Nutritional Factors during and after Cancer: Impacts on Survival and Quality of Life
Abstract
:1. Introduction
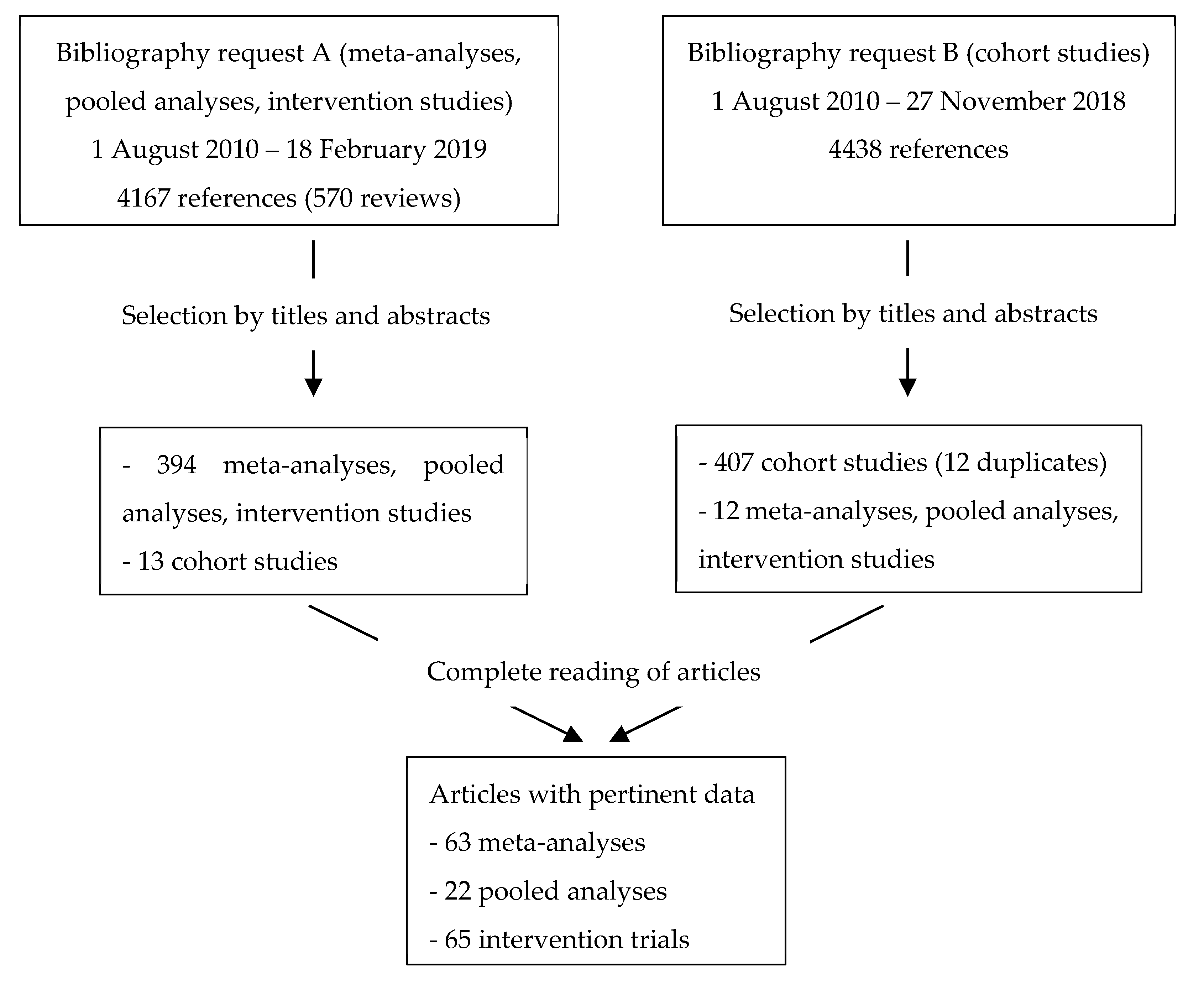
2. Materials and Methods
2.1. Evaluation Process
2.2. Definitions and Criteria for Inclusion/Exclusion of Publications
- -
- Population: cancer survivor or patient under neoplastic treatment.
- -
- Nutritional factors of interest: weight (loss or gain)/body mass index/cachexia/body composition (fat mass, muscle mass, body surface area), dietary patterns (a posteriori and a priori), alcoholic beverages, dietary supplements (vitamins, minerals and other nutrients or plants), foods, medicinal plant or mushroom products, nutritional advice and nutritional advice combined with physical activity.
