Comparing the Effects of Concord Grape (Vitis labrusca L.) Puree, Juice, and Pomace on Intestinal Morphology, Functionality, and Bacterial Populations In Vivo (Gallus gallus)
Abstract
:1. Introduction
2. Materials and Methods
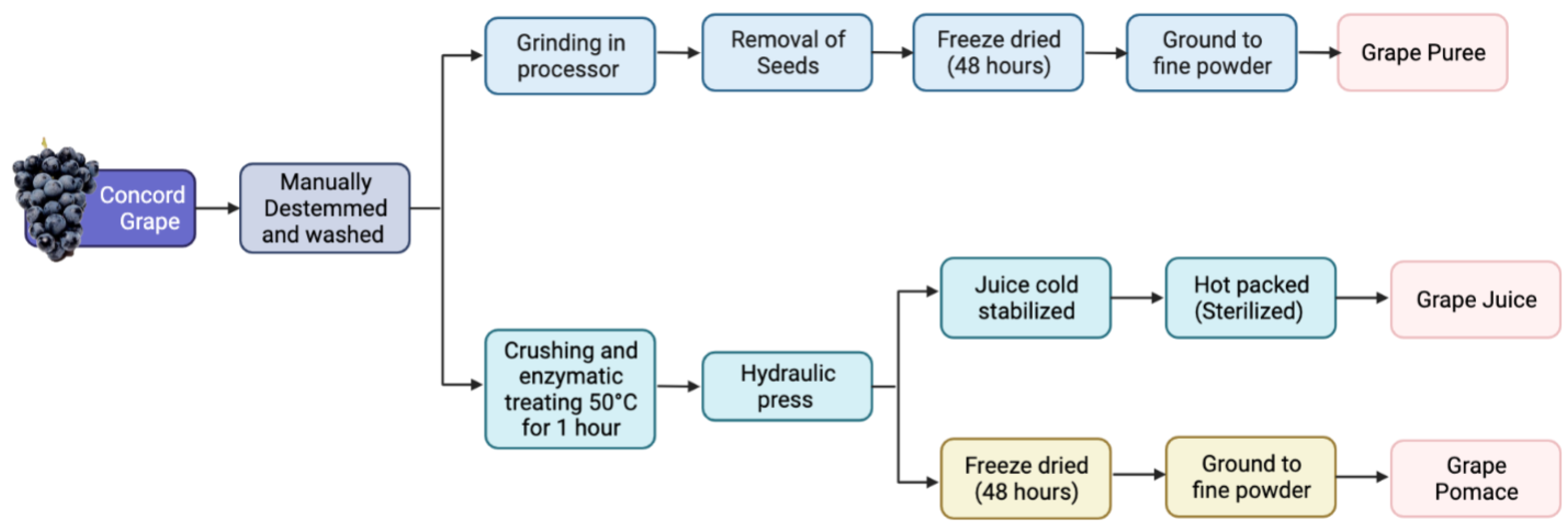
2.1. Sample Preparation
2.2. Polyphenols and Carbohydrate Analysis
2.2.1. Grape Sample Preparation
2.2.2. Polyphenol Analysis
2.2.3. Fibrous and Non-Fibrous Carbohydrate Analysis
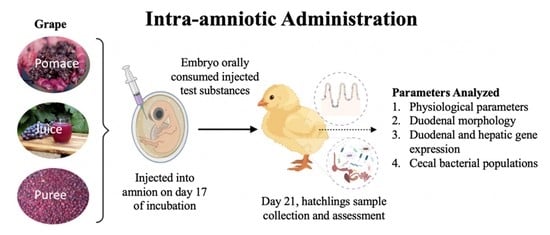
2.3. Animals and Study Design
2.3.1. Water Extract Preparation
2.3.2. Intra-Amniotic Administration and Sample Collection
2.4. Blood Glucose Measurements
2.5. Pectoral Muscle–Glycogen Content
2.6. Gene Expression Analysis
2.6.1. Isolation of Total RNA from Duodenum and Liver Tissue Samples
2.6.2. Real-Time Polymerase Chain Reaction (RT-PCR)
2.6.3. Primer Design
2.6.4. Real-Time qPCR Design
2.7. Intestinal Bacterial Population Assessment
2.7.1. Intestinal Sample Collection and DNA Extraction
2.7.2. Primer Design and PCR Amplification
