Perception and Understanding of Guideline Daily Amount and Warning Labeling among Mexican Adults during the Law Modification Period
Abstract
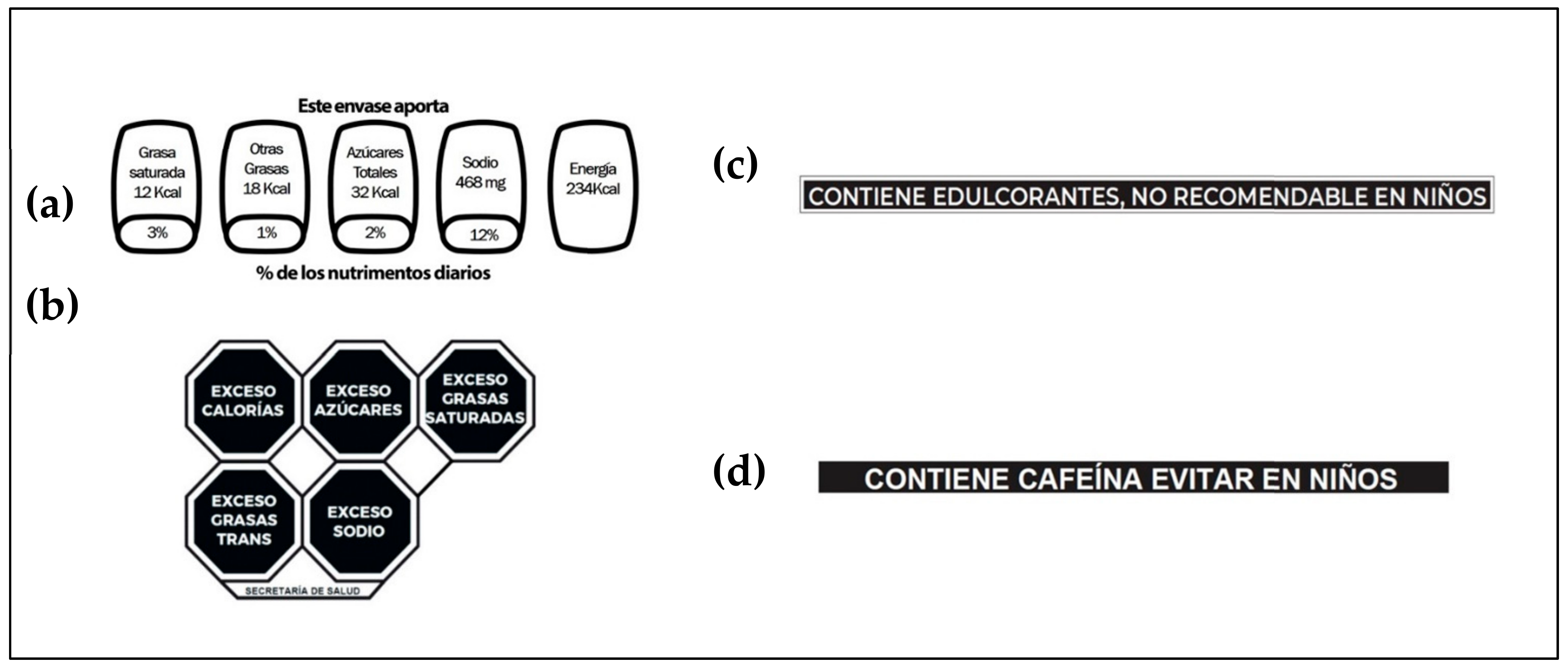
:1. Introduction
2. Materials and Methods
2.1. Recruitment and Procedures
2.2. Perception of the FOPL
2.3. Subjective Understanding of FOPL
2.4. Knowledge and Perception of Non-Caloric Sweeteners and Caffeine Disclaimers
2.5. Covariates
2.6. Determination of Sample Size and Sampling Procedure
2.7. Ethical Considerations
2.8. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organización Mundial de la Salud. Alimentos y Bebidas Ultraprocesados en América Latina: Tendencias, Efecto Sobre la Obesidad e Implicaciones Para las Políticas Públicas; Organización Panamericana de la Salud: Washington, DC, USA, 2015; ISBN 9789275718643. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; da Costa Louzada, M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019; ISBN 9789251317013. [Google Scholar]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef]
- Van Kleef, E.; Dagevos, H. The growing role of front-of-pack nutrition profile labeling: A consumer perspective on key issues and controversies. Crit. Rev. Food Sci. Nutr. 2015, 55, 291–303. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.-C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The Star Shines Bright. World Nutr. (Food Classification. Public Health). 2016, 7, 28–38. [Google Scholar]
- Organización Panamericana de la Salud. Modelo de Perfil de Nutrientes de la Organización Panamericana de la Salud; Organización Panamericana de la Salud: Washington, DC, USA, 2016. [Google Scholar]
- Lawrence, M.A.; Baker, P.I. Ultra-processed food and adverse health outcomes. BMJ 2019, 365, l2289. [Google Scholar] [CrossRef]
- Cecchini, M.; Warin, L. Impact of food labelling systems on food choices and eating behaviours: A systematic review and meta-analysis of randomized studies. Obes. Rev. 2016, 17, 201–210. [Google Scholar] [CrossRef]
- Khandpur, N.; de Morais Sato, P.; Mais, L.A.; Bortoletto Martins, A.P.; Spinillo, C.G.; Garcia, M.T.; Urquizar Rojas, C.F.; Jaime, P.C. Are front-of-package warning labels more effective at communicating nutrition information than traffic-light labels? A randomized controlled experiment in a Brazilian sample. Nutrients 2018, 10, 688. [Google Scholar] [CrossRef]
- Méjean, C.; Macouillard, P.; Péneau, S.; Hercberg, S.; Castetbon, K. Perception of front-of-pack labels according to social characteristics, nutritional knowledge and food purchasing habits. Public Health Nutr. 2013, 16, 392–402. [Google Scholar] [CrossRef]
