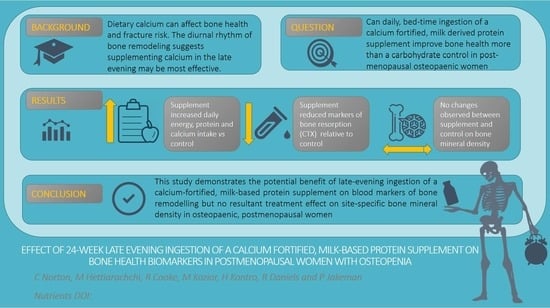
Effect of 24-Week, Late-Evening Ingestion of a Calcium-Fortified, Milk-Based Protein Matrix on Biomarkers of Bone Metabolism and Site-Specific Bone Mineral Density in Postmenopausal Women with Osteopenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
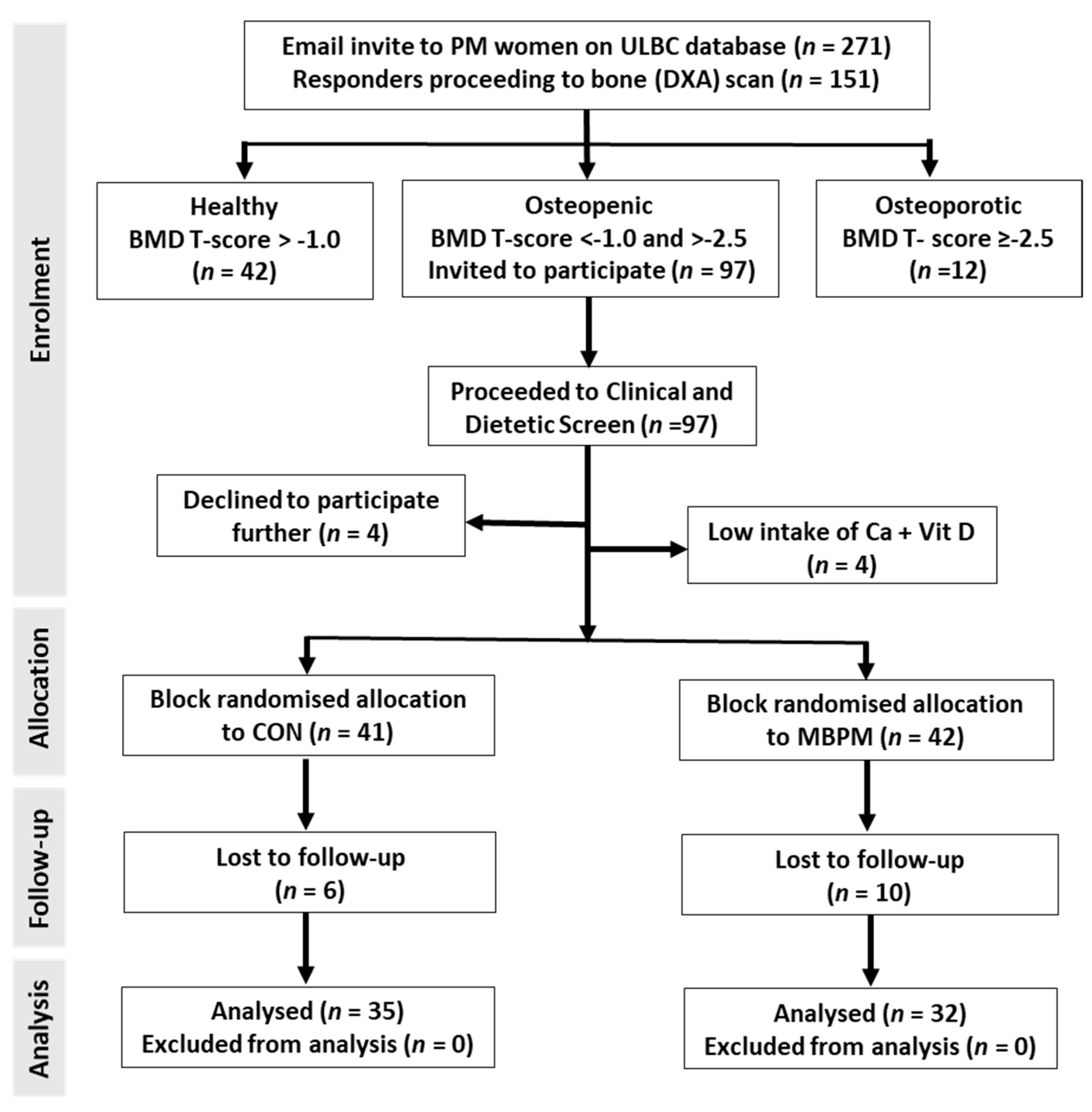
2.2. Participant Recruitment and Progression
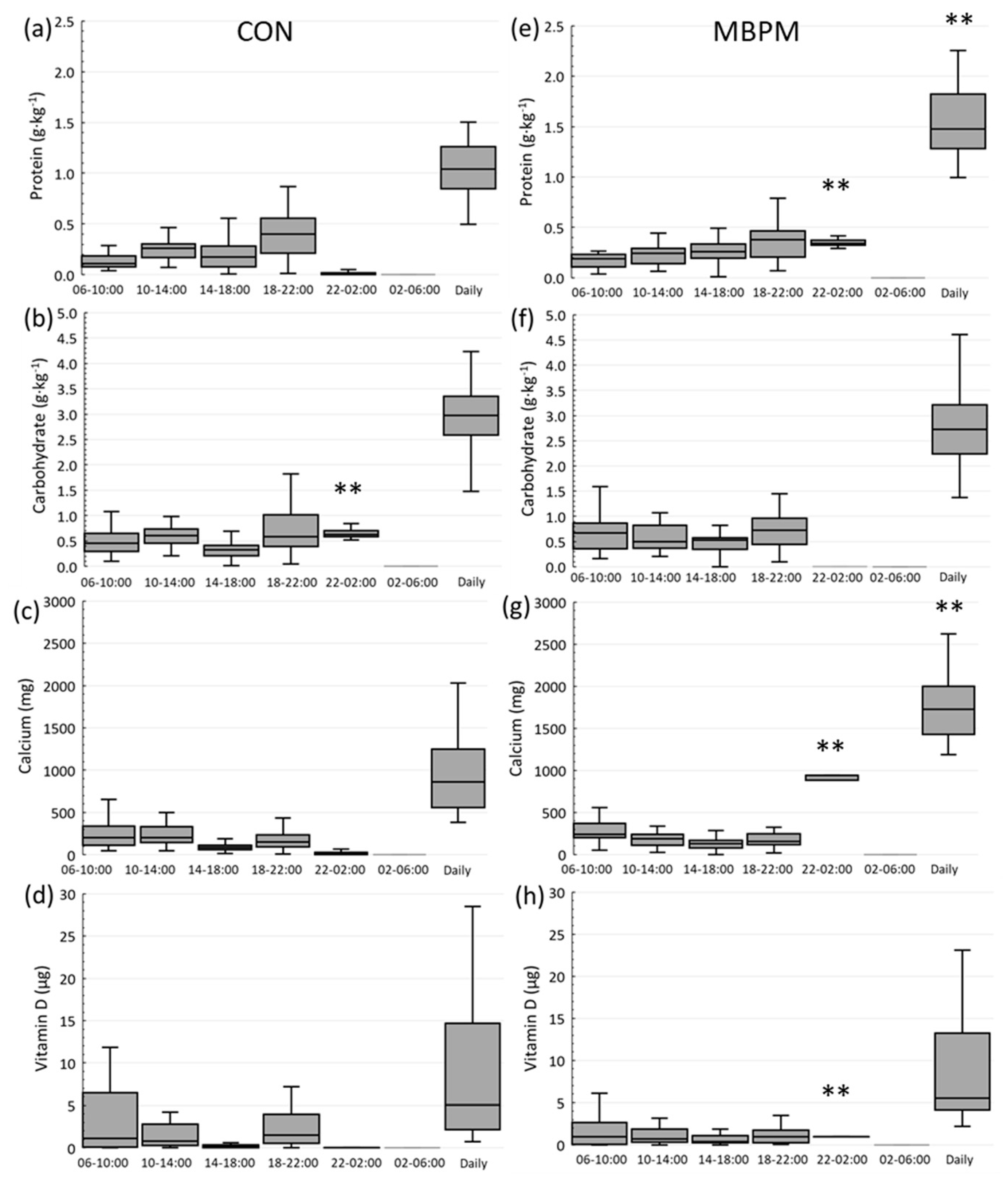
2.3. Mean Daily Intake (MDI) and Supplement Intake
2.4. Anthropometry and Bone Densitometry
2.5. Clinical Biochemistry
2.6. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Habitual and Intervention Dietary Intake
3.3. Vitamin D Status
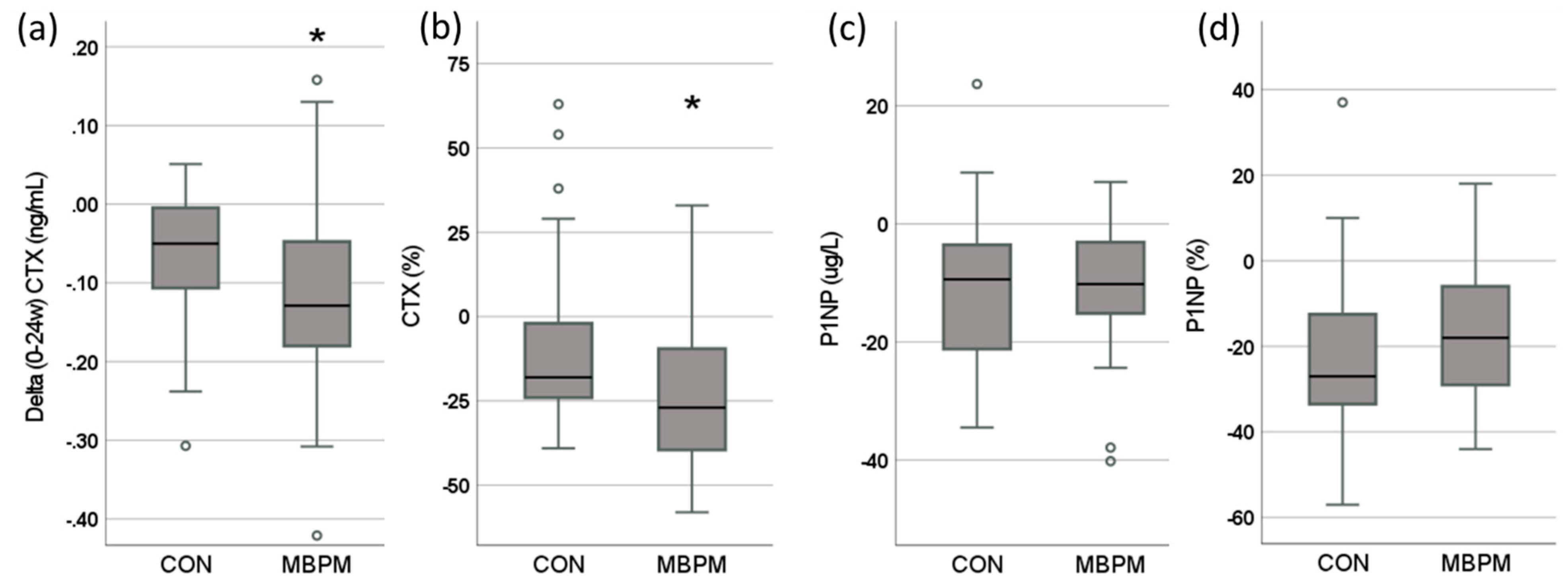
3.4. Change in Biomarkers of Bone Turnover (BTM)
3.5. Bone Mineral Density (BMD) and Trabecular Bone Score (TBS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunt, C.D.; Johnson, L.K. Calcium requirements: New estimations for men and women by cross-sectional statistical analyses of calcium balance data from metabolic studies. Am. J. Clin. Nutr. 2007, 86, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. International Osteoporosis Foundation Calcium Steering C. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Sahni, S.; Chocano-Bedoya, P.; Daly, R.M.; Welch, A.A.; Bischoff-Ferrari, H.; Weaver, C.M. Best Practices for Conducting Observational Research to Assess the Relation between Nutrition and Bone: An International Working Group Summary. Adv. Nutr. 2019, 10, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.G. Calcium intake and bone mass: A quantitative review of the evidence. Calcif. Tissue. Int. 1990, 47, 194–201. [Google Scholar] [CrossRef]
- Shea, B.; Wells, G.; Cranney, A.; Zytaruk, N.; Robinson, V.; Griffith, L.; Ortiz, Z.; Peterson, J.; Adachi, J.; Tugwell, P.; et al. The osteoporosis methodology group and the osteoporosis research advisory group. VII. Meta-Analysis of Calcium Supplementation for the Prevention of Postmenopausal Osteoporosis. Endocr. Rev. 2002, 2, 552–559. [Google Scholar] [CrossRef]
- Nordin, B.E. The effect of calcium supplementation on bone loss in 32 controlled trials in postmenopausal women. Osteoporos. Int. 2009, 20, 2135–2143. [Google Scholar] [CrossRef]
- Anderson, J.J.B.; Roggenkamp, K.J.; Suchindran, C.M. Calcium intakes and femoral and lumbar bone density of elderly US men and women: National Health and Nutrition Examination Survey 2005–2006 Analysis. J. Clin. Endocrinol. Metab. 2012, 97, 4531–4539. [Google Scholar] [CrossRef]
- Bristow, S.M.; Horne, A.M.; Gamble, G.D.; Mihov, B.; Stewart, A.; Reid, I.R. Dietary Calcium Intake and Bone Loss Over 6 Years in Osteopenic Postmenopausal Women. J. Clin. Endocrinol. Metab. 2019, 104, 3576–3584. [Google Scholar] [CrossRef]
- Bjarnason, N.H.; Henriksen, E.E.G.; Alexandersen, P.; Christgau, S.; Henriksen, D.B.; Christiansen, C. Mechanism of circadian variation in bone resorption. Bone 2002, 30, 307–313. [Google Scholar] [CrossRef]
- Qvist, P.; Christgau, S.; Pedersen, B.J.; Schlemmer, A.; Christiansen, C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): Effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone 2002, 31, 57–61. [Google Scholar] [CrossRef]
- Heaney, R.P. Calcium, Dairy Products and Osteoporosis. J. Am. Coll. Nutr. 2013, 19, 83S–99S. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Bertram, H.C.; Bonjour, J.