treatment: A Randomized Controlled Trial Study. Nutr. Cancer 2020, 72, 627–634. [Google Scholar] [CrossRef]
- Khodabakhshi, A.; Seyfried, T.N.; Kalamian, M.; Beheshti, M.; Davoodi, S.H. Does a ketogenic diet have beneficial effects on quality of life, physical activity or biomarkers in patients with breast cancer: A randomized controlled clinical trial. Nutr. J. 2020, 19, 87. [Google Scholar] [CrossRef]
- Tan-Shalaby, J.L.; Carrick, J.; Edinger, K.; Genovese, D.; Liman, A.D.; Passero, V.A.; Shah, R.B. Modified Atkins diet in advanced malignancies—Final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr. Metab. 2016, 13, 52. [Google Scholar] [CrossRef]
- Fine, E.J.; Segal-Isaacson, C.J.; Feinman, R.D.; Herszkopf, S.; Romano, M.C.; Tomuta, N.; Bontempo, A.F.; Negassa, A.; Sparano, J.A. Targeting insulin inhibition as a metabolic therapy in advanced cancer: A pilot safety and feasibility dietary trial in 10 patients. Nutrition 2012, 28, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Pfetzer, N.; Schwab, M.; Strauss, I.; Kammerer, U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr. Metab. 2011, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorff, T.B.; Groshen, S.; Garcia, A.; Shah, M.; Tsao-Wei, D.; Pham, H.; Cheng, C.W.; Brandhorst, S.; Cohen, P.; Wei, M.; et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer 2016, 16, 360. [Google Scholar] [CrossRef] [PubMed]
- Iyikesici, M.S. Feasibility study of metabolically supported chemotherapy with weekly carboplatin/paclitaxel combined with ketogenic diet, hyperthermia and hyperbaric oxygen therapy in metastatic non-small cell lung cancer. Int. J. Hyperth. 2019, 36, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Iyikesici, M.S. Long-Term Survival Outcomes of Metabolically Supported Chemotherapy with Gemcitabine-Based or FOLFIRINOX Regimen Combined with Ketogenic Diet, Hyperthermia, and Hyperbaric Oxygen Therapy in Metastatic Pancreatic Cancer. Complement Med. Res. 2020, 27, 31–39. [Google Scholar] [CrossRef]
- Kirkham, A.A.; King, K.; Joy, A.A.; Pelletier, A.B.; Mackey, J.R.; Young, K.; Zhu, X.; Meza-Junco, J.; Basi, S.K.; Hiller, J.P.; et al. Rationale and design of the Diet Restriction and Exercise-induced Adaptations in Metastatic breast cancer (DREAM) study: A 2-arm, parallel-group, phase II, randomized control trial of a short-term, calorie-restricted, and ketogenic diet plus exercise during intravenous chemotherapy versus usual care. BMC Cancer 2021, 21, 1093. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, M.X.; Jiang, L.; Jia, Y.F.; Ying, E.; Cao, H.; Guo, X.Y.; Sun, T. Does a ketogenic diet as an adjuvant therapy for drug treatment enhance chemotherapy sensitivity and reduce target lesions in patients with locally recurrent or metastatic Her-2-negative breast cancer? Study protocol for a randomized controlled trial. Trials 2020, 21, 487. [Google Scholar] [CrossRef]
| Author | Year | N | Study Type | Site | Intervention | Findings |
|---|---|---|---|---|---|---|
| Khodabakhshi [58,59,60] | 2020, 2021 | 80 | RCT | Breast, any stage | Eucaloric KD (6% calories from carbohydrates, 19% from protein, 20% from medium-chain triglycerides, 55% from fat) × 90 days | ↓BMI, ↓weight, ↓fat%, ↓fasting glucose, ↓insulin, ↑QOL, no difference in response rate in metastatic patients |
| Freedland [51] | 2019, 2021 | 42 | RCT | Prostate, any stage | ≤20 g carbohydrate/day diet + walking (≥30 min for ≥5 days/week) × 6 months | ↓weight, ↓fat mass, ↓% body fat, ↓insulin resistance, ↓hemoglobin A1c, ↑HDL, ↓TG, ↓HDL, no differences in PSA |
| Tan-Shalaby [61] | 2016 | 17 | Single-arm safety/feasibility study | Any site, advanced stage | 20–40 g carbohydrates/day diet × 16 weeks | ↓weight, ↑QOL, no unsafe adverse events, 36% achieved SD/PR, compliance was difficult |
| Fine [62] | 2012 | 12 | Single-arm safety/feasibility study | Any site, advanced stage | Carbohydrate dietary restriction to 5% of total kilocalories × 28 days | ↓weight, no unsafe adverse effects, 42% achieved SD/PR; extent of ketosis correlated with response |
