Efficacy of an Irritable Bowel Syndrome Diet in the Treatment of Small Intestinal Bacterial Overgrowth: A Narrative Review
Abstract
:1. Introduction
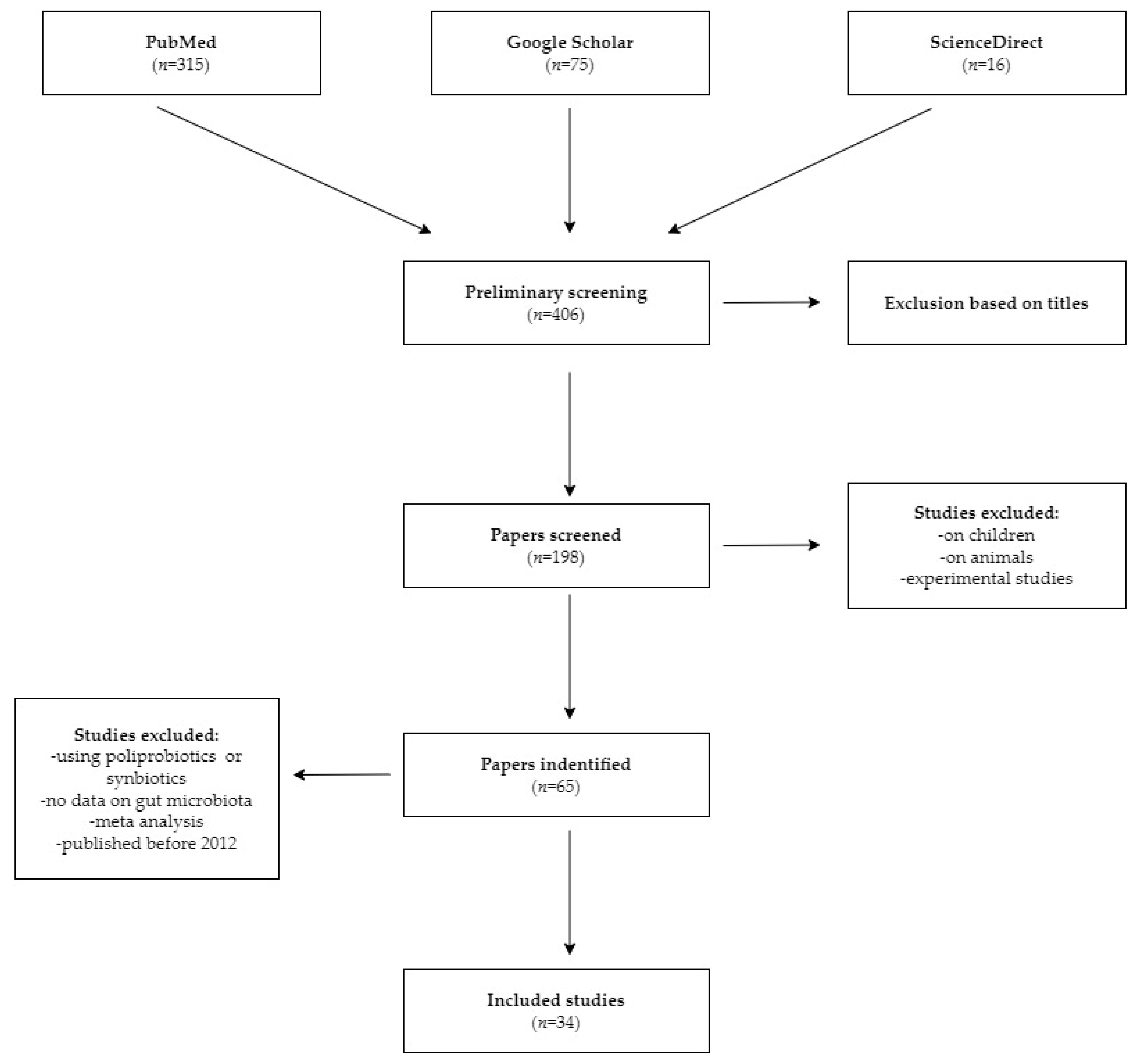
2. Materials and Methods
3. Results and Discussion
3.1. Low-FODMAP Diet
3.2. Monoprobiotics
3.3. Fiber
| Author, Year Type of Study | Period | Study Group | Intervention/Control | Methods |
|---|---|---|---|---|
| Saffouri et al., 2019 [50] Pilot interventional study | 7 days | 16 healthy | <11 g fiber/ 1000 cal/day | Breath test |
| Jalanka et al.,2019 [20] Randomized, controlled trail | 7 days | 16 constipated 8 healthy | 21 g/day Psyllium husk/ maltodextrin | 16S rRNA |
| Reider et al., 2020 [52] Clinical trial | 9 weeks | 20 healthy | 5g PHGG/ 3 time per day | 16S rRNA |
| Holscher et al., 2015 [54] Randomized, controlled trail | 21 days | 29 healthy | 5.0g/7.5 g/0.0g/day agave inulin | 16S rRNA |
| Linetzky et al., 2012 [55] Randomized clinical trial | 3 weeks | 60 constipated | 15g/day inulin+ PHGG, maltodextrin | PCR |
| Niv et al., 2016 [51] Randomized clinical trial | 18 weeks | 121 IBS | 6g PHGG group/ placebo | Francis severity IBS score |
| Polymeros et al., 2013 [53] Uncontrolled open-label trial | 4 weeks | 49 chronic constipated | 5 mg PHGG/day | Bristol stool scale |
3.4. Mindful Eating
4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome: A Bridge between Functional Organic Dichotomy. Gut Liver 2017, 11, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Deng, F.; Li, Y.; Zhao, J. Identification of Gut Microbiome Signatures Associated with Longevity Provides a Promising Modulation Target for Healthy Aging. Gut Microbes 2019, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Minhoo Kim, B.A.B. The Microbiome: An Emerging Key Player in Aging and Longevity. Transl. Med. Aging 2020, 4, 103–116. [Google Scholar] [PubMed]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef]
- Pimentel, M.; Saad, R.J.; Long, M.D.; Rao, S.S.C. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Takakura, W.; Pimentel, M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome—An Update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’Homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional Dysbiosis within the Gut Microbiota of Patients with Constipated-Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.; Siwiec, R.M.; Wo, J.M. Diagnosis and Management of Small Intestinal Bacterial Overgrowth. Nutr. Clin. Pract. 2013, 28, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Avelar Rodriguez, D.; Ryan, P.M.D.; Toro Monjaraz, E.M.; Ramirez Mayans, J.A.; Quigley, E.M. Small Intestinal Bacterial Overgrowth in Children: A State-Of-The-Art Review. Front. Pediatr. 2019, 7, 363. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, E.C.; Gabrielli, M.; Scarpellini, E.; Lupascu, A.; Novi, M.; Sottili, S.; Vitale, G.; Cesario, V.; Serricchio, M.; Cammarota, G.; et al. Small Intestinal Bacterial Overgrowth Recurrence after Antibiotic Therapy. Am. J. Gastroenterol. 