-P.; de Groot, L.; Dupont, D.; Feeney, E.; Ipsen, R.; Lecerf, J.M.; Mackie, A.; McKinley, M.C.; et al. Whole dairy matrix or single nutrients in assessment of health effects: Current evidence and knowledge gaps. Am. J. Clin. Nutr. 2017, 105, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, M.; Cooke, R.; Norton, C.; Jakeman, P. Temporal Change in Biomarkers of Bone Turnover Following Late Evening Ingestion of a Calcium-Fortified, Milk-Based Protein Matrix in Postmenopausal Women with Osteopenia. Nutrients 2019, 11, 1413. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.; Toomey, C.; McCormack, W.G.; Francis, P.; Saunders, J.; Kerin, E.; Jakeman, P. Protein Supplementation at Breakfast and Lunch for 24 Weeks beyond Habitual Intakes Increases Whole-Body Lean Tissue Mass in Healthy Older Adults. J. Nutr. 2016, 146, 65–69. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Schousboe, J.T.; Broy, S.B.; Engelke, K.; Leslie, W.D. Executive Summary of the 2015 ISCD Position Development Conference on Advanced Measures From DXA and QCT: Fracture Prediction Beyond BMD. J. Clin. Densitom. 2015, 18, 274–286. [Google Scholar] [CrossRef]
- Irish Universities Nutrition Alliance. 2011. Available online: http://www.iuna.net/ (accessed on 15 October 2021).