| Schmidt [63] | 2011 | 16 | Single-arm feasibility study | Any site, advanced stage | KD (<70 g carbohydrates/day, <20 g carbohydrates/meal) | Only 5/16 patients completed KD × 3 months, others discontinued due to difficult adherence or PD, mixed QOL changes, no severe adverse effects |
| Caffa [41] | 2020 | 36 | 2 safety/feasibility studies | Breast, any stage | Periodic 5-day FMD every 4 weeks | ↑fat-free mass, ↓fat mass, ↓blood glucose, ↓serum IGF1, ↓leptin, ↓C-peptide |
| Dorff [64] | 2016 | 20 | Single-arm safety/feasibility study | Any site, any stage | Fasting for 24, 48, or 72 h before platinum-based chemotherapy × 2 cycles | ↓IGF1 levels, non-significant trend toward less grade 3/4 neutropenia |
| Iyikesici [65] | 2018 | 44 | Retrospective | NSCLC, stage IV | Metabolically supported chemotherapy (fasting- and insulin-induced hypoglycemia, local hyperthermia, hyperbaric oxygen therapy) + KD | ORR 61%, median OS 42.9 months, median PFS 41 months, no significant toxicity or adverse events due to KD |
| Iyikesici [66] | 2020 | 25 | Retrospective | Pancreatic, stage IV | Metabolically supported chemotherapy + KD | Median OS 15.8 months, median PFS 12.9 months, no significant toxicity or adverse events due to KD |
| NCT Number | Cancer Type | Target Accrual | Intervention | Primary and Secondary Endpoints |
|---|---|---|---|---|
| 03795493 [67] | Breast cancer | 50 | CR (50% measured energy requirements) + aerobic exercise × 48–72 h prior to chemotherapy × 6 cycles vs. usual care | Tumor size on CT and MRI, treatment side effects, quality of life, PFS and OS |
| 05090358 | Breast cancer | 106 | KD vs. LCD vs. SGLT2 inhibitor × 12 weeks | Grade 3/4 hyperglycemia-free rate, ORR, PFS, alpelisib adherence and discontinuation rate, changes in systemic hormones and metabolites related to glucose homeostasis, changes in body weight and composition, and QOL measures |
| ChiCTR1900024597 [68] | Breast cancer | 518 | KD + irinotecan vs. normal diet + irinotecan | ORR, sensitivity to irinotecan, PFS, OS, QOL, incidence of grade 3–4 adverse events |
| 04316520 | Renal cell carcinoma | 20 | KD × 1 year | KD tolerance and adverse events, compliance, PFS, OS |
| 05119010 | Renal cell carcinoma | 60 | Continuous KD vs. intermittent KD (15 days on/15 days off) vs. intermittent oral liquid ketone supplement vs. standard diet × 3 months | ORR, grade 3–4 adverse events, weight, albuminemia/prealbuminemia, CRP levels, sarcopenia, QOL, PFS, OS |
| 04631445 | Pancreatic cancer | 40 | KD + triplet chemotherapy vs. normal diet + triplet chemotherapy | PFS, ORR, disease control rate, cancer biomarkers, BMI, insulin, A1c, serum metabolites, QOL |
| 04708860 | Breast cancer | 30 | Prolonged nightly fasting (13 h) × 12 weeks | Feasibility, adherence, metabolic biomarkers, QOL |
| 04387084 | Skin malignancies | 16 | Short-term fasting × 48 h prior to and 24 h after immunotherapy | Safety/feasibility, adherence, adverse events, ORR, immune-related toxicity, QOL, fasting-related biomarkers, immune biomarkers |
| 02710721 | Prostate cancer | 60 | 60-h modified fasting (36 h prior and 24 h after chemotherapy) vs. Mediterranean diet | QOL, differential blood counts, chemotherapy-related adverse effects |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Iyengar, N.M. Insulin-Lowering Diets in Metastatic Cancer. Nutrients 2022, 14, 3542. https://doi.org/10.3390/nu14173542
Shen S, Iyengar NM. Insulin-Lowering Diets in Metastatic Cancer. Nutrients. 2022; 14(17):3542. https://doi.org/10.3390/nu14173542
Chicago/Turabian StyleShen, Sherry, and Neil M. Iyengar. 2022. "Insulin-Lowering Diets in Metastatic Cancer" Nutrients 14, no. 17: 3542. https://doi.org/10.3390/nu14173542
APA StyleShen, S., & Iyengar, N. M. (2022). Insulin-Lowering Diets in Metastatic Cancer. Nutrients, 14(17), 3542. https://doi.org/10.3390/nu14173542