- -
- Type of study: intervention trials, meta-analyses, pooled analyses published until February 2019. For nutritional factors and cancer sites with insufficient data from meta-analyses, pooled analyses and intervention trials (Request A in Appendix A) cohort studies with more than 300 subjects published until November 2018 (Request B in Appendix A) were included. This was the case for:
- -
- Weight and associated factors: endometrium, cervix, liver, pancreas;
- -
- Weight, undernutrition and associated factors: head and neck, pharynx, nasopharynx;
- -
- Dietary patterns: all cancer sites;
- -
- Alcoholic beverages: all cancer sites except breast;
- -
- Foods: all cancer sites (except soya, fruit and vegetables and fibre for breast cancer only);
- -
- Vitamins and minerals (except vitamin C and antioxidants for breast cancer only) or herbs: all cancer sites;
- -
- Timing of exposure: at or after diagnosis;
- -
- Clinical outcomes: overall mortality, cancer-specific mortality, second primary cancer, cancer progression, cancer recurrence, quality of life.
- -
- Precancerous lesions as outcomes: colonic adenomas, polyps, polycystic ovary syndrome, cervical dysplasia (Cervical Intraepithelial Neoplasia Grade 1 or CIN1);
- -
- Primary prevention studies;
- -
- Animal or in vitro studies;
- -
- Studies in children;
- -
- Studies with nutritional exposure measured before diagnosis; for meta-analyses, publications not distinguishing pre- and post-diagnosis exposures.
- -
- Nutritional factors excluded: therapeutic food modes, chewing gum, water, non-validated criteria for the diagnosis of undernutrition (Inflammatory Nutrition Index, Nutritional Index, Subjective Global Assessment, albumin...); this expertise did not include the impacts of medical nutrition therapy such as enteral/parenteral nutrition, immuno-nutrition, peri-operative nutrition or oral nutritional supplements. In this field, recommendations for the nutritional management of adult cancer patients have been developed [8]. As physical activity during and after cancer has already been the subject of a recent collective expertise report [7], this factor was not included in this work, and only interventions combining nutritional advice and physical activity were considered.
- -
- Clinical outcomes/events excluded: biomarkers (inflammation, albumin, immune function, Prostate-specific antigen, oxidative stress, etc.), side or intermediate effects (diarrhoea, mucositis, intestinal function, arthralgia, neuropathies, pain, weight loss, loss of muscle mass, etc.), pathologic complete response, short-term mortality, biological recurrence, quality of life criteria other than overall quality of life.
- -
- Absence of hazard ratio, relative risk or odds ratio and of their 95% confidence interval.
- -
- Non-randomized intervention trials.
2.3. Bibliographic Queries, Data Extraction and Analyses
2.4. Terminology and Definitions
- -
- Body Surface Area: the external surface area of the skin covering the body (m²). The most common calculation formula is the square root of (weight x height/3600), with weight in kg, height in cm and Body Surface Area in m². The standard is 1.73 m².
- -
- Skeletal Muscle Index: muscle mass index (in cm²/m²), calculated as the ratio of skeletal muscle area (cm²) to the square of body size (m²) or the square of body surface area (m²) depending on the indices used. The cross-sectional skeletal muscle area is measured on a CT scan cross-section at the third lumbar vertebra and is a reliable representation of the total muscle mass of the body.
- -
- Skeletal Muscle radioDensity: muscle radiodensity, the average of the attenuation coefficient in the muscle (expressed in Hounsfield unit, which is measured on a CT scan cross-section at the third lumbar vertebra. The attenuation of the muscle is expressed in relation to that of the water taken as a reference).