2008, 103, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology Guidelines on the Management of Irritable Bowel Syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, S.; Fagoonee, S.; Battaglia, E.; Grassini, M.; Saracco, G.M.; Pellicano, R. Recent Advances in the Treatment of Irritable Bowel Syndrome. Pol. Arch. Intern. Med. 2021, 131, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Algera, J.; Colomier, E.; Simrén, M. The Dietary Management of Patients with Irritable Bowel Syndrome: A Narrative Review of the Existing and Emerging Evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Juntrapirat, A.; Lakananurak, N.; Gonlachanvit, S. Effect of Structural Individual Low-FODMAP Dietary Advice vs. Brief Advice on a Commonly Recommended Diet on IBS Symptoms and Intestinal Gas Production. Nutrients 2019, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, W.S.; Skidmore, P.M.; O’Brien, L.; Wilkinson, T.J.; Gearry, R.B. Efficacy of the Low FODMAP Diet for Treating Irritable Bowel Syndrome: The Evidence to Date. Clin. Exp. Gastroenterol. 2016, 9, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, Prebiotics and Synbiotics—A Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; de Vos, W.M.; Spiller, R. The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Scholz, M.; Lomer, M.C.; Ralph, F.S.; Irving, P.M.; Lindsay, J.O.; Fava, F.; Tuohy, K.; Whelan, K. Gut Microbiota Associations with Diet in Irritable Bowel Syndrome and the Effect of Low FODMAP Diet and Probiotics. Clin. Nutr. 2021, 40, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Huaman, J.W.; Mego, M.; Manichanh, C.; Cañellas, N.; Cañueto, D.; Segurola, H.; Jansana, M.; Malagelada, C.; Accarino, A.; Vulevic, J.; et al. Effects of Prebiotics vs a Diet Low in FODMAPs in Patients With Functional Gut Disorders. Gastroenterology 2018, 155, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, L.; Wang, X.; Fox, M.; Luo, L.; Du, L.; Chen, B.; Chen, X.; He, H.; Zhu, S.; et al. Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet Compared with Traditional Dietary Advice for Diarrhea-Predominant Irritable Bowel Syndrome: A Parallel-Group, Randomized Controlled Trial with Analysis of Clinical and Micr. Am. J. Clin. Nutr. 2021, 113, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of Varying Dietary Content of Fermentable Short-Chain Carbohydrates on Symptoms, Fecal Microenvironment, and Cytokine Profiles in Patients with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets That Differ in Their FODMAP Content Alter the Colonic Luminal Microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate Modelling of Faecal Bacterial Profiles of Patients with IBS Predicts Responsiveness to a Diet Low in FODMAPs. Gut 2018, 67, 872–881. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs Alter Symptoms and the Metabolome of Patients with IBS: A Randomised Controlled Trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Rossi, M.; Kanno, T.; Parkes, G.C.; Anderson, S.; Mason, A.J.; Irving, P.M.; Lomer, M.C.; Whelan, K. β-Galactooligosaccharide in Conjunction with Low FODMAP Diet Improves Irritable Bowel Syndrome Symptoms but Reduces Fecal Bifidobacteria. Am. J. Gastroenterol. 2020, 115, 906–915. [Google Scholar] [CrossRef]
