Abstract
There is limited evidence to support the relationship between the consumption of animal-source foods other than red meat and processed meat and colorectal cancer (CRC) risk. We aimed to examine the recent available evidence from observational studies about the association between these food groups’ intake and CRC risk. For this systematic review, we searched the PubMed database for the last five years. A total of fourteen cohort studies and seven case–control studies comprising a total of >60,000 cases were included. The studies showed a consistent significant decrease in CRC risk, overall and by subsites, associated with a high consumption of total dairy products. Less strong effects associated with the consumption of any subtype of dairy product were observed. Fish consumption, overall and by subtypes (oily or non-oily and fresh or canned), showed a mild inverse association with CRC risk. The association between white meat and egg intake and CRC risk was low and based on a small number of studies; thus, these findings should be interpreted with caution. In conclusion, a high consumption of total dairy products was associated with a lower CRC risk. However, evidence for fish, white meat, and eggs and the CRC risk were not as strong.
Keywords:
adults; case–control studies; colorectal cancer; dairy; eggs; fish; poultry; cohort studies; systematic review; white meat 1. Introduction
Colorectal cancer (CRC) is the third-most frequent cancer and the second-highest mortality in cancer patients worldwide [1]. The global cancer statistics in 2020 showed there were about 1932 million new cases and 935,000 deaths of CRC worldwide, accounting for 10.0% of the total new cases of cancer and 9.4% of the total cancer-related deaths, respectively [1].
During recent years, the mortality rates for CRC have been decreasing due to early screening programs [2,3] and better treatment options [4]. However, the aetiology of CRC is complex and still not fully understood. Both genetic and environmental factors play an important role in the aetiology of this disease [5]. Environmental and, in particular, diet and lifestyle factors are likely to be the main determinants of CRC development [6].
There is considerable evidence to suggest that the consumption of a diet with high intakes of vegetables, fruits, and whole grains may decrease the risk of CRC and that the consumption of processed meat and alcohol are risk factors for this type of cancer [7]. In addition, the Mediterranean dietary pattern could reduce the overall cancer risk [8] and, in particular, CRC risk [9]. On the other hand, recently, CRC innovative treatments deriving from natural extracts, termed nutraceuticals, have gained interest [10]. In this sense, for example, anti-inflammatory and reparative properties have been attributed to the nutraceutical grape seed extract [11].
However, evidence for the consumption of animal-source foods other than red meat and processed meat, as is the case with dairy products, fish, white meat, and eggs, is not as strong [7]. The latest report from the Continuous Update Project (CUP), led by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), concluded that there is strong evidence that consuming dairy products decreases the risk of cancer [7]. However, the evidence suggesting that the consumption of different types of dairy products (i.e., milk, yogurt, cheese or subtypes by fat content) is limited [12]. Regarding fish consumption, the evidence suggesting that the consumption of fish decreases the risk of CRC is limited but generally consistent [7], although the risk of CRC associated with the consumption of different types of fish (oily or non-oily fish) remains unclear [13]. In addition, to date, studies on the possible association between white meat (such as poultry—chicken, turkey, duck, and goose—and rabbit) or eggs and CRC risk are scarce, and the results are unclear [14].
Even if the most current dietary guidelines advocate the consumption of moderate intakes of low-fat dairy foods, fish, white meats, and eggs, in the context of a healthy diet to prevent chronic disease [15,16], the American Cancer Society does not issue specific recommendations on the consumption of these food groups for cancer prevention. Therefore, further research on the association between the consumption of these foods and CRC risk should be of considerable interest in terms of public health. A better understanding of the impact of these categories of animal-source foods would be of great help to make recommendations regarding these products.
This systematic review aims to examine the recent available evidence from observational studies (cohorts and case–control) in adults about the association between the consumption of animal-source foods other than red meat and processed meat, as is the case with dairy products, fish, white meat, and eggs, and the CRC risk. We also investigated associations with specific types of these food groups (e.g., whole-fat dairy products or canned fish, and whether the associations with CRC risk depended on the CRC subsite (colon or rectal and colon cancer location (proximal or distal colon)). The reason the review was based on the publications during the last five years rather than the entire past is that the latest report from the CUP about diet, nutrition, physical activity, and CRC was revised in 2018 [7].
2. Methods
2.1. Search Strategy
A systematic search was conducted in the PubMed database from the last five years (from 1 January 2018 to 15 July 2022) for published case–control or cohort studies evaluating the associations between the consumption of dairy products, fish, white meat, or eggs and CRC (total CRC, colon cancer (CC), or rectal cancer (RC) and proximal or distal colon cancer). For this purpose, a search for the relevant keywords and medical subject heading terms related to the consumption of the abovementioned food groups and subtypes in combination with the keywords related to CRC events was conducted. The search was expanded through citation chaining (forward and backward) of the included studies. Reference lists of all the identified articles and other related review articles, systematic reviews, and meta-analyses were hand-searched for additional articles. The present search was developed according to the “PRISMA Statement” guidelines (see the PRISMA checklist in the Supplementary Materials) (www.prisma-statement.org, accessed on 3 May 2022). For this review, a protocol was not prepared or registered.