3. Results
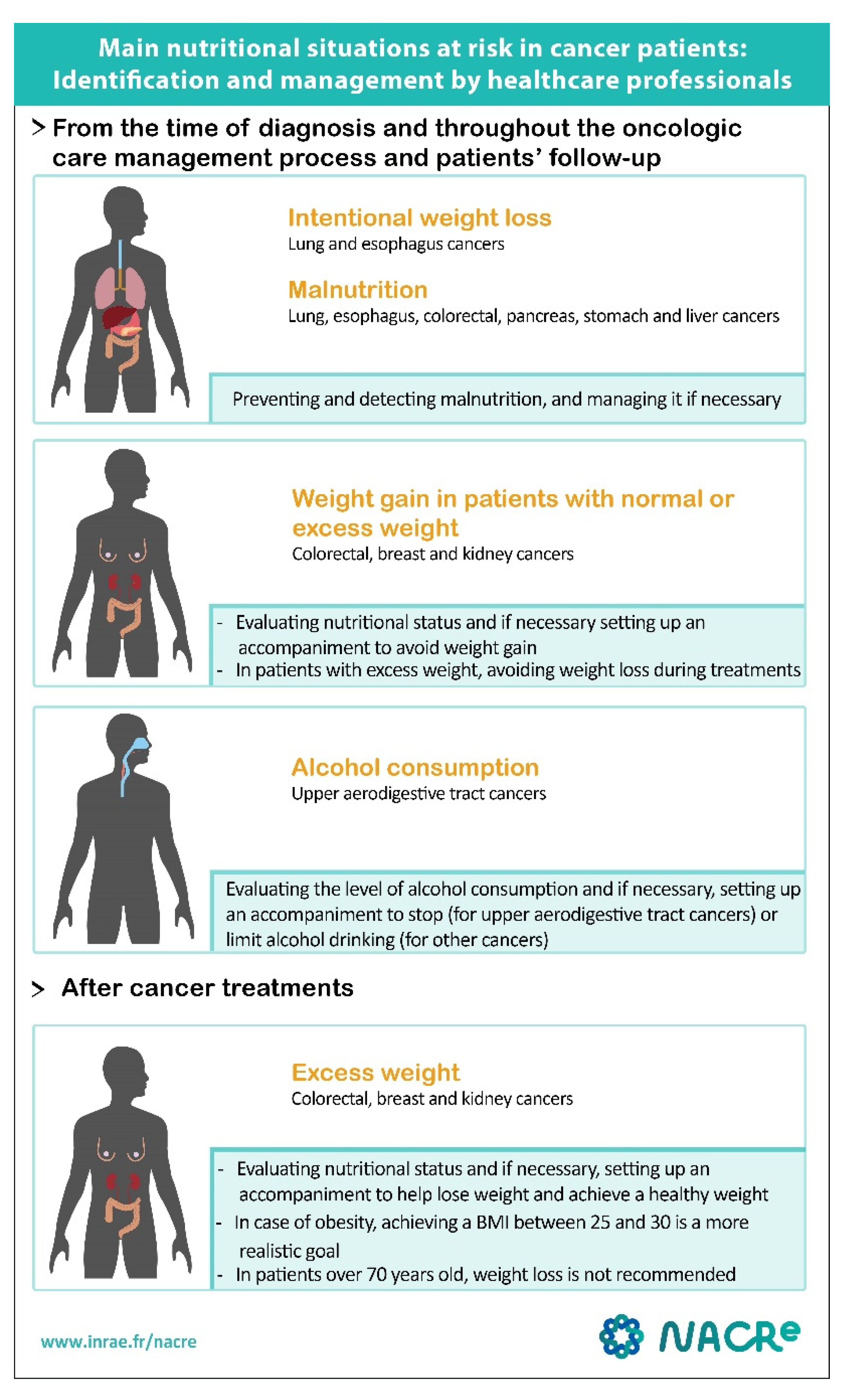
3.1. Overweight and Obesity
3.2. Malnutrition and Sarcopenia
3.3. Alcoholic Beverages
3.4. Dietary Patterns
3.5. Foods
3.6. Dietary Supplements
3.7. Medicinal Plants and Chinese Mushrooms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
Appendix A. Bibliographic Requests
- Request A: #1 AND (#2.1 OR #2.2 OR #2.3 OR #2.4 OR #2.5) AND #3.1 AND #4.1 AND #5
- Request B: #1 AND (#2.1 OR #2.2 OR #2.3 OR #2.4 OR #2.5) AND #3.2 AND #4.2 AND #5
- Request Nutritional advices: #1 AND #2.6 AND #3.1 AND #4.1 AND #5
References
- Institut National du Cancer. Les Cancers en France—L’essentiel des Faits et Chiffres; Edition 2019; Institut National du Cancer: Boulogne-Billancourt, France, 2019. [Google Scholar]
- Institut National du Cancer. Survie Nette Conditionnelle Chez les Personnes Atteintes de Cancer en France Métropolitaine; Institut national du Cancer: Boulogne-Billancourt, France, 2016. [Google Scholar]
- Demark-Wahnefried, W.; Aziz, N.M.; Rowland, J.H.; Pinto, B.M. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J. Clin. Oncol. 2005, 23, 5814–5830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institut National du Cancer. La vie Cinq ans Après un Diagnostic de Cancer; Institut National du Cancer: Boulogne-Billancourt, France, 2018. [Google Scholar]
- Institut National du Cancer. Nutrition et Prévention Primaire des Cancers: Actualisation des Données, Collection État des Lieux et des Connaissances; Institut National du Cancer: Boulogne-Billancourt, France, 2015. [Google Scholar]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- INCa. Bénéfices de l’activité physique pendant et après cancer. In Des Connaissances Scientifiques aux Repères Pratiques—Collection Etats des Lieux et des Connaissances; Institut National du Cancer: Boulogne-Billancourt, France, 2017. [Google Scholar]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Nechuta, S.; Chen, W.Y.; Cai, H.; Poole, E.M.; Kwan, M.L.; Flatt, S.W.; Patterson, R.E.; Pierce, J.P.; Caan, B.; Shu, X.O. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int. J. Cancer 2016, 138, 2088–2097. [Google Scholar] [CrossRef] [Green Version]
- Pajares, B.; Pollán, M.; Martín, M.; Mackey, J.R.; Lluch, A.; Gavila, J.; Vogel, C.; Ruiz-Borrego, M.; Calvo, L.; Pienkowski, T.; et al. Obesity and survival in operable breast cancer patients treated with adjuvant anthracyclines and taxanes according to pathological subtypes: A pooled analysis. Breast Cancer Res. BCR 2013, 15, R105. [Google Scholar] [CrossRef] [Green Version]
- Fontanella, C.; Lederer, B.; Gade, S.; Vanoppen, M.; Blohmer, J.U.; Costa, S.D.; Denkert, C.; Eidtmann, H.; Gerber, B.; Hanusch, C.; et al. Impact of body mass index on neoadjuvant treatment outcome: A pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res. Treat. 2015, 150, 127–139. [Google Scholar] [CrossRef]
- Druesne-Pecollo, N.; Touvier, M.; Barrandon, E.; Chan, D.S.M.; Norat, T.; Zelek, L.; Hercberg, S.; Latino-Martel, P. Excess body weight and second primary cancer risk after breast cancer: A systematic review and meta-analysis of prospective studies. Breast Cancer Res. Treat. 2012, 135, 647–654. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Wang, X.; Li, M.; Gan, Y.; Tang, Y. Association of obesity and overweight with overall survival in colorectal cancer patients: A meta-analysis of 29 studies. Cancer Causes Control 2014, 25, 1489–1502. [Google Scholar] [CrossRef]