2.8. Morphometric Examination of Duodenal Tissue
2.9. Statistical Analysis
3. Results
3.1. Polyphenol and Carbohydrate Analysis
3.2. Hatchability and Body Weight
3.3. Blood Glucose and Pectoral Glycogen Analysis
3.4. Duodenal Gene Expression
3.5. Liver Gene Expression
3.6. Duodenal Morphometric Parameters
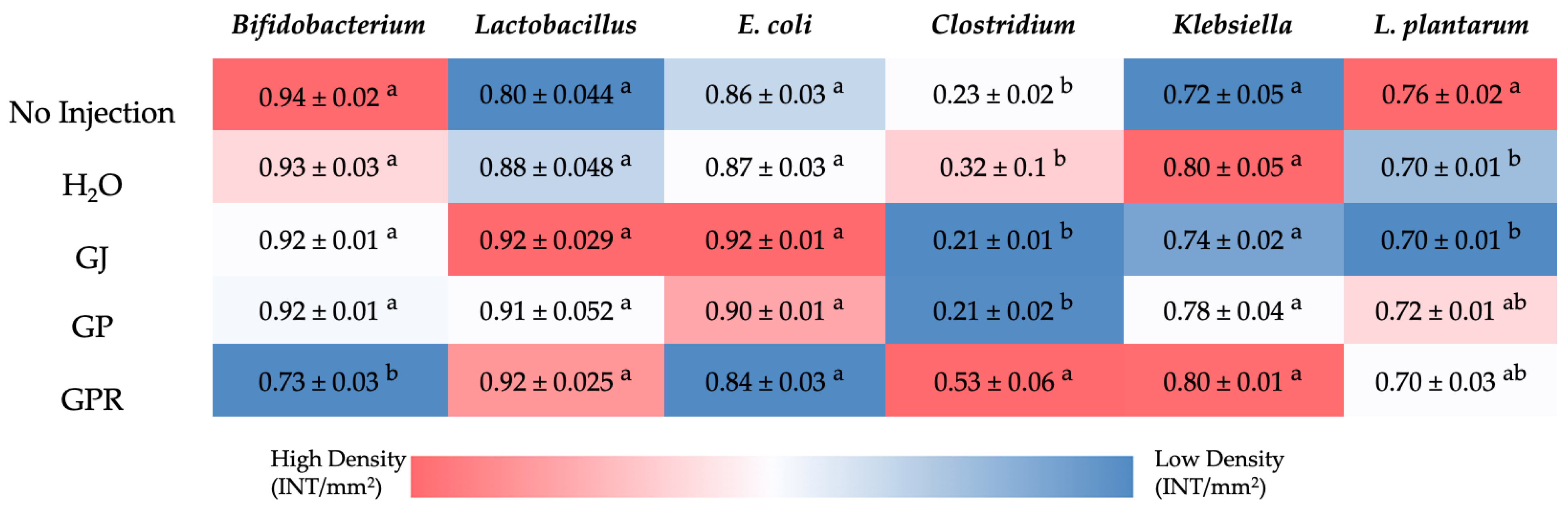
3.7. Analysis of the Gut Bacterial Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Padilla-Zakour, O.I. High Pressure Processing vs. Thermal Pasteurization of Whole Concord Grape Puree: Effect on Nutritional Value, Quality Parameters and Refrigerated Shelf Life. Foods 2021, 10, 2608. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.J.; Gómez, M.I.; Martin, K. Cost of Establishment and Production of Concord Grapes in the Lake Erie Region of New York-2020. 2020. Available online: https://dyson.cornell.edu/wp-content/uploads/sites/5/2020/11/Cost_of_Establishment_and_Production_Concord_Grapes-VD.pdf (accessed on 6 July 2022).
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 August 2022).