- Harvie, R.M.; Chisholm, A.W.; Bisanz, J.E.; Burton, J.P.; Herbison, P.; Schultz, K.; Schultz, M. Long-Term Irritable Bowel Syndrome Symptom Control with Reintroduction of Selected FODMAPs. World J. Gastroenterol. 2017, 23, 4632–4643. [Google Scholar] [CrossRef] [PubMed]
- Naseri, K.; Dabiri, H.; Rostami-Nejad, M.; Yadegar, A.; Houri, H.; Olfatifar, M.; Sadeghi, A.; Saadati, S.; Ciacci, C.; Iovino, P.; et al. Influence of Low FODMAP-Gluten Free Diet on Gut Microbiota Alterations and Symptom Severity in Iranian Patients with Irritable Bowel Syndrome. BMC Gastroenterol. 2021, 21, 292. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low Fermentable, Oligo-, Di-, Mono-Saccharides and Polyol Diet in the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Halmos, E.P.; Muir, J.G. Review Article: FODMAPS, Prebiotics and Gut Health-the FODMAP Hypothesis Revisited. Aliment. Pharmacol. Ther. 2020, 52, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Madempudi, R.S.; Neelamraju, J.; Ahire, J.J.; Gupta, S.K.; Shukla, V.K. Bacillus Coagulans Unique IS2 in Constipation: A Double-Blind, Placebo-Controlled Study. Probiotics Antimicrob. Proteins 2020, 12, 335–342. [Google Scholar] [CrossRef]
- Gupta, A.K.; Maity, C. Efficacy and Safety of Bacillus Coagulans LBSC in Irritable Bowel Syndrome: A Prospective, Interventional, Randomized, Double-Blind, Placebo-Controlled Clinical Study [CONSORT Compliant]. Medicine (Baltimore) 2021, 100, e23641. [Google Scholar] [CrossRef]
- Eskesen, D.; Jespersen, L.; Michelsen, B.; Whorwell, P.J.; Müller-Lissner, S.; Morberg, C.M. Effect of the Probiotic Strain Bifidobacterium Animalis Subsp. Lactis, BB-12®, on Defecation Frequency in Healthy Subjects with Low Defecation Frequency and Abdominal Discomfort: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Br. J. Nutr. 2015, 114, 1638–1646. [Google Scholar] [CrossRef]
- García-Collinot, G.; Madrigal-Santillán, E.O.; Martínez-Bencomo, M.A.; Carranza-Muleiro, R.A.; Jara, L.J.; Vera-Lastra, O.; Montes-Cortes, D.H.; Medina, G.; Cruz-Domínguez, M.P. Effectiveness of Saccharomyces Boulardii and Metronidazole for Small Intestinal Bacterial Overgrowth in Systemic Sclerosis. Dig. Dis. Sci. 2020, 65, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Saadi, M.; Ramsey, F.V.; Schey, R.; Parkman, H.P. Effect of Bifidobacterium Infantis 35624 (Align) on the Lactulose Breath Test for Small Intestinal Bacterial Overgrowth. Dig. Dis. Sci. 2018, 63, 989–995. [Google Scholar] [CrossRef]