Next, the search strategy is detailed:
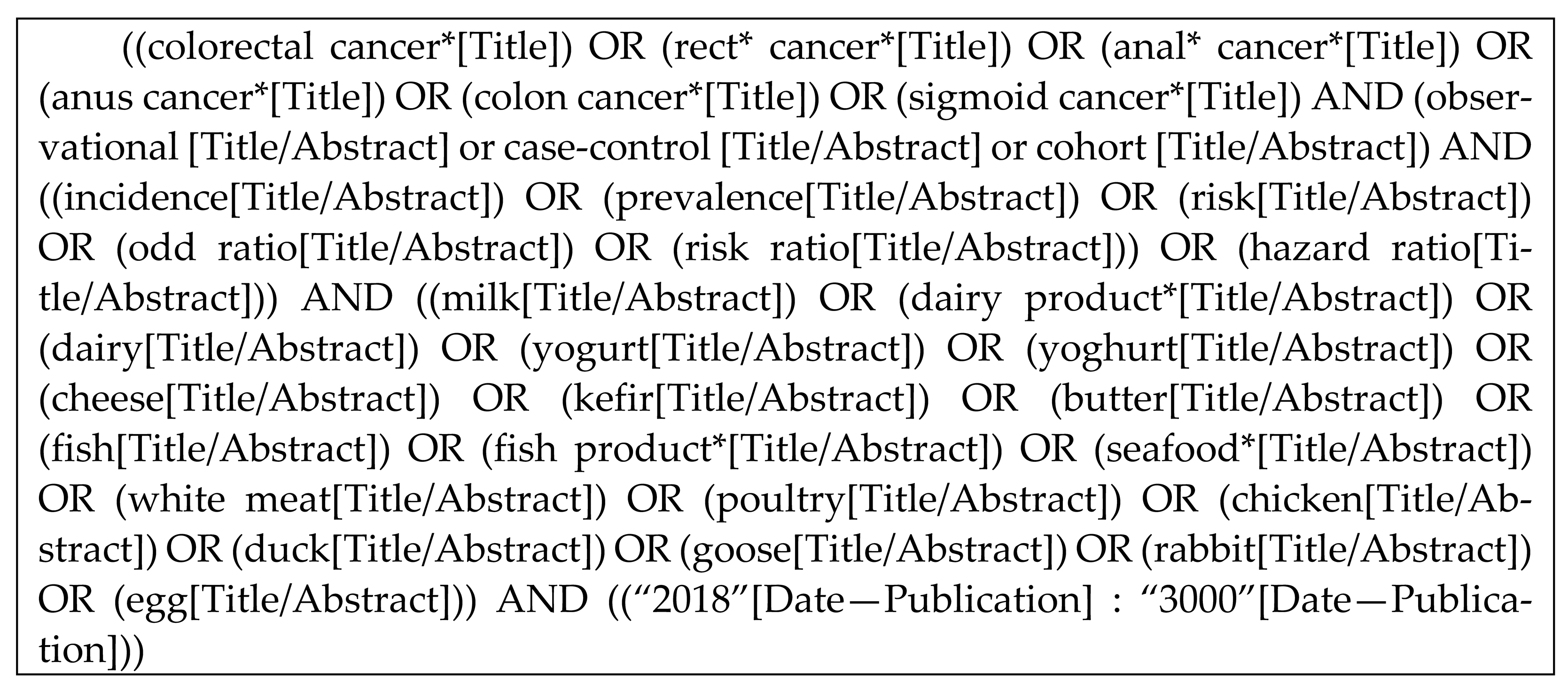
2.2. Review Process and Selection Criteria
Two researchers independently screened the titles and abstracts of the articles to identify potentially relevant studies. Studies that passed the title/abstract review were retrieved for a full-text review. An article was retained for the analysis only if: (i) it was published between 2018 and 2022; (ii) it was conducted on humans (>18 y old); (iii) it was written in English or Spanish; (iv) it was an observational study (i.e., case–control or cohort); (v) the exposure variable was at least one of the following dietary components: milk, dairy products, fish, white meat, or eggs; (vi) colorectal adenocarcinoma incidence was the outcome (studies investigating benign adenomas or polyps were excluded); (vii) provision of adjusted odds ratios (ORs) or risk ratios (RRs) or hazard ratios (HRs) with 95% confidence intervals (CIs); (viii) the estimates were adjusted for age; and (ix) Newcastle–Ottawa scale (NOS) ≥ 4, indicating sufficient study quality [17]. Template data collection forms and data extracted from the included studies will be made available upon request from the corresponding author.
The following types of publications were excluded: (i) nonoriginal papers (e.g., reviews, meta-analyses, book chapters, commentaries, editorials, proceedings, or letters to the editor); (ii) ecologic assessments and correlation studies; (iii) non-peer-reviewed articles; (iv) off-topic studies (e.g., those who analysed dietary patterns); (v) studies on CRC mortality; (vi) studies lacking specific CRC data; (vii) animal and mechanistic studies; (viii) studies conducted at stages of life other than adulthood (e.g., childhood and adolescence); (ix) supplements to the main manuscript; (x) duplicate publications; and (xi) those with a NOS score < 4.
2.3. Study Quality Assessment
To evaluate the validity of the individual studies, two reviewers worked independently to determine the quality of the included studies based on the use of the NOS for case–control or cohort studies [17]. The maximum score was 9, and a high score (≥6) indicated a high methodologic quality; however, given the lack of studies on the subject under study, it was agreed to select those that had a score equal to or greater than 4. A consensus was reached between the reviewers if there were any discrepancies.
2.4. Data Extraction
The data extracted for each individual study included the following: study design; name of the first author; year of publication; characteristics of the study population (age range or mean age, sex, and country); dietary exposure; dietary assessment instrument used; outcomes (including cancer site); OR; RR or HR (95% CI); adjusted variables; NOS; and funding sources. For case–control studies, the following additional information was extracted: number of cases and number of controls. For cohort studies, the following additional information was extracted: number of participants at the baseline, number of diagnosed cases of CRC, and length of follow-up. The most relevant variables to the outcome studied, thus, when the researchers used multiple estimates, the one with the highest number of adjusted variables was recorded. Where multiple estimates for the association of the same outcome were used, the one with the highest number of adjusted variables was extracted.
3. Results and Discussion
3.1. Study Selection
Figure 1 shows the PRISMA flow diagram summarizing the identification and selection of the relevant publications. Twenty-one studies were included in the systematic review: fourteen cohort studies [18,19,20,21,22,23,24,25,26,27,28,29,30,31] and seven case–control studies [32,33,34,35,36,37,38].
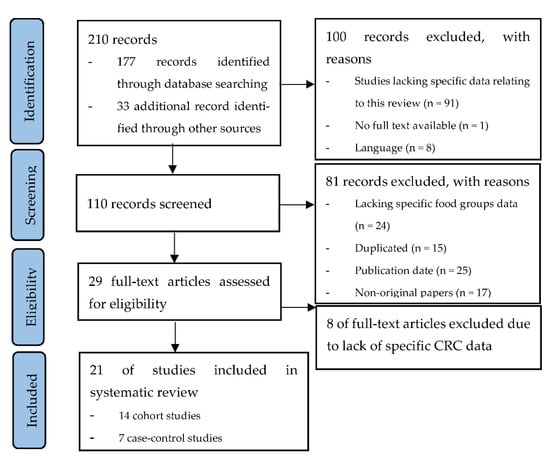
Figure 1.