- Lee, J.; Meyerhardt, J.A.; Giovannucci, E.; Jeon, J.Y. Association between Body Mass Index and Prognosis of Colorectal Cancer: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0120706. [Google Scholar] [CrossRef] [Green Version]
- Sinicrope, F.A.; Foster, N.R.; Yoon, H.H.; Smyrk, T.C.; Kim, G.P.; Allegra, C.J.; Yothers, G.; Nikcevich, D.A.; Sargent, D.J. Association of Obesity with DNA Mismatch Repair Status and Clinical Outcome in Patients with Stage II or III Colon Carcinoma Participating in NCCTG and NSABP Adjuvant Chemotherapy Trials. J. Clin. Oncol. 2012, 30, 406–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinicrope, F.A.; Ms, N.R.F.; Yothers, G.; Benson, A.; Seitz, J.F.; Labianca, R.; Goldberg, R.M.; Degramont, A.; O’Connell, M.J.; Sargent, D.; et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer 2013, 119, 1528–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chen, Q.; Li, Z.-M.; Xu, X.-D.; Song, A.-F.; Wang, L.S. Association of body mass index with mortality and postoperative survival in renal cell cancer patients, a meta-analysis. Oncotarget 2018, 9, 13959–13970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, L.; Zhang, H.; Zhao, Q.; Han, Y.; Yang, J.; Brain, L. Relation of excess body weight and survival in patients with esophageal adenocarcinoma: A meta-analysis. Dis. Esophagus 2013, 26, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, H.; Zhou, S.; Wang, D.; Zhu, L.; Hou, J.; Tang, J.; Zhao, J.; Zhong, S. Body mass index and mortality in lung cancer patients: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 4–17. [Google Scholar] [CrossRef]
- Wu, X.-S.; Wu, W.-G.; Li, M.-L.; Yang, J.-H.; Ding, Q.-C.; Zhang, L.; Mu, J.-S.; Gu, J.; Dong, P.; Lu, J.-H.; et al. Impact of being overweight on the surgical outcomes of patients with gastric cancer: A meta-analysis. World J. Gastroenterol. 2013, 19, 4596–4606. [Google Scholar] [CrossRef]
- Patel, J.; Pereira, J.; Chen, J.; Liu, J.; Guba, S.; John, W.; Orlando, M.; Scagliotti, G.; Bonomi, P. Relationship between efficacy outcomes and weight gain during treatment of advanced, non-squamous, non-small-cell lung cancer patients. Ann. Oncol. 2016, 27, 1612–1619. [Google Scholar] [CrossRef]
- Renfro, L.A.; Loupakis, F.; Adams, R.; Seymour, M.T.; Heinemann, V.; Schmoll, H.-J.; Douillard, J.-Y.; Hurwitz, H.I.; Fuchs, C.S.; Diaz-Rubio, E.; et al. Body Mass Index Is Prognostic in Metastatic Colorectal Cancer: Pooled Analysis of Patients From First-Line Clinical Trials in the ARCAD Database. J. Clin. Oncol. 2016, 34, 144–150. [Google Scholar] [CrossRef]
- Aparicio, T.; Ducreux, M.; Faroux, R.; Barbier, E.; Manfredi, S.; Lecomte, T.; Etienne, P.-L.; Bedenne, L.; Bennouna, J.; Phelip, J.-M.; et al. Overweight is associated to a better prognosis in metastatic colorectal cancer: A pooled analysis of FFCD trials. Eur. J. Cancer 2018, 98, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Boshier, P.R.; Heneghan, R.; Markar, S.R.; Baracos, V.E.; Low, D.E. Assessment of body composition and sarcopenia in patients with esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2018, 31, doy047. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, J.; Zhang, J.; Zou, S.; Luo, R.; Xu, H.; Huang, B. The Impact of Preoperative Underweight Status on Postoperative Complication and Survival Outcome of Gastric Cancer Patients: A Systematic Review and Meta-analysis. Nutr. Cancer 2018, 70, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Bundred, J.; Tan, B.H.L. Body composition assessment and sarcopenia in patients with gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2018, 22, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.-V.; Chen, J.-D.; Wu, W.-T.; Huang, K.-C.; Hsu, C.-T.; Han, D.-S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2017, 7, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Mintziras, I.; Miligkos, M.; Wächter, S.; Manoharan, J.; Maurer, E.; Bartsch, D.K. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int. J. Surg. 2018, 59, 19–26. [Google Scholar] [CrossRef]
- Ren, G.; Cai, W.; Wang, L.; Huang, J.; Yi, S.; Lu, L.; Wang, J. Impact of body mass index at different transplantation stages on postoperative outcomes in patients with hematological malignancies: A meta-analysis. Bone Marrow Transplant. 2018, 53, 708–721. [Google Scholar] [CrossRef]
- Vrieling, A.; Kampman, E.; Knijnenburg, N.C.; Mulders, P.F.; Sedelaar, J.M.; Baracos, V.E.; Kiemeney, L.A. Body Composition in Relation to Clinical Outcomes in Renal Cell Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 420–434. [Google Scholar] [CrossRef] [Green Version]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Clark, L.H.; Jackson, A.L.; Soo, A.E.; Orrey, D.C.; Gehrig, P.A.; Kim, K.H. Extremes in body mass index affect overall survival in women with cervical cancer. Gynecol. Oncol. 2016, 141, 497–500. [Google Scholar] [CrossRef]
- Kizer, N.T.; Thaker, P.H.; Gao, F.; Zighelboim, I.; Powell, M.A.; Rader, J.S.; Mutch, D.G.; Grigsby, P.W. The effects of body mass index on complications and survival outcomes in patients with cervical carcinoma undergoing curative chemoradiation therapy. Cancer 2010, 117, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Oei, R.W.; Ye, L.; Huang, J.; Kong, F.; Xu, T.; Shen, C.; Wang, X.; He, X.; Kong, L.; Hu, C.-S.; et al. Prognostic value of nutritional markers in nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy: A propensity score matching study. OncoTargets Ther. 2018, 11, 4857–4868. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Shen, L.-J.; Guo, X.; Guo, X.-M.; Qian, C.-N.; Wu, P.-H. Critical weight loss predicts poor prognosis in nasopharyngeal carcinoma. BMC Cancer 2016, 16, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pai, P.-C.; Chuang, C.-C.; Tseng, C.-K.; Tsang, N.-M.; Chang, K.-P.; Yen, T.-C.; Liao, C.-T.; Hong, J.-H.; Chang, J.T.-C. Impact of Pretreatment Body Mass Index on Patients with Head-and-Neck Cancer Treated with Radiation. Int. J. Radiat. Oncol. 2012, 83, e93–e100. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Monteiro-Grillo, I.; Camilo, M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: Long-term follow-up of a randomized controlled trial of nutritional therapy. Am. J. Clin. Nutr. 2012, 96, 1346–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druesne-Pecollo, N.; Keita, Y.; Touvier, M.; Chan, D.S.; Norat, T.; Hercberg, S.; Latino-Martel, P. Alcohol Drinking and Second Primary Cancer Risk in Patients with Upper Aerodigestive Tract Cancers: A Systematic Review and Meta-analysis of Observational Studies. Cancer Epidemiol. Biomark. Prev. 2014, 23, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.-Y.; Xu, S.-Z.; Shen, P. Effect of Low-fat Diet on Breast Cancer Survival: A Meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 1141–1144. [Google Scholar] [CrossRef] [Green Version]