- Ojetti, V.; Petruzziello, C.; Migneco, A.; Gnarra, M.; Gasbarrini, A.; Franceschi, F. Effect of Lactobacillus Reuteri (DSM 17938) on Methane Production in Patients Affected by Functional Constipation: A Retrospective Study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S.K. Bacillus Coagulans MTCC 5856 Supplementation in the Management of Diarrhea Predominant Irritable Bowel Syndrome: A Double Blind Randomized Placebo Controlled Pilot Clinical Study. Nutr. J. 2016, 15, 21. [Google Scholar] [CrossRef]
- Ducrotté, P.; Sawant, P.; Jayanthi, V. Clinical Trial: Lactobacillus Plantarum 299v (DSM 9843) Improves Symptoms of Irritable Bowel Syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, R.; Aruna, T.; Malar, S.; Shilpa, B.; Dhanasekar, K.R. Efficacy of Saccharomyces Cerevisiae CNCM I-3856 as an Add-on Therapy for Irritable Bowel Syndrome. Int. J. Colorectal Dis. 2020, 35, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Akhondi-Meybodi, M.; Rahimian, M.; Salmanroghani, H.; Amirbeigy, M.; Baghbanian, M.; Ghelmani, S. Study of the Effect of Probiotic Saccharomyces Boulardii on the Treatment of Irritable Bowel Syndrome. J. Biol. Today’s World 2014, 3, 152–156. [Google Scholar] [CrossRef]
- Lewis, E.D.; Antony, J.M.; Crowley, D.C.; Piano, A.; Bhardwaj, R.; Tompkins, T.A.; Evans, M. Efficacy of Lactobacillus Paracasei HA-196 and Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients 2020, 12, 1159. [Google Scholar] [CrossRef]
- Attar, A.; Flourie, B.; Rambaud, J.C.; Franchisseur, C.; Ruszniewski, P.; Bouhnik, Y. Antibiotic Efficacy in Small Intestinal Bacterial Overgrowth-Related Chronic Diarrhea: A Crossover, Randomized Trial. Gastroenterology 1999, 117, 794–797. [Google Scholar] [CrossRef]
- Bubnov, R.V.; Babenko, L.P.; Lazarenko, L.M.; Mokrozub, V.V.; Spivak, M.Y. Specific Properties of Probiotic Strains: Relevance and Benefits for the Host. EPMA J. 2018, 9, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Qu, C.; Wang, B.; Liang, S.; Zeng, B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth. J. Clin. Gastroenterol. 2017, 51, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Saffouri, G.B.; Shields-Cutler, R.R.; Chen, J.; Yang, Y.; Lekatz, H.R.; Hale, V.L.; Cho, J.M.; Battaglioli, E.J.; Bhattarai, Y.; Thompson, K.J.; et al. Small Intestinal Microbial Dysbiosis Underlies Symptoms Associated with Functional Gastrointestinal Disorders. Nat. Commun. 2019, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- Niv, E.; Halak, A.; Tiommny, E.; Yanai, H.; Strul, H.; Naftali, T.; Vaisman, N. Randomized Clinical Study: Partially Hydrolyzed Guar Gum (PHGG) versus Placebo in the Treatment of Patients with Irritable Bowel Syndrome. Nutr. Metab. 2016, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Reider, S.J.; Moosmang, S.; Tragust, J.; Trgovec-Greif, L.; Tragust, S.; Perschy, L.; Przysiecki, N.; Sturm, S.; Tilg, H.; Stuppner, H.; et al. Prebiotic Effects of Partially Hydrolyzed Guar Gum on the Composition and Function of the Human Microbiota—Results from the PAGODA Trial. Nutrients 2020, 12, 1257. [Google Scholar] [CrossRef]