PRISMA flow diagram summarizing the identification and selection of the relevant publications about the associations between the consumption of dairy products, fish, white meat, and eggs and the risk of colorectal cancer.
3.2. Study Characteristics about Milk and Dairy Products
Table 1 and Table 2 show the main characteristics of the studies selected about the association between the consumption of dairy products and CRC risk. In total, the cohort studies included 1,847,899 participants with 18,880 cases recorded during follow-up periods that ranged from 6 to 32 y. The case–control studies included 3553 cases and 5902 controls. Table 3 summarizes the association results obtained in the articles reviewed.

Table 1.
Characteristics of the ten cohort studies included in the systematic review examining the association between the consumption of dairy products and the risk of colorectal cancer.

Table 2.
Characteristics of the three case–control studies included in the systematic review examining the association between the consumption of dairy products and the risk of colorectal cancer.

Table 3.
Summary of the findings found in the reviewed articles examining the association between the consumption of dairy products and the risk of colorectal cancer.
Of the cohort studies, six were conducted in Europe (one in various European countries, one in France, one in Norway, one in Spain, and two in Sweden); one in China; and two in the United States. The case–control studies were conducted in three countries (China, Iran, and Spain). Most of the studies obtained funding only from agencies (in particular, nine of eleven studies), and in the rest, no information was provided on funding resources or grants.
3.3. Dairy Products
3.3.1. Total Dairy Products in Overall and by Fat Content
Five cohort study [19,20,21,26,27] and three case–control study [32,33,34] comparisons were used to assess the association between the total dairy consumption and CRC risk. In three of the cohort studies [19,20,21], a significant inverse association was observed for CRC, in addition to CC and RC separately and, also, in subtypes according to tumour localisation (distal and proximal). Previous studies have shown a significant decrease in the risk of CRC risk associated with a high consumption of total dairy products [12], supporting the conclusions of the last systematic review and meta-analysis of prospective studies, which updated the evidence of the WCRF-AICR Continuous Update Project [39]. Regarding the total dairy product consumption and CRC risk, the aforementioned meta-analysis observed similar associations in men and women. This result agrees with the study of Zhang et al. [33] included in the present search.
Observed inverse associations between intake of dairy products and CRC development were mostly attributed to their high calcium content. Additionally, the casein and lactose may increase calcium bioavailability, and lactic acid-producing bacteria may also protect against CRC. Other nutrients or bioactive compounds present in dairy products, such as lactoferrin, the short-chain fatty acid butyrate, or vitamin D (from fortified products), could also be protective factors for this type of cancer; however, further studies are required to confirm this effect [40].
However, in the other two cohort investigations [26,27] and one of the case–control researches [34] analysed in the present review, CRC did not show clear associations with the total dairy consumption. Moreover, in one case–control study [32], a direct association was found between total dairy intake and CRC risk. The discrepancies between the findings of these studies could be due to differences in the amount and types of products consumed, as well as differences in the overall lifestyle between the population studied (for example, in China, generally, the levels of dairy consumption are low, but people who have a high consumption of dairy products can also have a high intake of red and processed meat, due to the Westernisation of eating habits). Moreover, these discrepancies could be due to differences in the adjustment variables included in the statistical models.
Regarding the associations between dairy products by fat content and the risk of CRC, only one of the analysed studies [19] researched this aspect and concluded that whole-fat dairy consumption was not associated with an increased CRC risk. This finding agrees with those previously reported [41,42] and allows us to deduce that there are no reasons to not recommend whole-fat dairy consumption. The associations between the consumption of dairy products with different fat contents and CRC risk were not documented in the latest report by the CUP panel [7]. In a recent meta-analysis, it was observed that a high consumption of high-fat dairy products was a significant inverse association with the CRC risk [12]. However, due to the substantial heterogeneity among the few studies analysed in the aforementioned meta-analysis, Barrubés et al. [12] concluded that these observations should be interpreted cautiously.
3.3.2. Total Milk, Whole, and Low-Fat Milk
The analysis of the association of the total milk consumption with CRC risk included six cohort study [18,19,22,24,25,26] and two case–control study [33,34] comparisons. No significant association was found in any of them, except for a weak inverse association with CC risk in one of the cohort studies [18] and a significant inverse association with the CRC risk in one of the case–control studies [33] and one of the cohort studies [25], this last association independent of sex and cancer sites [25,33]. Previously, cohort studies on the association between milk intake and the risk of CRC have reported different results [43,44,45,46,47,48]; in some of them, an inverse association between milk intake and CC risk was also found, in particular among women in an EPIC study [41]. In this sense, it should be noted that the aetiological factors may differ between CC and RC [49]; in fact, different genetic features between CC and RC have been observed [50]. In addition, milk may have different effects on CC and RC risk [51].
Continuing with the study of milk consumption, but in this case, depending on the fat content, one of the analysed studies [19] found suggestive evidence that a high consumption of low-fat milk is associated with lower CRC incidence, this beverage as the main contributor to total dairy product consumption in the studied population. Barrubés et al. [19] explained that they found no significant association between the whole-fat milk intake and CRC risk, due probably to the low consumption of this subtype of milk in the population studied. In any case, the fat content in whole-fat milk might mitigate the potential benefits of the other bioactive components [52].
3.3.3. Yogurt and Other Fermented Dairy Products
Concerning fermented dairy products, five cohort studies [19,23,24,25,26], and one case–control study [34] were included in this review of the association between the consumption of these products and CRC risk. In four of these five cohort investigations [19,24,25,26], no significant associations were found for fermented dairy products or for yogurt and different types of yogurts (low-fat and whole-fat yogurt).