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.v.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity—A Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape Seed Extract: Having a Potential Health Benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Gomes, T.M.; Toaldo, I.M.; Haas, I.C.d.S.; Burin, V.M.; Caliari, V.; Luna, A.S.; de Gois, J.S.; Bordignon-Luiz, M.T. Differential Contribution of Grape Peel, Pulp, and Seed to Bioaccessibility of Micronutrients and Major Polyphenolic Compounds of Red and White Grapes through Simulated Human Digestion. J. Funct. Foods 2019, 52, 699–708. [Google Scholar] [CrossRef]
- Abdrabba, S.; Hussein, S. Chemical Composition of Pulp, Seed and Peel of Red Grape from Lybia. Glob. J. Sci. Res. 2015, 3, 6–11. [Google Scholar]
- Shinagawa, F.B.; de Santana, F.C.; Torres, L.R.O.; Mancini-Filho, J. Grape Seed Oil: A Potential Functional Food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current Trends and Possibilities for Exploitation of Grape Pomace as a Potential Source for Value Addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic Content and Antioxidant Capacity of Muscadine Grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Poudel, P.R.; Tamura, H.; Kataoka, I.; Mochioka, R. Phenolic Compounds and Antioxidant Activities of Skins and Seeds of Five Wild Grapes and Two Hybrids Native to Japan. J. Food Compos. Anal. 2008, 21, 622–625. [Google Scholar] [CrossRef]
- Falchi, M.; Bertelli, A.; lo Scalzo, R.; Morassut, M.; Morelli, R.; Das, S.; Cui, J.; Das, D.K. Comparison of Cardioprotective Abilities between the Flesh and Skin of Grapes. J. Agric. Food Chem. 2006, 54, 6613–6622. [Google Scholar] [CrossRef] [PubMed]
- Mohansrinivasan, V.; Devi, C.S.; Deori, M.; Biswas, A.; Naine, S.J. Exploring the Anticancer Activity of Grape Seed Extract on Skin Cancer Cell Lines A431. Braz. Arch. Biol. Technol. 2015, 58, 540–546. [Google Scholar] [CrossRef]
- Morré, D.M.; Morré, D.J. Anticancer Activity of Grape and Grape Skin Extracts Alone and Combined with Green Tea Infusions. Cancer Lett. 2006, 238, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, G.F.; Santos, I.B.; de Bem, G.F.; Cordeiro, V.S.C.; da Costa, C.A.; de Carvalho, L.C.R.M.; Ognibene, D.T.; Resende, A.C.; de Moura, R.S. The Beneficial Effect of Anthocyanidin-Rich Vitis vinifera L. Grape Skin Extract on Metabolic Changes Induced by High-Fat Diet in Mice Involves Antiinflammatory and Antioxidant Actions. Phytother. Res. 2017, 31, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of Extraction Solvent and Temperature on Polyphenol Profiles, Antioxidant and Anti-Inflammatory Effects of Red Grape Skin By-Product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef]
- de Andrade, R.B.; Machado, B.A.S.; Barreto, G.d.A.; Nascimento, R.Q.; Corrêa, L.C.; Leal, I.L.; Tavares, P.P.L.G.; Ferreira, E.d.S.; Umsza-Guez, M.A. Syrah Grape Skin Residues Has Potential as Source of Antioxidant and Anti-Microbial Bioactive Compounds. Biology 2021, 10, 1262. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.v.; Furtado, A.A.L.; Cabral, L.M.C. Grape By-Product Extracts against Microbial Proliferation and Lipid Oxidation: A Review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in Grape Seeds: An Updated Review of Their Health Benefits and Potential Uses in the Food Industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Nowshehri, J.A.; Bhat, Z.A.; Shah, M.Y. Blessings in Disguise: Bio-Functional Benefits of Grape Seed Extracts. Food Res. Int. 2015, 77, 333–348. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds—Biochemistry and Functionality. J. Med. Food 2004, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors Affecting the Composition of the Gut Microbiota, and Its Modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. 2012, 27, 201. [Google Scholar] [CrossRef] [PubMed]
- Brial, F.; le Lay, A.; Dumas, M.E.; Gauguier, D. Implication of Gut Microbiota Metabolites in Cardiovascular and Metabolic Diseases. Cell. Mol. Life Sci. 2018, 75, 3977–3990. [Google Scholar] [CrossRef]
- Solis, A.G.; Klapholz, M.; Zhao, J.; Levy, M. The Bidirectional Nature of Microbiome-Epithelial Cell Interactions. Curr. Opin. Microbiol. 2020, 56, 45. [Google Scholar] [CrossRef]
- Gassler, N. Paneth Cells in Intestinal Physiology and Pathophysiology. World J. Gastrointest. Pathophysiol. 2017, 8, 150. [Google Scholar] [CrossRef]
- Agarwal, N.; Kolba, N.; Jung, Y.; Cheng, J.; Tako, E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus Gallus). Nutrients 2022, 14, 220. [Google Scholar] [CrossRef]
- Agarwal, N.; Kolba, N.; Khen, N.; Even, C.; Turjeman, S.; Koren, O.; Tako, E. Quinoa Soluble Fiber and Quercetin Alter the Composition of the Gut Microbiome and Improve Brush Border Membrane Morphology In Vivo (Gallus Gallus). Nutrients 2022, 14, 448. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Kolba, N.; Agarwal, N.; Kim, D.; Eshel, A.; Koren, O.; Tako, E. Modifications in the Intestinal Functionality, Morphology and Microbiome Following Intra-Amniotic Administration (Gallus Gallus) of Grape (Vitis Vinifera) Stilbenes (Resveratrol and Pterostilbene). Nutrients 2021, 13, 3247. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.T.; Johnson, A.A.T.; Kolba, N.; Bonneau, J.P.; Glahn, R.P.; Ozeri, L.; Koren, O.; Tako, E. Nicotianamine-Chelated Iron Positively Affects Iron Status, Intestinal Morphology and Microbial Populations in Vivo (Gallus Gallus). Sci. Rep. 2020, 10, 2297. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.; Kolba, N.; Tako, E. Low Phytate Peas (Pisum sativum L.) Improve Iron Status, Gut Microbiome, and Brush Border Membrane Functionality in Vivo (Gallus Gallus). Nutrients 2020, 12, 2563. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Kolba, N.; Tako, E. Yacon (Smallanthus Sonchifolius) Flour Soluble Extract Improve Intestinal Bacterial Populations, Brush Border Membrane Functionality and Morphology in Vivo (Gallus Gallus). Food Res. Int. 2020, 137, 109705. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Knez, M.; Uzan, A.; Stangoulis, J.C.R.; Glahn, R.P.; Koren, O.; Tako, E. Alterations in the Gut (Gallus Gallus) Microbiota Following the Consumption of Zinc Biofortified Wheat (Triticum aestivum)-Based Diet. J. Agric. Food Chem. 2018, 66, 6291–6299. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Kolba, N.; Martino, H.S.D.; Hart, J.; Tako, E. Soluble Extracts from Chia Seed (Salvia hispanica L.) Affect Brush Border Membrane Functionality, Morphology and Intestinal Bacterial Populations in Vivo (Gallus Gallus). Nutrients 2019, 11, 2457. [Google Scholar] [CrossRef]