- Polymeros, D.; Beintaris, I.; Gaglia, A.; Karamanolis, G.; Papanikolaou, I.S.; Dimitriadis, G.; Triantafyllou, K. Partially Hydrolyzed Guar Gum Accelerates Colonic Transit Time and Improves Symptoms in Adults with Chronic Constipation. Dig. Dis. Sci. 2014, 59, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C.; Swanson, K.S. Agave Inulin Supplementation Affects the Fecal Microbiota of Healthy Adults Participating in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Linetzky Waitzberg, D.; Alves Pereira, C.C.; Logullo, L.; Manzoni Jacintho, T.; Almeida, D.; Teixeira da Silva, M.d.L.; Matos de Miranda Torrinha, R.S. Microbiota Benefits after Inulin and Partially Hydrolized Guar Gum Supplementation: A Randomized Clinical Trial in Constipated Women. Nutr. Hosp. 2012, 27, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Oskouie, F.H.; Vahedi, H.; Shahrbaf, M.A.; Sadeghi, A.; Rashidkhani, B.; Hekmatdoost, A. Gastroenterology and Hepatology from Bed to Bench. Dietary Fiber and Risk of Irritable Bowel Syndrome: A Case-Control Study. Gastroenterol. Hepatol. Bed Bench 2018, 11, 20–24. [Google Scholar]
- Garg, P. Inflammation in Irritable Bowel Syndrome (IBS): Role of Psyllium Fiber Supplementation in Decreasing Inflammation and Physiological Management of IBS. Turk. J. Gastroenterol. 2021, 32, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Ginnebaugh, B.; Chey, W.D.; Saad, R. Small Intestinal Bacterial Overgrowth: How to Diagnose and Treat (and Then Treat Again). Gastroenterol. Clin. N. Am. 2020, 49, 571–587. [Google Scholar] [CrossRef]
- Khayyatzadeh, S.S.; Kazemi-Bajestani, S.M.R.; Mirmousavi, S.J.; Heshmati, M.; Khoshmohabbat, S.; Ferns, G.A.; Ghayour-Mobarhan, M. Dietary Behaviors in Relation to Prevalence of Irritable Bowel Syndrome in Adolescent Girls. J. Gastroenterol. Hepatol. 2018, 33, 404–410. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome as Well as Traditional Dietary Advice: A Randomized Controlled Trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef] [PubMed]
- Zaribaf, F.; Keshteli, A.H.; Esmaillzadeh, A.; Saneei, P.; Feizi, A.; Daghaghzadeh, H.; Feinle-Bisset, C.; Adibi, P. Empirically Derived Dietary Habits Are Associated with Irritable Bowel Syndrome. Eur. J. Clin. Nutr. 2018, 72, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Vakhshuury, M.; Khoshdel, A. The Relation between Dietary Patterns and Functional Gastrointestinal Disorders among Iranian Military Men. Adv. Biomed. Res. 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Type of Study | Period | Study Group | Intervention/Control | Methods | Outcome |
|---|---|---|---|---|---|
| Patcharatrakul et al., 2019 [17] Randomized controlled trial | 4 weeks | 62 IBS | SILFD/BRD | Breath test | ↓H2 volume |
| McIntoshet al., 2017 [28] Randomized controlled trial | 3 weeks | 37 IBS | LFD/HFD | Breath test 16S rRNA | ↓H2 volume ↓Bifidobacterium ↑Porphyromonadaceae |
| Bennet et al., 2018 [27] Randomized controlled trial | 4 weeks | 67 IBS | LFD/TDA | GA-map Dysbiosis test | ↓Bifidobacterium, ↑Dysbiosis index |