- Vargas-Meza, J.; Jaúregui, A.; Contreras-Manzano, A.; Nieto, C.; Barquera, S. Acceptability and understanding of front-of-pack nutritional labels: An experimental study in Mexican consumers. BMC Public Health 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud. El Etiquetado Frontal Como Instrumento de Política Para Prevenir Enfermedades No Transmisibles en la Región de las Américas; Organización Panamericana de la Salud: Washington, DC, USA, 2020. [Google Scholar]
- Federación, D.O. de la Modificación de la Norma Oficial Mexicana NOM-051-SCFI/SSA1-2010, Especificaciones Generales de Etiquetado Para Alimentos y BEBIDAS No alcohólicas Preenvasados-Información Comercial y Sanitaria, Publicada el 5 de abril de 2010. Available online: http://www.dof.gob.mx/nota_detalle.php?codigo=5356328&fecha=14/08/2014 (accessed on 26 July 2016).
- Vargas-Meza, J.; Jáuregui, A.; Pacheco-Miranda, S.; Contreras-Manzano, A.; Barquera, S. Front-of-pack nutritional labels: Understanding by low- and middle-income Mexican consumers. PLoS ONE 2019, 14, e0225268. [Google Scholar] [CrossRef]
- Secretaría de Economía MODIFICACIÓN a la Norma Oficial Mexicana NOM-051-SCFI/SSA1-2010, Especificaciones Generales de Etiquetado Para Alimentos y Bebidas No Alcohólicas Preenvasados-Información Comercial y Sanitaria, Publicada el 5 de abril de 2010. Available online: https://www.dof.gob.mx/2020/SEECO/NOM_051.pdf (accessed on 12 June 2020).
- Nieto, C.; Jauregui, A.; Contreras-Manzano, A.G.; Arillo-Santillán, E.; Barquera, S.; White, C.; Hammond, D.; Trasher, J. Understanding of food labeling systems among White, Latinos, and Mexican population: Data from the International Food Policy Study 2017. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 87. [Google Scholar] [CrossRef]
- Taillie, L.S.; Hall, M.G.; Popkin, B.M.; Ng, S.W.; Murukutla, N. Experimental studies of front-of-package nutrient warning labels on sugar-sweetened beverages and ultra-processed foods: A scoping review. Nutrients 2020, 12, 569. [Google Scholar] [CrossRef]
- Contreras-Manzano, A.; Alejandra, J.; Velasco-Bernal, A.; Vargas-Meza, J.; Rivera, J.A.; Tolentino-Mayo, L.; Barquera, S. Comparative Analysis of the Classification of Food Different Nutrient Profiling Systems. Nutrients 2018, 10, 737. [Google Scholar] [CrossRef]
- Talati, Z.; Egnell, M.; Hercberg, S.; Julia, C.; Pettigrew, S. Consumers’ perceptions of five front-of-package nutrition labels: An experimental study across 12 countries. Nutrients 2019, 11, 1934. [Google Scholar] [CrossRef]
- Bearth, A.; Cousin, M.-E.; Siegrist, M. The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perceptions. Food Qual. Prefer. 2014, 38, 14–23. [Google Scholar] [CrossRef]
- Mexican Association of Market and Opinion Intelligence Agencies AMAI Questionnaire 2018. Available online: http://www.amai.org/nse/wp-content/uploads/2018/04/Nota-Metodoloόgico-NSE-2018-v3.pdf (accessed on 14 January 2019).