Only in one of the analysed cohort studies [23], yogurt consumption was associated with a reduced risk of proximal CC with a long latency period, which confirmed the results from the previously conducted European Prospective Investigation into Cancer and Nutrition (EPIC). Similar results were found in a previous analysis [53] and a pooled analysis of prospective cohorts [54]. In this same vein, one case–control study analysed did find a significant inverse association between yogurt consumption and proximal CC, with a dose–response relationship [34]. This result agrees with that of previous case–control investigations—in particular, with one study in Los Angeles in which the authors observed an inverse association between regular yogurt consumption and CC [55] and another in the Italian EPIC population after controlling for the calcium and other nutrients intake [56]. However, no relation was evident in either the PREvention with Mediterranean Diet Study [19] or the EPIC after adjustment for dietary calcium [41]. Possible explanations for the diverse findings are among other differences in the study designs, dietary assessment methods, genetic backgrounds, lifestyle, and dietary habits of study populations. The consumption of yogurt varies greatly worldwide, both the consumption of any type of yogurt and plain yogurt without added sugar, fruit, or flavourings is more frequent in Europe than in the United States [57,58,59,60]. Moreover, specifically, the findings related to distal CC could be due, at least in part, to the confounding effect of calcium intake.
3.3.4. Cheese
Five cohort [19,22,24,25,26] and two case–control studies [28,30] were used to analyse the associations between the consumption of cheese and CRC risk. These cohort studies did not support any major adverse or beneficial effects of cheese in the diet from the CRC risk perspective, which agrees with the conclusions of the WCRF/AICR [41], which indicates the association with cheese consumption was not clear. In any case, two results found in the analysed cohort studies should be highlighted. First, a high cheese consumption was associated with a modestly decreased risk of CRC [24,25], which has been attributed to positive effects of lactoferrin [61] and dairy lipids [62], as well as changes in the gut microbiome [63]. Second, the consumption of “fromage blanc” (a French type of quark/cottage cheese resulting from lactic coagulation and draining without further processing or additives) was associated with an increased risk of CRC [26]. The authors did not find any clear mechanism to explain the observed association with the consumption of “fromage blanc”, so they suggested that their results be interpreted with caution, because it could result from an artifact of the study sample. On the other hand, in one of the case–control studies [32], a direct association was found for high-fat cheese consumption, which was explained by the saturated fat content and by the increased bile acid discharge. It has been reported that an increase in bile acid production above the physiological levels promotes CRC [64,65].
3.3.5. Other Dairy Products: Butter, Sugary Dairy Products, Cream, and Ice Cream
Three cohort studies [19,24,26] were used to compare the overall risk of CRC between the highest and lowest consumption of butter and sugary dairy products. As for the butter, no significant associations were found in two of these manuscripts [19,24]. Regarding sugary dairy products, Barrubés et al. [19] did not find an association with the CRC risk. However, Deschasaux-Tanguy et al. [26] observed a direct association with CRC risk. These authors argued that the observed association could be due to the fact that these products often contain elevated amounts of sugar, as well as additives (for instance, emulsifiers or texturizers) [66], which could increase the CRC risk through body weight gain and, consequently, increase the insulin resistance [7].
Regarding cream and ice cream intake, only in one of the case–control studies analysed were they assessed [34]. Collatuzzo et al. [34] found a direct association for cream and CRC in the overall CC, as well as proximal CC, while ice cream was inversely associated with the risk of distal CC. In relation to the first of these associations, researchers pointed out that the literature reports a direct association between high-fat dairy product intake and overall cancer mortality and between a low-fat dairy intake and cancer mortality but no relation with CRC in particular [19]. As mentioned above in the subsection on the total dairy products by the fat content, there was no evidence of an increased CRC risk derived from whole-fat dairy consumption [19].
3.4. Study Characteristics about Fish, White Meat, and Eggs
Table 4 and Table 5 show the main characteristics of the studies selected about the association between the consumption of fish, white meat, and eggs and CRC risk. In total, the cohort studies included 1,438,288 participants with 38,508 cases recorded during follow-up periods that ranged from 5.7 to 30 y. The case–control studies included 5203 cases and 8430 controls. Table 6 summarises the associated results obtained in the articles reviewed. Of the cohort studies, two were conducted in Europe (one in various European countries and one in Denmark), two in the UK, and one in the United States. The case–control studies were conducted in Asia (one in China and one in the Republic of Korea), Europe (one in Italy and one in Spain), and in Morocco. Most of the studies obtained funding only from agencies (in particular, nine out of ten studies), and in one, no information was provided on the funding resources or grants.

Table 4.
Characteristics of the five cohort studies included in the systematic review examining the association between the consumption of fish, white meat, and eggs and the risk of colorectal cancer.

Table 5.
Characteristics of the five case–control studies included in the systematic review examining the association between the consumption of fish, white meat, and eggs and the risk of colorectal cancer.

Table 6.
Summary of the findings found in the reviewed articles examining the association between the consumption of fish, white meat, and eggs and the risk of colorectal cancer.
3.5. Fish, White Meat, and Eggs
3.5.1. Fish
Three cohort [22,28,31] and three case–control studies [32,36,38] were used to analyse the associations between the highest and lowest consumption of total fish and CRC risk. In two of these cohort investigations [28,31] and in one of these case–control studies [38], fish consumption was associated with a significant CRC risk reduction. A protective effect was also observed for oily fish in both the cohort [28,31] and case–control studies [32]. Aglago et al. [28] found an inverse association between oily fish and non-oily fish consumption, separately, both for the overall CRC risk, as well as for CC and distal CC alone. Moreover, in the cohort study [31] and the case–control study [38] that analysed the consumption of canned fish, the results were significant and showed an inverse association between said intake and CRC risk.
These findings agree with those previously reported in two recent meta-analyses [67,68]. In the meta-analysis of Caini et al. [67], it is suggested that the aforementioned association may be attributable to several biological mechanisms, such as those related to ω-3 polyunsaturated fatty acids (PUFAs) that: (i) affect eicosanoids metabolism [69], (ii) are incorporated into membrane phospholipids, and (iii) that do not enhance the luminal concentration of secondary bile acids and the lower colon and liver activity of ornithine decarboxylase and tyrosine-specific protein kinase, all these mechanisms involved in colon carcinogenesis [70].
In addition, ω-3 PUFA has been associated with a higher intestinal microbial diversity, thus improving the host immune function and eventually decreasing the risk of the development of CRC [71,72]. Mechanisms related to eicosapentaenoic acid and docosahexaenoic acid that produce lipid mediators endowed with pro-resolving, immunomodulatory, and anti-inflammatory properties could also be involved [73]. On the other hand, the association between fish consumption and CRC risk could also be due, in part, to: (i) a replacement effect, since those who eat more fish generally eat less red meat, whose causal link with CRC is well-known, and (ii) the fact that preferring fish instead of meat may be part of a generally healthier lifestyle, including protective habits against this type of cancer [74,75].