| Zhang et al., 2021 [24] Parallel-group, randomized controlled trial | 3 weeks | 100 IBS | LFD/TDA | 16S rRNA | ↓Bifidobacterium, ↓Fusobacterium, ↓Bacterioides ↑Bilophila |
| Huaman et al., 2018 [23] Randomized controlled trial | 4 weeks | 40 FGIDs | LFD/MD +(B-GOS) | 16S rRNA | ↓Bifidobacterium ↑Bilophila |
| Halmos et al., 2015 [26] Single-blinded, randomized, crossover trial | 3 weeks | 27 IBS 6 Healthy | LFD/AD | 16S rRNA | ↓Akkermansia Muciniphila ↓Faecalibacterium prausnitzii ↓Ruminococcus torques |
| Staudacher et al., 2012 [22] Randomized controlled trial | 4 weeks | 41 IBS | LFD/HD | FISH | ↓Bifidobacterium |
| Naseri et al., 2021 [31] Clinical trial | 6 weeks | 42 IBS | LF-GFD | 16S rRNA | ↑Bacterioides ↑Bifidobacterium, Lactobacillus |
| Wilson et al., 2020 [29] Randomized placebo-controlled trial | 4 weeks | 69 IBS | LFD +(B-GOS/placebo)/sham diet + placebo supplement | 16S rRNA | ↓Bifidobacterium, ↓Actinobacteria |
| Staudacher et al., 2021 [21] Randomized controlled trial | 4 weeks | 95 IBS | LFD/sham diet +(probiotic/placebo) | 16S rRNA | ↓Bifidobacterium ↑Bacterioides |
| Hustoft et al., 2017 [25] Randomized, double- blinded, placebo-controlled crossover study | 9 weeks | 20 IBS | LFD/HFD + FOS/maltodextrin | 16S rRNA |
↓Bifidobacterium, ↓Clostridium, Faecalibacterium ↑Bilophila |
| Harvie et al., 2017 [30] Randomized controlled trial | 6 months | 50 IBS | LFD/TDA + reintroduction | 16S rRNA | No change in the microbiota |
| Authors, Year, Type of Study | Duration of Study | Study Group | Intervention/Control | Dose/Day |
|---|---|---|---|---|
| Gayathri et al., 2021 [44] Randomized controlled trial | 8 weeks | 100 IBS | S. cerevisiae CNCM I-3856/placebo | 2 × 109 CFU |
| Gupta et al., 2021 [37] Randomized controlled trial | 80 days | 40 IBS | Bacillus coagulans LBSC (DSM17654)/placebo | 2 × 1012 CFU |
| Madempud et al., 2020 [36] Randomized controlled trial | 4 weeks | 100 FC | B. coagulans Unique IS2/placebo | 2 × 1012 CFU |
| García-Collinot et al., 2020 [39] Clinical trial | 2 months | 75 SIBO + SSc | S.Boulardii(SB)/metronidazole (M)SB + M | 200 mg |
| Lewis et al., 2020 [46] Randomized controlled trial | 8 weeks | 251 IBS | Lactobacillus paracasei HA-196/Bifidobacterium longum R0175/placebo | 10 × 109 CFU |
| Kumar et al., 2018 [40] Randomized controlled trial | 2 weeks | 19 healthy | B.infantis 35624/placebo | No data |
| Ojetti et al., 2017 [41] Retrospective study | 4 weeks | 20 constipated | L. reuteri (DSM 17938) | 2 × 108 CFU |
| Majeed et al., 2016 [42] Randomized controlled trial | 3 months | 36 IBS-D | B. coagulans(MTCC 5856)/placebo | 2 × 109 CFU |
| Eskesen et al., 2015 [38] Randomized controlled trial | 4 weeks | 1248 with low defecation frequency | Bifidobacterium animalis subsp. lactis, BB-12/placebo | 1 × 1012 or 10× 1012 CFU |
| Akhondi-Meybodi et al., 2014 [45] Randomized clinical trial | 3 weeks | 60 IBS | Saccharomyces boulardii CNCM I 745/placebo | 200mg |