- Carboni, J.; Reed, S.; Kolba, N.; Eshel, A.; Koren, O.; Tako, E. Alterations in the Intestinal Morphology, Gut Microbiota, and Trace Mineral Status Following Intra-Amniotic Administration (Gallus Gallus) of Teff (Eragrostis Tef) Seed Extracts. Nutrients 2020, 12, 3020. [Google Scholar] [CrossRef]
- Pacifici, S.; Song, J.; Zhang, C.; Wang, Q.; Glahn, R.P.; Kolba, N.; Tako, E. Intra Amniotic Administration of Raffinose and Stachyose Affects the Intestinal Brush Border Functionality and Alters Gut Microflora Populations. Nutrients 2017, 9, 304. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Barnes, K.W.; Eisele, T.; Giusti, M.M.; Haché, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. Aoac Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Dreiling, C.E.; Brown, D.E.; Casale, L.; Kelly, L. Muscle Glycogen: Comparison of Iodine Binding and Enzyme Digestion Assays and Application to Meat Samples. Meat Sci. 1987, 20, 167–177. [Google Scholar] [CrossRef]
- Dias, D.; Kolba, N.; Binyamin, D.; Ziv, O.; Regini Nutti, M.; Martino, H.; Glahn, R.; Koren, O.; Tako, E. Iron Biofortified Carioca Bean (Phaseolus vulgaris L.)—Based Brazilian Diet Delivers More Absorbable Iron and Affects the Gut Microbiota In Vivo (Gallus Gallus). Nutrients 2018, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Pisula, A. Detecting a Probiotic Product within the Gut of Broiler Chickens; California Polytechnic State University: San Luis Obispo, CA, USA, 2018. [Google Scholar]
- Zhu, X.Y.; Zhong, T.; Pandya, Y.; Joerger, R.D. 16S RRNA-Based Analysis of Microbiota from the Cecum of Broiler Chickens. Appl. Environ. Microbiol. 2002, 68, 124. [Google Scholar] [CrossRef] [PubMed]
- Amit-Romach, E.; Sklan, D.; Uni, Z. Microflora Ecology of the Chicken Intestine Using 16S Ribosomal DNA Primers. Poult. Sci. 2004, 83, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, P.S.; Schut, F.; Jansen, G.J.; Raangs, G.C.; Kamphuis, G.R.; Wilkinson, M.H.F.; Welling, G.W. Quantitative Fluorescence in Situ Hybridization of Bifidobacterium Spp. with Genus-Specific 16S RRNA-Targeted Probes and Its Application in Fecal Samples. Appl. Environ. Microbiol. 1995, 61, 3069. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Welch, R.M.; Lei, X.; Yasuda, K.; Miller, D.D. Dietary Inulin Affects the Expression of Intestinal Enterocyte Iron Transporters, Receptors and Storage Protein and Alters the Microbiota in the Pig Intestine. Br. J. Nutr. 2008, 99, 472–480. [Google Scholar] [CrossRef]
- Goff, J.P. Invited Review: Mineral Absorption Mechanisms, Mineral Interactions That Affect Acid–Base and Antioxidant Status, and Diet Considerations to Improve Mineral Status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef]
- Reboul, E. Absorption of Vitamin A and Carotenoids by the Enterocyte: Focus on Transport Proteins. Nutrients 2013, 5, 3563. [Google Scholar] [CrossRef]