- Hill, T.; O’Brien, M.; Lamberg-Allardt, C.; Jakobsen, J.; Kiely, M.; Flynn, A.; Cashman, K. Vitamin D status of 51–75-year-old Irish women: Its determinants and impact on biochemical indices of bone turnover. Public Health Nutr. 2006, 9, 225–233. [Google Scholar] [CrossRef]
- Vasikaran, S.; Eastell, R.; Bruyère, O.; Foldes, A.J.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T.; et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef]
- Bauer, D.C.; Black, D.M.; Garnero, P.; Hochberg, M.; Ott, S.; Orloff, J.; Thompson, D.E.; Ewing, S.K.; Delmas, P.D. Fracture Intervention Trial Study Group. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: The fracture intervention trial. J. Bone Miner. Res. 2004, 19, 1250–1258. [Google Scholar] [CrossRef]
- Wichers, M.; Schmidt, E.; Bidlingmaier, E.; Klingmüller, D. Diurnal Rhythm of CrossLaps in Human Serum. Clin. Chem. 1999, 45, 1858–1860. [Google Scholar] [CrossRef]
- Brabraj, J.A.; Smith, K.; Cuthbertson, D.J.R.; Rickhuss, P.; Dorling, J.S.; Rennie, M.J. Human bone collagen is a rapid, nutritionally modulated process. J. Bone Miner. Res. 2005, 20, 930–937. [Google Scholar] [CrossRef]
- Smeets, J.S.J.; Horstman, A.M.H.; Vles, G.F.; Emans, P.J.; Goessens, J.P.B.; Gijsen, A.P.; van Kranenburg, J.M.X.; van Loon, L.J.C. Protein synthesis rates of muscle, tendon, ligament, cartilage, and bone tissue in vivo in humans. PLoS ONE 2019, 14, e0224745. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.E.; Jacques, R.M.; Paggiosi, M.; Gossiel, F.; Peel, N.F.; McCloskey, E.V.; Walsh, J.S.; Eastell, R. Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: The TRIO study. Osteoporosis Int. 2016, 27, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. The bone remodelling transient: Interpreting interventions involving bone-related nutrients. Nutr. Rev. 2001, 59, 327–334. [Google Scholar] [CrossRef]
- Zikán, V.; Haas, T.; Stepan, J.J. Acute effects in healthy women of oral calcium on the calcium-parathyroid axis and bone resorption as assessed by serum b-CrossLaps. Calcif. Tissue Int. 2001, 68, 352–357. [Google Scholar] [CrossRef]
- Matkovic, V.; Heaney, R.P. Calcium balance during human growth: Evidence for threshold behavior. Am. J. Clin. Nutr. 1992, 55, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Tai, V.; Leung, W.; Grey, A.; Reid, I.R.; Bolland, M.J. Calcium intake and bone mineral density: Systematic review and meta-analysis. BMJ 2015, 351, h4183. [Google Scholar] [CrossRef]
- Reid, I.R.; Mason, B.; Horne, A.; Ames, R.; Reid, H.E.; Bava, U.; Bolland, M.J.; Gamble, G.D. Randomized controlled trial of calcium in healthy older women. Am. J. Med. 2006, 119, 777–785. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Saville, P.D. Menopausal changes in bone remodelling. J. Lab. Clin. Med. 1978, 92, 964–970. [Google Scholar]
- Winter, E.M.; Kooijman, S.; Appelman-Dijkstra, N.M.; Meijer, O.C.; Rensen, P.C.; Schilperoort, M. Chronobiology and Chronotherapy of Osteoporosis. JBMR Plus 2021, 5, e10504. [Google Scholar] [CrossRef]
- Michalska, D.; Luchavova, M.; Zikan, V.; Raska, I., Jr.; Kubena, A.A.; Stepan, J.J. Effects of morning vs. evening teriparatide injection on bone mineral density and bone turnover markers in postmenopausal osteoporosis. Osteoporosis. Int. 2012, 23, 2885–2891. [Google Scholar] [CrossRef]
| ALL (n = 67) | CON (n = 35) | MBPM (n = 32) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Unit | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range |