- Chlebowski, R.T.; Aragaki, A.K.; Anderson, G.L.; Thomson, C.A.; Manson, J.E.; Simon, M.S.; Howard, B.V.; Rohan, T.E.; Snetselar, L.; Lane, D.; et al. Low-Fat Dietary Pattern and Breast Cancer Mortality in the Women’s Health Initiative Randomized Controlled Trial. J. Clin. Oncol. 2017, 35, 2919–2926. [Google Scholar] [CrossRef] [Green Version]
- Pettersson, A.; Kasperzyk, J.L.; Kenfield, S.A.; Richman, E.L.; Chan, J.M.; Willett, W.C.; Stampfer, M.J.; Mucci, L.A.; Giovannucci, E.L. Milk and Dairy Consumption among Men with Prostate Cancer and Risk of Metastases and Prostate Cancer Death. Cancer Epidemiol. Biomark. Prev. 2012, 21, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Kenfield, S.A.; Van Blarigan, E.L.; Wilson, K.M.; Batista, J.L.; Sesso, H.D.; Ma, J.; Stampfer, M.J.; Chavarro, J.E. Dairy intake after prostate cancer diagnosis in relation to disease-specific and total mortality. Int. J. Cancer 2015, 137, 2462–2469. [Google Scholar] [CrossRef] [Green Version]
- Richman, E.L.; Kenfield, S.A.; Chavarro, J.E.; Stampfer, M.J.; Giovannucci, E.L.; Willett, W.C.; Chan, J.M. Fat Intake After Diagnosis and Risk of Lethal Prostate Cancer and All-Cause Mortality. JAMA Intern. Med. 2013, 173, 1318–1326. [Google Scholar] [CrossRef] [Green Version]
- Van Blarigan, E.L.; Kenfield, S.A.; Yang, M.; Sesso, H.D.; Ma, J.; Stampfer, M.J.; Chan, J.; Chavarro, J.E. Fat intake after prostate cancer diagnosis and mortality in the Physicians’ Health Study. Cancer Causes Control 2015, 26, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Chi, F.; Wu, R.; Zeng, Y.-C.; Xing, R.; Liu, Y.; Xu, Z.-G. Post-diagnosis Soy Food Intake and Breast Cancer Survival: A Meta-analysis of Cohort Studies. Asian Pac. J. Cancer Prev. 2013, 14, 2407–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AFSSA/AFSSAPS. Sécurité et Bénéfices des Phyto-Estrogènes Apportés par L’alimentation; Recommandations; Rapport et Synthèse Afssa; Afssaps: Nancy, France, 2005. [Google Scholar]
- Académie Nationale de Pharmacie. Les Compléments Alimentaires Contenant des Plantes; Rapport de L’académie Nationale de Pharmacie; Académie Nationale de Pharmacie: Paris, France, 2018. [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Survivors of Breast Cancers. Continuous Update Project Expert Report: Diet, Nutrition, Physical Activity and Breast Cancer Survivors; World Cancer Research Fund/American Institute for Cancer Research: London, UK, 2014. [Google Scholar]
- Guercio, B.J.; Sato, K.; Niedzwiecki, D.; Ye, X.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Coffee Intake, Recurrence, and Mortality in Stage III Colon Cancer: Results from CALGB 89803 (Alliance). J. Clin. Oncol. 2015, 33, 3598–3607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Ding, M.; Yuan, C.; Wu, K.; Smith-Warner, S.A.; Hu, F.B.; Chan, A.T.; Meyerhardt, J.A.; Ogino, S.; Fuchs, C.S.; et al. Association between Coffee Intake after Diagnosis of Colorectal Cancer and Reduced Mortality. Gastroenterology 2018, 154, 916–926.e9. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Orsini, N.; Wolk, A. Vitamin C and survival among women with breast cancer: A Meta-analysis. Eur. J. Cancer 2014, 50, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Poole, E.M.; Shu, X.; Caan, B.J.; Flatt, S.W.; Holmes, M.D.; Lu, W.; Kwan, M.L.; Nechuta, S.J.; Pierce, J.P.; Chen, W.Y. Postdiagnosis supplement use and breast cancer prognosis in the After Breast Cancer Pooling Project. Breast Cancer Res. Treat. 2013, 139, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Chen, Y.; Wang, X.; Li, H.; Zhang, H.; Gong, J.; Shen, S.; Yin, W.; Hu, H. Efficacy and safety of oral branched-chain amino acid supplementation in patients undergoing interventions for hepatocellular carcinoma: A meta-analysis. Nutr. J. 2015, 14, 67. [Google Scholar] [CrossRef] [Green Version]
- Nojiri, S.; Fujiwara, K.; Shinkai, N.; Iio, E.; Joh, T. Effects of branched-chain amino acid supplementation after radiofrequency ablation for hepatocellular carcinoma: A randomized trial. Nutrition 2016, 33, 20–27. [Google Scholar] [CrossRef]
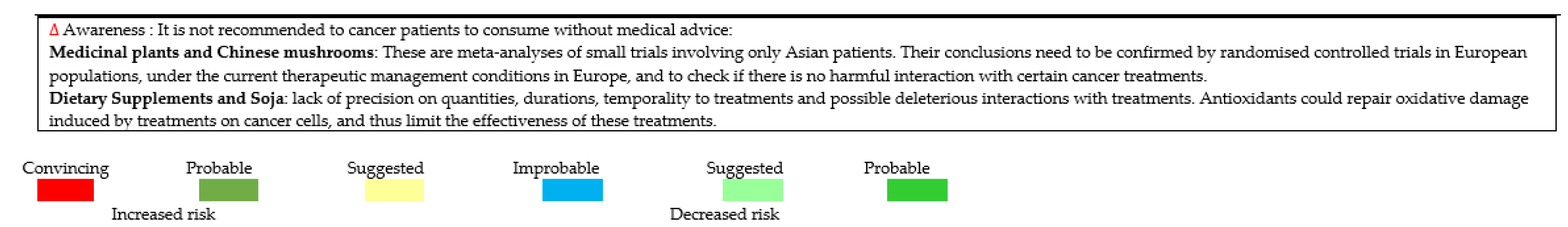
- Asher, G.N.; Corbett, A.H.; Hawke, R.L. Common Herbal Dietary Supplement-Drug Interactions. Am. Fam. Physician 2017, 96, 101–107. [Google Scholar]
- Fasinu, P.S.; Rapp, G.K. Herbal Interaction with Chemotherapeutic Drugs—A Focus on Clinically Significant Findings. Front. Oncol. 2019, 9, 1356. [Google Scholar] [CrossRef] [Green Version]
- Yetley, E.A. Multivitamin and multimineral dietary supplements: Definitions, characterization, bioavailability, and drug interactions. Am. J. Clin. Nutr. 2007, 85, 269S–276S. [Google Scholar] [CrossRef] [Green Version]
- Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus versicolor) on Survival in Cancer Patients: Systematic Review and Meta-Analysis. Recent Patents Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef]
- Shi, Q.; Li, W.; Le, Q.-Q.; Chen, W.-T.; Ren, J.-L.; Li, Q.; Hou, F.-G. Attenuated effects of Jianpi Qushi herbs on patients receiving FOLFOX4 after colorectal cancer surgery: A meta-analysis. Chin. J. Integr. Med. 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, L.; Mao, D.; Peng, W.; Sheng, C.; Ding, C.; Lin, F.; Lei, C.; Zhang, S. Use of Jianpi Jiedu Herbs in Patients with Advanced Colorectal Cancer: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2018, 2018, 6180810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Grade | Criteria Required |
|---|---|
| Convincing | MA or PA of intervention studies or at least 2 intervention studies with:
MA or PA of prospective cohort studies with:
|
| Probable | MA or PA of intervention studies or at least 2 intervention studies with:
MA or PA of prospective studies with:
|
| Suggestive | MA or PA of intervention studies or one intervention with:
MA or PA of prospective studies with:
At least 2 cohort studies with:
|
| Not conclusive |
|
| Improbable | MA or PA of intervention studies or at least 2 intervention studies with:
MA or PA of prospective studies with:
|
| Breast | Colo-rectum | Prostate | Lung | Esophagus | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SPE-M | R | SPC | M | SPE-M | R | P | QoL | M | SPE-M | M | SPE-M | P | M | |||||
| Excess body weight | Overweight | 1 | |||||||||||||||||
| Obesity | 2 | 3 | 4 | ||||||||||||||||
| Overweight + Obesity | |||||||||||||||||||
| Weight gain | |||||||||||||||||||
| Malnutrition | Underweight | ||||||||||||||||||
| Weight loss | |||||||||||||||||||
| Body composition | |||||||||||||||||||
| Sarcopenia | |||||||||||||||||||