- Moodi, V.; Abedi, S.; Esmaeilpour, M.; Asbaghi, O.; Izadi, F.; Shirinbakhshmasoleh, M.; Behrouzian, M.; Shahriari, A.; Ghaedi, E.; Miraghajani, M. The Effect of Grapes/Grape Products on Glycemic Response: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2021, 35, 5053–5067. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and Its Metabolic Effects. A Comprehensive Review of Clinical Trials. Metabolism 2018, 83, 102–119. [Google Scholar] [CrossRef]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of Dietary Polyphenol-Rich Plant Products from Grape or Hop on pro-Inflammatory Gene Expression in the Intestine, Nutrient Digestibility and Faecal Microbiota of Weaned Pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimzadeh, S.K.; Navidshad, B.; Farhoomand, P.; Farhoomand, P.; Aghjeh-Gheshlagh, F.M. Effects of Grape Pomace and Vitamin E on Performance, Antioxidant Status, Immune Response, Gut Morphology and Histopathological Responses in Broiler Chickens. S. Afr. J. Anim. Sci. 2018, 48, 324. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G. Effect of Raw and Fermented Grape Pomace on the Growth Performance, Antioxidant Status, Intestinal Morphology, and Selected Bacterial Species in Broiler Chicks. Animals 2021, 11, 364. [Google Scholar] [CrossRef]
- Chee, S.H.; Iji, P.A.; Choct, M.; Mikkelsen, L.L.; Kocher, A. Characterisation and Response of Intestinal Microflora and Mucins to Manno-Oligosaccharide and Antibiotic Supplementation in Broiler Chickens. Br. Poult. Sci. 2010, 51, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of Dietary Fructooligosaccharide on Digestive Enzyme Activities, Intestinal Microflora and Morphology of Male Broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef]
- Hou, Y.; Wei, W.; Guan, X.; Liu, Y.; Bian, G.; He, D.; Fan, Q.; Cai, X.; Zhang, Y.; Wang, G.; et al. A Diet-Microbial Metabolism Feedforward Loop Modulates Intestinal Stem Cell Renewal in the Stressed Gut. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Singh, S.J.; Chaturvedi, R.K. Stem Cells as Potential Targets of Polyphenols in Multiple Sclerosis and Alzheimer’s Disease. BioMed Res. Int. 2018, 2018, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.T.; Centeno, C.; Treviño, J. Tannins in Faba Bean Seeds: Effects on the Digestion of Protein and Amino Acids in Growing Chicks. Anim. Feed. Sci. Technol. 1993, 41, 271–278. [Google Scholar] [CrossRef]
- Academic Publishers, W. Recent Advances of Research in Antinutritional Factors in Legume Seeds and Oilseeds. In Proceedings of the Fourth International Workshop on Antinutritional Factors in Legume Seeds and Oilseeds, Toledo, Spain, March 2004; p. 110. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell Cycle Proteins as Promising Targets in Cancer Therapy. Nat. Rev. Cancer 2017, 17, 93. [Google Scholar] [CrossRef]
- Lu, F.; Li, Y.; Zhou, B.; Guo, Q.; Zhang, Y. Early-Life Supplementation of Grape Polyphenol Extract Promotes Polyphenol Absorption and Modulates the Intestinal Microbiota in Association with the Increase in MRNA Expression of the Key Intestinal Barrier Genes. Food Funct. 2021, 12, 602–613. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Wu, M.L.; Wu, J.; Sun, Y.; Zhang, K.L.; Liu, J. Resveratrol Reverses Retinoic Acid Resistance of Anaplastic Thyroid Cancer Cells via Demethylating CRABP2 Gene. Front. Endocrinol. 2019, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.R.; Mahalakshmi, R. Mitochondrial VDAC2 and Cell Homeostasis: Highlighting Hidden Structural Features and Unique Functionalities. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1843–1858. [Google Scholar] [CrossRef]