3.5.2. White Meat
Five cohort studies [22,29,30,31,37] and one case–control study [35] were used to analyse the association between high and low white meat intake and CRC risk. In one of the cohort studies [29], poultry consumption was inversely associated with RC risk in women but positively in men. The authors argued that these differences by sex could be due to a lower incidence of this type of cancer among women and also to a less frequent consumption of meat among women. In the case–control study [35], a positive association was observed between the poultry intake and CC risk among men. In contrast, no significant association was observed between white meat and CRC risk among women. The same as Knuppel et al. [29], Deoula et al. [35] considered that their results may be due to a lower consumption of the total meat among women compared to men.
3.5.3. Eggs
Two case–control studies [32,37] were used to analyse the association between the high and low consumption of eggs and CRC risk. In one of them, an inverse association was found, and in the other, no association was found. Although Shen et al. [37] did not provide arguments to explain why egg consumption is a protective factor against CRC risk, it should be noted that eggs contain several antioxidants (including selenium, carotenoids, and vitamin E), which reduce the free radicals arising from cellular metabolism [76]. Positive associations between oxidative stress and the incidence of chronic diseases such as cancers have been reported [77]. In addition, eggs are a good source of choline and B vitamins directly or tangentially involved in one-carbon metabolism [76]. Disorders in one-carbon metabolism can lead to decreased DNA synthesis, genomic instability, and decreased methyl donor production [78]. Genomic instability and DNA hypomethylation are common traits of CRC [79]. On the contrary, in the meta-analysis of Schwingshackl et al. [14], based on a small number of studies (n = 3), the results showed a positive significant association in the high vs. low meta-analysis. A plausible hypothesis to explain these observations is that a high cholesterol intake would increase the formation of secondary bile acid and enhance the induction of colorectal tumours [80].
3.6. Strengths and Limitations
This systematic review has several strengths: (i) cohort and case–control studies through a systematic search have been identified, (ii) a quantitative NOS scale was used to evaluate the quality of the individual studies, and (iii) all of the studies used a validated questionnaire to assess the food consumption.
The limitations of this review include: (i) the heterogeneous nature of the studies, including the study population characteristics, sample size, study design, and follow-up periods; (ii) being observational studies, residual confounding can be a problem, and not all studies were adjusted for important confounders; (iii) in the same way, since these are observational studies, the results cannot support the causal relationship between food group consumption and CRC risk; and (iv) the fact that some studies used self-reported data on the dietary intake may affect the reliability of the reported data, although the use of validated questionnaires could reduce this bias.
4. Conclusions
This systematic review of observational studies supports the protective role of the consumption of total dairy products against the CRC risk in all CRC subsites. Therefore, it makes sense to suggest that the dairy intake might be associated with a lower risk of CRC and, furthermore, that there are no reasons to advise against whole-fat dairy products. However, the effect associated with the consumption of any subtype of dairy product, including milk, fermented dairy products (including yogurt), cheese, and other dairy products, was less strong. As regards fish consumption, a mild inverse association with CRC risk was observed, with a similar effect for CC and RC, according to the tumour location. This same relationship was found for different types of fish (oily and non-oily fish), both fresh and processed (canned fish). Finally, this review on white meat and eggs was based on a small number of studies, and the evidence was low; thus, the findings for these food groups should be interpreted with caution. The current findings related to dairy products and fish confirm those from previous meta-analyses [7,12,67,68]. The main new findings of the present review are those related to specific subtypes of fish (in particular, canned fish), white meat, and eggs. Further studies are needed to confirm these results and to clarify the mechanism to explain the observed associations, with special emphasis on the differences in the subtypes of foods and CRC subsite-specific risk.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu14163430/s1: Table S1: PRISMA checklist.
Author Contributions
Conceptualisation, M.A.-I., I.A.-L. and L.B.; methodology, M.A.-I. and I.A.-L.; investigation, M.A.-I. and I.A.-L.; resources, M.A.-I. and I.A.-L.; data curation, M.A.-I. and I.A.-L.; writing—original draft preparation, M.A.-I. and I.A.-L.; writing—review and editing, M.A.-I., I.A.-L. and L.B.; visualisation, M.A.-I. and I.A.-L.; supervision, M.A.-I.; project administration, M.A.-I.; funding acquisition, M.A.-I. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. CIBERehd is funded by the Instituto de Salud Carlos III. BIOMICS Research Group, Microfluidics & BIOMICS Cluster of the UPV/EHU is funded by the Basque Government. Funding of article processing charges provided by the Spanish Gastroenterology Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLO-BOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [PubMed]
- Roquette, R.; Painho, M.; Nunes, B. Geographical patterns of the incidence and mortality of colorectal cancer in mainland Portugal municipalities (2007–2011). BMC Cancer 2019, 19, 512. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Levi, F.; Rosato, V.; Bertuccio, P.; Lucchini, F.; Negri, E.; La Vecchia, C. Recent trends in colorectal cancer mortality in Europe. Int. J. Cancer 2011, 129, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [Green Version]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [Green Version]
- Center, M.M.; Jemal, A.; Smith, R.A.; Ward, E. Worldwide Variations in Colorectal Cancer. CA Cancer J. Clin. 2009, 59, 366–378. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Colorectal Cancer. 2017. Available online: https://www.wcrf.org/sites/default/files/Colorectal-cancer-report.pdf (accessed on 1 May 2022).