| Alcoholic beverages | |||||||||||||||||||
| Foods | Soja | ||||||||||||||||||
| Fibers | |||||||||||||||||||
| Coffee | |||||||||||||||||||
| High-fat dairy products | |||||||||||||||||||
| Saturated Fatty Acids | |||||||||||||||||||
| Vegetable fats | |||||||||||||||||||
| Dietary Patterns | Low-fat diet | ||||||||||||||||||
| Nutritional advices | To limit weight loss | ||||||||||||||||||
| Dietary supplements | Vitamin C | ||||||||||||||||||
| Vitamin D | |||||||||||||||||||
| Vitamin E | |||||||||||||||||||
| Branched chain amino acids | |||||||||||||||||||
| Medicinal plants and Chinese mushrooms | Coriolus versicolor (extracts) | ||||||||||||||||||
| Jianpi Qushi (decoctions) | |||||||||||||||||||
| Jianpi Jiedu (decoctions) | |||||||||||||||||||
| Liver | Pancreas | Stomach | Cervix | Bladder | UADT | Nasopharynx | Solid tumors | Hematological tumors | |||||||||||
| M | R | M | M | SPE-M | R | M | M | R | SPC | M | R | M | SPE-M | R | M | P | |||
| Excess body weight | Overweight | ||||||||||||||||||
| Obesity | |||||||||||||||||||
| Overweight + Obesity | |||||||||||||||||||
| Weight gain | |||||||||||||||||||
| Malnutrition | Underweight | ||||||||||||||||||
| Weight loss | |||||||||||||||||||
| Body composition | |||||||||||||||||||
| Sarcopenia | |||||||||||||||||||
| Alcoholic beverages | |||||||||||||||||||
| Foods | Soja | ||||||||||||||||||
| Fibers | |||||||||||||||||||
| Coffee | |||||||||||||||||||
| High-fat dairy products | |||||||||||||||||||
| Saturated Fatty Acids | |||||||||||||||||||
| Vegetable fats | |||||||||||||||||||
| Dietary Patterns | Low-fat diet | ||||||||||||||||||
| Nutritional advices | To limit weight loss | ||||||||||||||||||
| Dietary supplements | Vitamin C | ||||||||||||||||||
| Vitamin D | |||||||||||||||||||
| Vitamin E | |||||||||||||||||||
| Branched chain amino acids | |||||||||||||||||||
| Medicinal plants and Chinese mushrooms | Coriolus versicolor (extracts) | ||||||||||||||||||
| Jianpi Qushi (decoctions) | |||||||||||||||||||
| Jianpi Jiedu (decoctions) | |||||||||||||||||||
 | |||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas, S.; Cottet, V.; Dossus, L.; Fassier, P.; Ginhac, J.; Latino-Martel, P.; Romieu, I.; Schneider, S.; Srour, B.; Touillaud, M.; et al. Nutritional Factors during and after Cancer: Impacts on Survival and Quality of Life. Nutrients 2022, 14, 2958. https://doi.org/10.3390/nu14142958
Salas S, Cottet V, Dossus L, Fassier P, Ginhac J, Latino-Martel P, Romieu I, Schneider S, Srour B, Touillaud M, et al. Nutritional Factors during and after Cancer: Impacts on Survival and Quality of Life. Nutrients. 2022; 14(14):2958. https://doi.org/10.3390/nu14142958
Chicago/Turabian StyleSalas, Sébastien, Vanessa Cottet, Laure Dossus, Philippine Fassier, Julie Ginhac, Paule Latino-Martel, Isabelle Romieu, Stéphane Schneider, Bernard Srour, Marina Touillaud, and et al. 2022. "Nutritional Factors during and after Cancer: Impacts on Survival and Quality of Life" Nutrients 14, no. 14: 2958. https://doi.org/10.3390/nu14142958
APA StyleSalas, S., Cottet, V., Dossus, L., Fassier, P., Ginhac, J., Latino-Martel, P., Romieu, I., Schneider, S., Srour, B., Touillaud, M., Touvier, M., & Ancellin, R. (2022). Nutritional Factors during and after Cancer: Impacts on Survival and Quality of Life. Nutrients, 14(14), 2958. https://doi.org/10.3390/nu14142958