- Ungvari, Z.; Sonntag, W.E.; de Cabo, R.; Baur, J.A.; Csiszar, A. Mitochondrial Protection by Resveratrol. Exerc. Sport Sci. Rev. 2011, 39, 128. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Hsu, T.M.; Jones, R.B.; Fodor, A.A.; Goran, M.I.; Kanoski, S.E. Early-Life Sugar Consumption Affects the Rat Microbiome Independently of Obesity. J. Nutr. 2017, 147, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 1–17. [Google Scholar] [CrossRef]
- Magnusson, K.R.; Hauck, L.; Jeffrey, B.M.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L.E. Relationships between Diet-Related Changes in the Gut Microbiome and Cognitive Flexibility. Neuroscience 2015, 300, 128–140. [Google Scholar] [CrossRef]


| Analyte | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Base Pairs Length | GI Number |
|---|---|---|---|---|
| Iron Metabolism | ||||
| DMT1 | TTGATTCAGAGCCTCCCATTAG | GCGAGGAGTAGGCTTGTATTT | 101 | 751817 |
| Ferroportin | CTCAGCAATCACTGGCATCA | ACTGGGCAACTCCAGAAATAAG | 98 | 423984 |
| DcytB | CATGTGCATTCTCTTCCAAAGTC | CTCCTTGGTGACCGCATTAT | 103 | 20380692 |
| Hepcidin * | AGACGACAATGCAGACTAACC | CTGCAGCAATCCCACATTTC | 132 | SAMN08056490 |
| Immune Response | ||||
| NF-κB | CACAGCTGGAGGGAAGTAAAT | TTGAGTAAGGAAGTGAGGTTGAG | 100 | 396033 |
| IL-6 | ACCTCATCCTCCGAGACTTTA | GCACTGAAACTCCTGGTCTT | 105 | 395337 |
| TNF-𝛂 | GACAGCCTATGCCAACAAGTA | TTACAGGAAGGGCAACTCATC | 109 | 374125 |
| Zinc Metabolism | ||||
| ZnT1 | GGTAACAGAGCTGCCTTAACT | GGTAACAGAGCTGCCTTAACT | 105 | 423089 |
| ZnT7 | GGAAGATGTCAGGATGGTTCA | CGAAGGACAAATTGAGGCAAAG | 87 | 424464 |
| ZIP4 | TCTCCTTAGCAGACAATTGAG | GTGACAAACAAGTAGGCGAAAC | 95 | 107050877 |
| ZIP1 | TGCCTCAGTTTCCCTCAC | GGCTCTTAAGGGCACTTCT | 144 | 121112053 |
| Vitamin A Metabolism | ||||
| CRBP2 | GGCTACATGGTTGCACTAGACA | AACCACCCGGTTATCGAGTC | 195 | NM_001277417.1 |
| LRAT | GATTTTGCCTATGGCGGCAG | TTGTCGGTCTGGAAGCTGAC | 197 | 22403 |
| STRA6 * | GTGCGCTGAACTTTGTCTGC | TTCTTCCTGCTCCCGACCT | 116 | 415301 |
| RBP4 * | TGCCACCAACACAGAACTCTC | CTTTGAAGCTGCTCACACGG | 149 | 396454 |
| BBM Functionality | ||||
| VDAC2 | CAGCACTCGCTTTGGAATTG | GTGTAACCCACTCCAACTAGAC | 99 | 395498 |
| SI | CCAGCAATGCCAGCATATTG | CGGTTTCTCCTTACCACTTCTT | 95 | 425007 |
| OCLN | GTCTGTGGGTTCCTCATCGT | GTTCTTCACCCACTCCTCCA | 124 | 396026 |
| MUC6 | CCAACTTGCAGTGTTCCAAAG | CTGACAGTGTAGAGCAAGTACAG | 106 | 414878 |
| 18s rRNA | GCAAGACGAACTAAAGCGAAAG | TCGGAACTACGACGGTATCT | 100 | 7262899 |
| Sample | TPC (mg/g GAE) | MA (CE/g) | ADF (%/DM) | NDF (%/DM) | NFC (%/DM) |
|---|---|---|---|---|---|
| GJ | 2.4 ± 0.00 c | 858 ± 256 c | NA | NA | NA |
| GP | 11.6 ± 0.05 a | 2353 ± 159 b | 41.1 | 43.7 | 29.4 |
| GPR | 7.1 ± 0.30 b | 2544 ± 91 a | 5.7 | 6.3 | 82.8 |
| Treatment Group | Hatch/Injected | Body Weight (g) | Blood Glucose (mg/dL) | Glycogen (mg/g) |
|---|---|---|---|---|
| No Injection | 9/10 | 40.8 ± 1.2 a | 254 ± 24 a | 0.40 ± 0.10 a |
| H2O | 9/10 | 38.3 ± 4.3 a | 234 ± 11 a | 0.30 ± 0.09 a |
| GJ | 13/13 | 38.9 ± 1.7 a | 226 ± 12 a | 0.30 ± 0.06 a |