- Couto, E.; Boffetta, P.; Lagiou, P.; Ferrari, P.; Buckland, G.; Overvad, K.; Dahm, C.; Tjonneland, A.; Olsen, A.; Clavelchapelon, F.; et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer 2011, 104, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43–44, 83–88. [Google Scholar] [CrossRef]
- Chartier, L.C.; Howarth, G.S.; Mashtoub, S. Combined nutraceuticals: A novel approach to colitis-associated colorectal cancer? Nutr. Cancer 2019, 71, 199–206. [Google Scholar] [CrossRef]
- Iannone, M.; Mare, R.; Paolino, D.; Gagliardi, A.; Froiio, F.; Cosco, D.; Fresta, M. Characterization and in vitro anticancer properties of chitosan-microencapsulated flavan-3-ols-rich grape seed extracts. Int. J. Biol. Macromol. 2017, 104, 1039–1045. [Google Scholar] [CrossRef]
- Barrubés, L.; Babio, N.; Becerra-Tomás, N.; Rosique-Esteban, N.; Salas-Salvadó, J. Association Between Dairy Product Con-sumption and Colorectal Cancer Risk in Adults: A Systematic Review and Meta-Analysis of Epidemiologic Studies. Adv. Nutr. 2019, 10, S190–S211. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.A.; Key, T.J.; Appleby, P.N.; Dahm, C.; Keogh, R.H.; Fentiman, I.S.; Akbaraly, T.; Brunner, E.; Burley, V.; Cade, J.; et al. Meat, poultry and fish and risk of colorectal cancer: Pooled analysis of data from the UK dietary cohort consortium. Cancer Causes Control 2010, 21, 1417–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Preterre, A.L.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer. Int. J. Cancer 2017, 142, 1748–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietary Guidelines Advisory Committee. 2020. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-07/ScientificReport_of_the_2020DietaryGuidelinesAdvisoryCommittee_first-print.pdf (accessed on 15 June 2022).
- National Health and Medical Research Council 2013. Australian Dietary Guidelines. Canberra: National Health and Medical Research Council. Available online: https://www.eatforhealth.gov.au/sites/default/files/content/n55_australian_dietary_guidelines.pdf (accessed on 15 June 2022).
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute, 2011. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 31 May 2022).
- Bakken, T.; Braaten, T.; Olsen, A.; Hjartåker, A.; Lund, E.; Skeie, G. Milk and risk of colorectal, colon and rectal cancer in the Norwegian Women and Cancer (NOWAC) Cohort Study. Br. J. Nutr. 2018, 119, 1274–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrubés, L.; Babio, N.; Mena-Sánchez, G.; Toledo, E.; Ramírez-Sabio, J.B.; Estruch, R.; Ros, E.; Fitó, M.; Arós, F.; Fiol, M.; et al. PREvención con DIeta MEDiterránea Study Investigators. Dairy product consumption and risk of colorectal cancer in an older mediter-ranean population at high cardiovascular risk. Int. J. Cancer 2018, 143, 1356–1366. [Google Scholar] [CrossRef] [Green Version]
- Vulcan, A.; Ericson, U.; Manjer, J.; Ohlsson, B. A colorectal cancer diet quality index is inversely associated with colorectal cancer in the Malmö diet and cancer study. Eur. J. Cancer Prev. 2019, 28, 463–471. [Google Scholar] [CrossRef]
- Um, C.Y.; Prizment, A.; Hong, C.P.; Lazovich, D.; Bostick, R.M. Associations of Calcium, Vitamin D, and Dairy Product Intakes with Colorectal Cancer Risk among Older Women: The Iowa Women’s Health Study. Nutr. Cancer 2019, 71, 739–748. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2019, 49, 246–258. [Google Scholar] [CrossRef] [Green Version]
- Michels, K.B.; Willett, W.C.; Vaidya, R.; Zhang, X.; Giovannucci, E. Yogurt consumption and colorectal cancer incidence and mortality in the Nurses’ Health Study and the Health Professionals Follow-Up Study. Am. J. Clin. Nutr. 2020, 112, 1566–1575. [Google Scholar] [CrossRef]
- Nilsson, L.M.; Winkvist, A.; Esberg, A.; Jansson, J.-H.; Wennberg, P.; van Guelpen, B.; Johansson, I. Dairy Products and Cancer Risk in a Northern Sweden Population. Nutr. Cancer 2020, 72, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, N.; Bouras, E.; Brandt, P.A.V.D.; Muller, D.C.; Papadopoulou, A.; Heath, A.K.; Critselis, E.; Gunter, M.J.; Vineis, P.; Ferrari, P.; et al. A Prospective Diet-Wide Association Study for Risk of Colorectal Cancer in EPIC. Clin. Gastroenterol. Hepatol. 2021, 20, 864–873.e13. [Google Scholar] [CrossRef] [PubMed]
- Deschasaux-Tanguy, M.; Barrubés Piñol, L.; Sellem, L.; Debras, C.; Srour, B.; Chazelas, E.; Wendeu-Foyet, G.; Hercberg, S.; Galan, P.; Kesse-Guyot, E.; et al. Dairy product consumption and risk of cancer: A short report from the NutriNet-Santé prospective cohort study. Int. J. Cancer 2022, 150, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Kakkoura, M.G.; Du, H.; Guo, Y.; Yu, C.; Yang, L.; Pei, P.; Chen, Y.; Sansome, S.; Chan, W.C.; Yang, X.; et al. Dairy consumption and risks of total and site-specific cancers in Chinese adults: An 11-year prospective study of 0.5 million people. BMC Med. 2022, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Aglago, E.K.; Huybrechts, I.; Murphy, N.; Casagrande, C.; Nicolas, G.; Pischon, T.; Fedirko, V.; Severi, G.; Boutron-Ruault, M.-C.; Fournier, A.; et al. Consumption of Fish and Long-chain n-3 Polyunsaturated Fatty Acids Is Associated With Reduced Risk of Colorectal Cancer in a Large European Cohort. Clin. Gastroenterol. Hepatol. 2020, 18, 654–666.e6. [Google Scholar] [CrossRef]
- Knuppel, A.; Papier, K.; Fensom, G.K.; Appleby, P.N.; A Schmidt, J.; Tong, T.Y.N.; Travis, R.C.; Key, T.J.; Perez-Cornago, A. Meat intake and cancer risk: Prospective analyses in UK Biobank. Int. J. Epidemiol. 2020, 49, 1540–1552. [Google Scholar] [CrossRef]
- Mejborn, H.; Møller, S.P.; Thygesen, L.C.; Biltoft-Jensen, A. Dietary Intake of Red Meat, Processed Meat, and Poultry and Risk of Colorectal Cancer and All-Cause Mortality in the Context of Dietary Guideline Compliance. Nutrients 2020, 13, 32. [Google Scholar] [CrossRef]
- Wang, F.; Chandler, P.D.; Zeleznik, O.A.; Wu, K.; Wu, Y.; Yin, K.; Song, R.; Avila-Pacheco, J.; Clish, C.B.; Meyerhardt, J.A.; et al. Plasma Metabolite Profiles of Red Meat, Poultry, and Fish Consumption, and Their Associations with Colorectal Cancer Risk. Nutrients 2022, 14, 978. [Google Scholar] [CrossRef]