| Ducrotté et al., 2012 [43] Clinical trial | 4 weeks | 214 IBS | L. plantarum 299v (DSM 9843)/placebo | 10 × 1012 CFU |
| Key Results | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fully Characterized Strains | Diarrhea | Stool Frequency/ Consistency | Bloating | Abdominal Pain | SBM | Gas Release | H2 Volume | CH4 Volume |
| B. infantis 35624 | ND 1 | ND | ND | ND | ND | ND | No change | ↑ |
| L. reuteri (DSM 17938) | ↓ | ND | ND | ND | ND | ND | No change | ↓ |
| B. coagulans(MTCC 5856) | ↓ | ↑ | ↓ | ↓ | ND | ND | ND | ND |
| B. coagulans LBSC (DSM17654) | ↓ | ↑ | ↓ | ↓ | ND | ND | ND | ND |
| B. coagulans Unique IS2 | ND | ↑ | ND | ↓ | ND | ND | ND | ND |
| L. plantarum 299v (DSM 9843) | ND | ↑ | ↓ | ↓ | ND | ND | ND | ND |
| S. cerevisiae CNCM I-3856 | ND | ↑ | ND | ↓ | ND | ND | ND | ND |
| S. boulardii CNCM I 745 | ↓ | ND | ↓ | ↓ | ND | ↓ | ↓ | ND |
| B. animalis subsp. lactis, BB-12 | ND | ↑ | ↓ | ↓ | ND | ND | ND | ND |
| L. paracasei HA-196 | ND | ND | ND | ND | ND | ND | ND | ND |
| Author, Year, Type of Study | Study Group | Characteristics of Group | Methods |
|---|---|---|---|
| Zaribaf et al., 2019 [61] Cross-sectional study | 4763 adults | Iranian | Rome III questionnaire Questions about: meal patterns, eating rate, chewing quality |
| Vakhshuury et al., 2019 [62] Cross-sectional study | 600 adults | Military personnel in Iran | Rome III questionnaire, FFQ Questions about: breakfast consumption, lunch intake time, chewing efficiency |
| Khayyatzadeh et al., 2018 [59] Randomized controlled trial | 988 adults | Iranian | Rome III questionnaire, FFQ, dietary behaviors assessment Questions about: meal pattern, chewing quality |
| Böhn et al., 2015 [60] Randomized controlled trial | 75 adults | 18–70 year old Swedish met Rome III criteria for IBS | Rome III criteria, IBS-SSS questionnaire 4-day food diary |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wielgosz-Grochowska, J.P.; Domanski, N.; Drywień, M.E. Efficacy of an Irritable Bowel Syndrome Diet in the Treatment of Small Intestinal Bacterial Overgrowth: A Narrative Review. Nutrients 2022, 14, 3382. https://doi.org/10.3390/nu14163382
Wielgosz-Grochowska JP, Domanski N, Drywień ME. Efficacy of an Irritable Bowel Syndrome Diet in the Treatment of Small Intestinal Bacterial Overgrowth: A Narrative Review. Nutrients. 2022; 14(16):3382. https://doi.org/10.3390/nu14163382
Chicago/Turabian StyleWielgosz-Grochowska, Justyna Paulina, Nicole Domanski, and Małgorzata Ewa Drywień. 2022. "Efficacy of an Irritable Bowel Syndrome Diet in the Treatment of Small Intestinal Bacterial Overgrowth: A Narrative Review" Nutrients 14, no. 16: 3382. https://doi.org/10.3390/nu14163382
APA StyleWielgosz-Grochowska, J. P., Domanski, N., & Drywień, M. E. (2022). Efficacy of an Irritable Bowel Syndrome Diet in the Treatment of Small Intestinal Bacterial Overgrowth: A Narrative Review. Nutrients, 14(16), 3382. https://doi.org/10.3390/nu14163382