| Age | y | 62.8 | 6.0 | 51 to 74 | 63.6 | 6.3 | 51 to 75 | 61.9 | 5.8 | 51 to 74 |
| Height | cm | 161.0 | 5.2 | 148 to 175 | 160.7 | 4.2 | 151 to 171 | 161.3 | 6.1 | 148 to 175 |
| Weight | kg | 67.8 | 11.8 | 44 to 113 | 70.2 | 12.7 | 54 to 113 | 65.3 | 10.4 | 44 to 96 |
| BMI | kg∙m−2 | 26.2 | 4.3 | 18 to 41 | 27.2 | 4.7 | 20 to 41 | 25.1 | 3.6 | 18 to 34 |
| AP Spine (L1–L4) BMD | T-score | −1.5 | 0.4 | −2.4 to −1.0 | −2.3 | 0.4 | −2.3 to −1.0 | −1.5 | 0.4 | −2.4 to −1.0 |
| Trabecular Bone Score | T-Score | −1.7 | 0.9 | −3.7 to 0.1 | −1.5 | 0.8 | −3.1 to 0.1 | −2.0 | 0.9 | −3.7 to −0.5 |
| Average Habitual Daily Intake in CON Group | |||||||
|---|---|---|---|---|---|---|---|
| Energy (kcal·kg−1) | Protein (g·kg−1) | Carbohydrate (g·kg−1) | Fat (g·kg−1) | Protein (g) | Calcium (mg) | Vitamin D (ug) | |
| Mean | 22 | 1.0 | 2.4 | 0.8 | 73 | 951 | 13.3 |
| SD | 5 | 0.3 | 0.7 | 0.3 | 14 | 473 | 18.2 |
| Min | 10 | 0.5 | 1 | 0.3 | 43 | 384 | 1.0 |
| Max | 34 | 1.5 | 4.3 | 1.5 | 110 | 2032 | 80.0 |
| Average Habitual Daily Intake in MBPM Group | |||||||
| Mean | 25 | 1.2 | 2.8 | 0.9 | 75 | 957 | 11.6 |
| SD | 7 | 0.4 | 0.8 | 0.3 | 19 | 514 | 15.0 |
| Min | 12 | 0.7 | 1.4 | 0.3 | 53 | 505 | 1.0 |
| Max | 39 | 1.9 | 4.6 | 1.6 | 122 | 2805 | 62.0 |
| AP Spine BMD (g∙cm−2) | Dual Femur BMD (g∙cm−2) | TBS (g∙cm−2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | ∆0–24 | Week | ∆0–24 | Week | ∆0–24 | ||||||||
| 0 | 24 | g∙cm−2 | % | 0 | 24 | g∙cm−2 | % | 0 | 24 | g∙cm−2 | % | ||
| CON | mean | 1.016 | 1.017 | 0.001 | 0.10 | 0.910 | 0.904 | −0.004 | −0.47 | 1.339 | 1.329 | −0.009 | −0.72 |
| CI95% | 0.024 | 0.027 | 0.010 | 0.97 | 0.029 | 0.030 | 0.004 | 0.43 | 0.028 | 0.033 | 0.013 | 0.99 | |
| MBPM | mean | 1.041 | 1.030 | −0.005 | −0.51 | 0.897 | 0.903 | 0.0002 | 0.01 | 1.308 | 1.304 | −0.004 | −0.31 |
| CI95% | 0.043 | 0.040 | 0.011 | 0.97 | 0.035 | 0.036 | 0.004 | 0.48 | 0.032 | 0.035 | 0.017 | 1.32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, C.; Hettiarachchi, M.; Cooke, R.; Kozior, M.; Kontro, H.; Daniel, R.; Jakeman, P. Effect of 24-Week, Late-Evening Ingestion of a Calcium-Fortified, Milk-Based Protein Matrix on Biomarkers of Bone Metabolism and Site-Specific Bone Mineral Density in Postmenopausal Women with Osteopenia. Nutrients 2022, 14, 3486. https://doi.org/10.3390/nu14173486
Norton C, Hettiarachchi M, Cooke R, Kozior M, Kontro H, Daniel R, Jakeman P. Effect of 24-Week, Late-Evening Ingestion of a Calcium-Fortified, Milk-Based Protein Matrix on Biomarkers of Bone Metabolism and Site-Specific Bone Mineral Density in Postmenopausal Women with Osteopenia. Nutrients. 2022; 14(17):3486. https://doi.org/10.3390/nu14173486
Chicago/Turabian StyleNorton, Catherine, Manjula Hettiarachchi, Rachel Cooke, Marta Kozior, Hilkka Kontro, Rosemary Daniel, and Philip Jakeman. 2022. "Effect of 24-Week, Late-Evening Ingestion of a Calcium-Fortified, Milk-Based Protein Matrix on Biomarkers of Bone Metabolism and Site-Specific Bone Mineral Density in Postmenopausal Women with Osteopenia" Nutrients 14, no. 17: 3486. https://doi.org/10.3390/nu14173486
APA StyleNorton, C., Hettiarachchi, M., Cooke, R., Kozior, M., Kontro, H., Daniel, R., & Jakeman, P. (2022). Effect of 24-Week, Late-Evening Ingestion of a Calcium-Fortified, Milk-Based Protein Matrix on Biomarkers of Bone Metabolism and Site-Specific Bone Mineral Density in Postmenopausal Women with Osteopenia. Nutrients, 14(17), 3486. https://doi.org/10.3390/nu14173486