- Alegria-Lertxundi, I.; Aguirre, C.; Bujanda, L.; Fernández, F.J.; Polo, F.; Ordovás, J.M.; Etxezarraga, M.C.; Zabalza, I.; Larzabal, M.; Portillo, I.; et al. Food groups, diet quality and colorectal cancer risk in the Basque Country. World J. Gastroenterol. 2020, 26, 4108–4125. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, Y.J.; Feng, X.L.; Abulimiti, A.; Huang, C.Y.; Luo, H.; Zhang, N.Q.; Chen, Y.M.; Zhang, C.X. Higher intakes of dietary vitamin D, calcium and dairy products are inversely associated with the risk of colorectal cancer: A case-control study in China. Br. J. Nutr. 2020, 123, 699–711. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Seyyedsalehi, M.S.; Rezaeianzadeh, A.; Marzban, M.; Rashidian, H.; Hadji, M.; Kamangar, F.; Etemadi, A.; Pukkala, E.; Zendehdel, K.; et al. Consumption of Yoghurt and Other Dairy Products and Risk of Colorectal Cancer in Iran: The IROPICAN Study. Nutrients 2022, 14, 2506. [Google Scholar] [CrossRef]
- Deoula, M.S.; El Kinany, K.; Huybrechts, I.; Gunter, M.J.; Hatime, Z.; Boudouaya, H.A.; Benslimane, A.; Nejjari, C.; El Abkari, M.; Badre, W.; et al. Consumption of meat, traditional and modern processed meat and colorectal cancer risk among the Moroccan population: A large-scale case-control study. Int. J. Cancer 2020, 146, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.; Woo, H.D.; Kim, D.W.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Dietary mercury intake and colo-rectal cancer risk: A case-control study. Clin. Nutr. 2020, 39, 2106–2113. [Google Scholar] [CrossRef]
- Shen, W.; Sun, J.; Li, Z.; Yao, F.; Lin, K.; Jiao, X. Food intake and its effect on the species and abundance of intestinal flora in colorectal cancer and healthy individuals. Korean J. Intern. Med. 2021, 36, 568–583. [Google Scholar] [CrossRef] [PubMed]
- Franchi, C.; Ardoino, I.; Bosetti, C.; Negri, E.; Serraino, D.; Crispo, A.; Giacosa, A.; Fattore, E.; Dolci, A.; Bravi, F.; et al. Inverse Association between Canned Fish Consumption and Colorectal Cancer Risk: Analysis of Two Large Case-Control Studies. Nutrients 2022, 14, 1663. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Proyect Expert Report 2018. Meat, Fish and Dairy Products and the Risk of Cancer. Available online: https://www.wcrf.org/diet-activity-and-cancer/risk-factors/meat-fish-dairy-and-cancer-risk/ (accessed on 1 June 2022).
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-De-Mesquita, B.; Skeie, G.; Olsen, A.; Tjonneland, A.; Dahm, C.; Overvad, K.; et al. Consumption of Dairy Products and Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS ONE 2013, 8, e72715. [Google Scholar] [CrossRef] [Green Version]
- Larsson, S.C.; Bergkvist, L.; Wolk, A. High-fat dairy food and conjugated linoleic acid intakes in relation to colorectal cancer incidence in the Swedish Mammography Cohort. Am. J. Clin. Nutr. 2005, 82, 894–900. [Google Scholar] [CrossRef]
- Lee, S.-A.; Shu, X.O.; Yang, G.; Li, H.; Gao, Y.-T.; Zheng, W. Animal Origin Foods and Colorectal Cancer Risk: A Report From the Shanghai Women’s Health Study. Nutr. Cancer 2009, 61, 194–205. [Google Scholar] [CrossRef]
- Park, S.-Y.; Murphy, S.P.; Wilkens, L.R.; Nomura, A.M.; Henderson, B.E.; Kolonel, L.N. Calcium and Vitamin D Intake and Risk of Colorectal Cancer: The Multiethnic Cohort Study. Am. J. Epidemiol. 2007, 165, 784–793. [Google Scholar] [CrossRef]
- Kesse, E.; Boutron-Ruault, M.; Norat, T.; Riboli, E.; Clavel-Chapelon, F. E3N Group Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int. J. Cancer 2005, 117, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Larsson, S.C.; Bergkvist, L.; Rutegård, J.; Giovannucci, E.; Wolk, A. Calcium and dairy food intakes are inversely associated with colorectal cancer risk in the Cohort of Swedish Men. Am. J. Clin. Nutr. 2006, 83, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Tantamango-Bartley, Y.; Knutsen, S.F.; Jaceldo-Siegl, K.; Fan, J.; Mashchak, A.; Fraser, G.E. Independent associations of dairy and calcium intakes with colorectal cancers in the Adventist Health Study-2 cohort. Public Health Nutr. 2017, 20, 2577–2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, C.C.J.M.; Leurs, L.J.; Weijenberg, M.P.; Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P.A. Fluid Intake and Colorectal Cancer Risk in the Netherlands Cohort Study. Nutr. Cancer 2010, 62, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Frattini, M.; Balestra, D.; Suardi, S.; Oggionni, M.; Alberici, P.; Radice, P.; Costa, A.; Daidone, M.G.; Leo, E.; Pilotti, S.; et al. Different Genetic Features Associated with Colon and Rectal Carcinogenesis. Clin. Cancer Res. 2004, 10, 4015–4021. [Google Scholar] [CrossRef] [Green Version]
- Kapiteijn, E.; Liefers, G.J.; Los, L.C.; Kranenbarg, E.-K.; Hermans, J.; Tollenaar, R.A.; Moriya, Y.; Van De Velde, C.J.; Van Krieken, J.H. Mechanisms of oncogenesis in colon versus rectal cancer. J. Pathol. 2001, 195, 171–178. [Google Scholar] [CrossRef]
- Huncharek, M.; Muscat, J.; Kupelnick, B. Colorectal Cancer Risk and Dietary Intake of Calcium, Vitamin D, and Dairy Products: A Meta-Analysis of 26,335 Cases From 60 Observational Studies. Nutr. Cancer 2009, 61, 47–69. [Google Scholar] [CrossRef]
- Vargas, A.J.; Thompson, P.A. Diet and Nutrient Factors in Colorectal Cancer Risk. Nutr. Clin. Pract. 2012, 27, 613–623. [Google Scholar] [CrossRef]
- Zhang, X.; Keum, N.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Chan, A.T.; Fuchs, C.S.; Giovannucci, E.L. Calcium intake and colorectal cancer risk: Results from the nurses’ health study and health professionals follow-up study. Int. J. Cancer 2016, 139, 2232–2242. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.; Smith-Warner, S.A.; Spiegelman, D.; Beeson, W.L.; van den Brandt, P.A.; Colditz, G.; Folsom, A.R.; Fraser, G.E.; Freudenheim, J.L.; Giovannucci, E.; et al. Dairy Foods, Calcium, and Colorectal Cancer: A Pooled Analysis of 10 Cohort Studies. J. Natl. Cancer Inst. 2004, 96, 1015–1022, Erratum in: J. Natl. Cancer Inst. 2004, 96, 1724. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.K.; Pike, M.C.; Garabrant, D.; Mack, T.M. Diet and colon cancer in Los Angeles County, California. Cancer Causes Control 1992, 3, 457–473. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- USDA Agricultural Research Service, Beltsville Human Nutrition Research Center. MyPyramid Equivalents Product Downloads. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/mypyramid-equivalents-product-downloads/ (accessed on 17 October 2021).