| GP | 6/10 | 36.8 ± 1.2 a | 314 ± 0.1 a | 0.23 ± 0.11 a |
| GPR | 9/12 | 39.6 ± 0.8 a | 226 ± 12 a | 0.32 ± 0.10 a |
| Treatment Group | Villi Surface Area (µm2) | Crypt Depth (µm) | Paneth Cell Number | Paneth Cell Diameter (µm) |
|---|---|---|---|---|
| No Injection | 164.6 ± 7.7 ᵃ | 22.1 ± 0.8 a | 1.2 ± 0.03 ᵃ | 1.4 ± 0.02 ᵇ |
| H2O | 161.0 ± 3.8 ᵃᵇ | 21.9 ± 0.7 a | 1.0 ± 0.01 ᵇ | 1.5 ± 0.02 ᵃ |
| GJ | 153.3 ± 3.9 ᵃᵇᶜ | 16.2 ± 0.6 ᵇ | 1.3 ± 0.03 ᵃ | 1.4 ± 0.02 ᵇ |
| GP | 145.4 ± 4.3 ᶜ | 13.8 ± 0.5 ᶜ | 1.3 ± 0.04 ᵃ | 1.4 ± 0.02 ᵇ |
| GPR | 148.8± 3.9 ᵇᶜ | 15.4 ± 0.5 bc | 1.3 ± 0.04 ᵃ | 1.4 ± 0.02 ᵇ |
| Treatment Group | Villi Goblet Cell Diameter (µM) | Total Villi Goblet Cell Number | Villus Goblet Cell Type-Number | ||
|---|---|---|---|---|---|
| Acidic | Neutral | Mixed | |||
| No Injection | 3.5 ± 0.07 ᵃ | 20.1 ± 0.60 ᵈ | 18.3 ± 0.58 ᵈ | 0.06 ± 0.02 ᵇ | 1.8 ± 0.14 ᶜ |
| H2O | 3.2 ± 0.06 ᵇᶜ | 33.2 ± 0.73 ᶜ | 29.9 ± 0.69 ᶜ | 0.22 ± 0.04 ᵇ | 3.2 ± 0.20 ᵃ |
| GJ | 3.3 ± 0.07 ᵃᵇ | 41.7 ± 0.95 ᵃ | 39.0 ± 0.87 ᵃ | 0.14 ± 0.04 ᵇ | 2.6 ± 0.23 ᵇ |
| GP | 3.0 ± 0.06 ᶜ | 21.6 ± 1.22 ᵈ | 18.9 ± 1.20 ᵈ | 0.62 ± 0.09 ᵃ | 2.1 ± 0.14 ᵇᶜ |
| GPR | 3.3 ± 0.07 ᵇ | 38.4 ± 0.98 ᵇ | 34.7 ± 0.87 ᵇ | 0.21 ± 0.05 ᵇ | 3.5 ± 0.26 ᵃ |
| Treatment Group | Crypt Goblet Cell Diameter (µM) | Total Crypt Goblet Cell Number | Crypt Goblet Cell Type-Number | ||
|---|---|---|---|---|---|
| Acidic | Neutral | Mixed | |||
| No Injection | 3.0 ± 0.05 ᵇ | 7.0 ± 0.24 ᶜ | 5.8 ± 0.20 c | 0.02 ± 0.02 ᶜ | 1.2 ± 0.1 ᶜ |
| H2O | 2.9 ± 0.05 ᵇ | 8.6 ± 0.32 ᵇ | 6.9 ± 0.28 b | 0.13 ± 0.03 b | 1.5 ± 0.1 bc |
| GJ | 2.9 ± 0.07 ᵇ | 10.6 ± 0.36 ᵃ | 8.0 ± 0.29 a | 0.39 ± 0.05 a | 2.2 ± 0.1 a |
| GP | 2.7 ± 0.05 ᶜ | 8.1 ± 0.27 ᵇ | 6.4 ± 0.25 bc | 0.0 ± 0.0 c | 1.7 ± 0.1 b |
| GPR | 3.3 ± 0.05 ᵃ | 8.3 ± 0.28 ᵇ | 6.7 ± 0.21 ᵇ | 0.0 ± 0.0 c | 1.6 ± 0.1 ᵇ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, N.; Shukla, V.; Kolba, N.; Jackson, C.; Cheng, J.; Padilla-Zakour, O.I.; Tako, E. Comparing the Effects of Concord Grape (Vitis labrusca L.) Puree, Juice, and Pomace on Intestinal Morphology, Functionality, and Bacterial Populations In Vivo (Gallus gallus). Nutrients 2022, 14, 3539. https://doi.org/10.3390/nu14173539
Agarwal N, Shukla V, Kolba N, Jackson C, Cheng J, Padilla-Zakour OI, Tako E. Comparing the Effects of Concord Grape (Vitis labrusca L.) Puree, Juice, and Pomace on Intestinal Morphology, Functionality, and Bacterial Populations In Vivo (Gallus gallus). Nutrients. 2022; 14(17):3539. https://doi.org/10.3390/nu14173539
Chicago/Turabian StyleAgarwal, Nikita, Viral Shukla, Nikolai Kolba, Cydney Jackson, Jacquelyn Cheng, Olga I. Padilla-Zakour, and Elad Tako. 2022. "Comparing the Effects of Concord Grape (Vitis labrusca L.) Puree, Juice, and Pomace on Intestinal Morphology, Functionality, and Bacterial Populations In Vivo (Gallus gallus)" Nutrients 14, no. 17: 3539. https://doi.org/10.3390/nu14173539
APA StyleAgarwal, N., Shukla, V., Kolba, N., Jackson, C., Cheng, J., Padilla-Zakour, O. I., & Tako, E. (2022). Comparing the Effects of Concord Grape (Vitis labrusca L.) Puree, Juice, and Pomace on Intestinal Morphology, Functionality, and Bacterial Populations In Vivo (Gallus gallus). Nutrients, 14(17), 3539. https://doi.org/10.3390/nu14173539