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbandeh, M.U.S. Per Capita Consumption of Yogurt 2000–2018 Statista; 24 September 2019. Available online: https://www.statista.com/statistics/184309/per-capita-consumption-of-yogurt-in-the-us-since-2000/ (accessed on 17 May 2022).
- Trichia, E.; Luben, R.; Khaw, K.T.; Wareham, N.J.; Imamura, F.; Forouhi, N.G. The associations of longitudinal changes in consumption of total and types of dairy products and markers of metabolic risk and adiposity: Findings from the European Investigation into Cancer and Nutrition (EPIC)-Norfolk study, United Kingdom. Am. J. Clin. Nutr. 2020, 111, 1018–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, H.; Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Saito, D.; Akasu, T.; Alexander, D.B.; Futakuchi, M.; Fukamachi, K.; et al. Cancer prevention by bovine lactoferrin: From animal studies to human trial. BioMetals 2010, 23, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alcalá, L.M.; Castro-Gómez, M.P.; Pimentel, L.L.; Fontecha, J. Milk fat components with potential anticancer ac-tivity-a review. Biosci. Rep. 2017, 37, BSR20170705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Ou, J.; DeLany, J.P.; Zhang, M.; Sharma, S.; O’Keefe, S.J. Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutr. Cancer 2012, 64, 34–40. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Chazelas, E.; Deschasaux, M.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Alles, B.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; Latino-Martel, P.; et al. Food additives: Distribution and co-occurrence in 126,000 food products of the French market. Sci. Rep. 2020, 10, 3980. [Google Scholar] [CrossRef] [Green Version]
- Caini, S.; Chioccioli, S.; Pastore, E.; Fontana, M.; Tortora, K.; Caderni, G.; Masala, G. Fish Consumption and Colorectal Cancer Risk: Meta-Analysis of Prospective Epidemiological Studies and Review of Evidence from Animal Studies. Cancers 2022, 14, 640. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Chen, P.-F. High Consumption of Fish Tends to Be Inversely Associated with the Risk of Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Jiang, Y.; Fischer, S.M. Prostaglandin E3 metabolism and cancer. Cancer Lett. 2014, 348, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, B.S. Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ. Mol. Mutagen. 2004, 44, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T. Environmental Factors, Gut Microbiota, and Colorectal Cancer Prevention. Clin. Gastroenterol. Hepatol. 2019, 17, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, G.; Prossomariti, A.; Baldassarre, M.; Montagna, C.; Vitaglione, P.; Fogliano, V.; Biagi, E.; Candela, M.; Brigidi, P.; Balbi, T.; et al. A Mediterranean Diet Mix Has Chemopreventive Effects in a Murine Model of Colorectal Cancer Modulating Apoptosis and the Gut Microbiota. Front. Oncol. 2019, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Irún, P.; Lanas, A.; Piazuelo, E. Omega-3 Polyunsaturated Fatty Acids and Their Bioactive Metabolites in Gastrointestinal Malignancies Related to Unresolved Inflammation. A Review. Front. Pharmacol. 2019, 10, 852. [Google Scholar] [CrossRef]
- Wennberg, M.; Tornevi, A.; Johansson, I.; Hörnell, A.; Norberg, M.; Bergdahl, I.A. Diet and lifestyle factors associated with fish consumption in men and women: A study of whether gender differences can result in gender-specific confounding. Nutr. J. 2012, 11, 101. [Google Scholar] [CrossRef] [Green Version]
- Tani, S.; Kawauchi, K.; Atsumi, W.; Matsuo, R.; Ashida, T.; Imatake, K.; Suzuki, Y.; Yagi, T.; Takahashi, A.; Matsumoto, N.; et al. Association among daily fish intake, white blood cell count, and healthy lifestyle behaviors in an apparently healthy Japanese population: Implication for the anti-atherosclerotic effect of fish consumption. Heart Vessels 2021, 36, 924–933. [Google Scholar] [CrossRef]
- Zaheer, K. An Updated Review on Chicken Eggs: Production, Consumption, Management Aspects and Nutritional Benefits to Human Health. Food Nutr. Sci. 2015, 6, 1208–1220. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Gehrke, C.W.; Kuo, K.C.; Ehrlich, M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988, 48, 1159–1161. [Google Scholar] [PubMed]
- Aune, D.; De Stefani, E.; Ronco, A.L.; Boffetta, P.; Deneo-Pellegrini, H.; Acosta, G.; Mendilaharsu, M. Egg consumption and the risk of cancer: A multisite case-control study in Uruguay. Asian Pac. J. Cancer Prev. 2009, 10, 869–876. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).