- Graubard, B.I.; Korn, E.L. Predictive margins with survey data. Biometrics 1999, 55, 652–659. [Google Scholar] [CrossRef]
- Pauline, D.; Caroline, M.; Chantal, J.; Emmanuelle, K.G.; Mathilde, T.; Léopold, F.; Serge, H.; Sandrine, P. Effectiveness of front-of-pack nutrition labels in french adults: Results from the nutrinet-santé cohort study. PLoS ONE 2015, 10, e0140898. [Google Scholar] [CrossRef]
- Teresa, C.; Fierro, C.; Reyes, M.; Dillman-Carpentier, F.R.; Taillie, L.S.; Corvalan, C. Responses to the Chilean law of food labeling and advertising: Exploring knowledge, perceptions and behaviors of mothers of young children. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Freire, W.B.; Waters, W.F.; Rivas-Mariño, G. Semáforo nutricional de alimentos procesados: Estudio cualitativo sobre conocimientos, comprensión, actitudes y prácticas en el Ecuador. Rev. Peru. Med. Exp. Salud Publica 2017, 34, 11–18. [Google Scholar] [CrossRef]
- Jáuregui, A.; Vargas-Meza, J.; Nieto, C.; Contreras-Manzano, A.; Alejandro, N.Z.; Tolentino-Mayo, L.; Hall, M.G.; Barquera, S. Impact of front-of-pack nutrition labels on consumer purchasing intentions: A randomized experiment in low- and middle-income Mexican adults. BMC Public Health 2020, 20, 463. [Google Scholar] [CrossRef]
- Stern, D.; Tolentino, L.; Barquera, S. Revisión del Etiquetado Frontal: Análisis de las Guías Diarias de Alimentación (GDA) y su Comprensión por Estudiantes de Nutrición en México; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2011. [Google Scholar]
- De la Cruz-Góngora, V.; Torres, P.; Contreras-Manzano, A.; Jáuregui de la Mota, A.; Mundo-Rosas, V.; Villalpando, S.; Rodríguez-Oliveros, G. Understanding and acceptability by Hispanic consumers of four front-of-pack food labels. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Tolentino-Mayo, L.; Rincón-Gallardo Patiño, S.; Bahena-Espina, L.; Ríos, V.; Barquera, S. Conocimiento y uso del etiquetado nutrimental de alimentos y bebidas industrializados en México. Salud Publica Mex 2018, 60, 328–337. [Google Scholar] [CrossRef]
- Nieto, C.; Alcalde-Rabanal, J.; Mena, C.; Carriedo, Á.; Barquera, S. Perception of the use and understanding of nutrition labels among different socioeconomic groups in Mexico: A qualitative study. Salud Publica Mex. 2020, 62, 288–297. [Google Scholar] [CrossRef]
- Sagaceta-Mejía, J.; Tolentino-Mayo, L.; Cruz-Casarrubias, C.; Nieto, C.; Barquera, S. Understanding of front of package nutrition labels: Guideline daily amount and warning labels in Mexicans with non-communicable diseases. PLoS ONE 2022, 17, e0269892. [Google Scholar] [CrossRef]
- Mejia, C.R.; Aguilar-Pantaleón, C.; Alfaro, S.; Carranza, B.; Eulogio, P.; Gálvez-N, A.; Godo, G. Factors that influence the use of the octagon as a marker of nutritional information in consumers in the population of Lima-Peru. Nutr. Clin. y Diet. Hosp. 2020, 39, 65–71. [Google Scholar] [CrossRef]
- Ares, G.; Antúnez, L.; Otterbring, T.; Curutchet, M.R.; Galicia, L.; Moratorio, X.; Bove, I. Sick, salient and full of salt, sugar and fat: Understanding the impact of nutritional warnings on consumers’ associations through the salience bias. Food Qual. Prefer. 2020, 86, 103991. [Google Scholar] [CrossRef]
- Brasil, G. de DIÁRIO OFICIAL DA UNIÃO: RESOLUÇÃO DE DIRETORIA COLEGIADA—RDC No 429. Available online: https://www.in.gov.br/en/web/dou/-/resolucao-de-diretoria-colegiada-rdc-n-429-de-8-de-outubro-de-2020-282070599 (accessed on 19 August 2021).
- Bandeira, L.M.; Pedroso, J.; Toral, N.; Gubert, M.B. Performance and perception on front-of-package nutritional labeling models in Brazil. Rev. Saude Publica 2021, 55, 19. [Google Scholar] [CrossRef]
- Plasek, B.; Lakner, Z.; Temesi, Á. Factors that Influence the Perceived Healthiness of Food—Review. Nutrients 2020, 12, 1881. [Google Scholar] [CrossRef]
- Farhat, G.; Dewison, F.; Stevenson, L. Knowledge and Perceptions of Non-Nutritive Sweeteners Within the UK Adult Population. Nutrients 2021, 13, 444. [Google Scholar] [CrossRef]
- Goodman, S.; Vanderlee, L.; Jones, A.; White, C.; Hammond, D. Perceived Healthiness of Sweeteners among Young Adults in Canada. Can. J. Diet. Pract. Res. 2021, 82, 90–94. [Google Scholar] [CrossRef]
- Vargas-Meza, J.; Pacheco-Miranda, S.; Ayvar-Gama, Y.; Cervantes-Armenta, M.A.; Nieto, C.; Cruz-Casarrubias, C.; Munguía-Serrano, A.; Barquera, S.; Campos-Nonato, I. Perceptions associated with Mexican warning label: A qualitative study in Mexican Adults. Appetite Under review. 2022. [Google Scholar]
- Shim, S.-M.; Seo, S.H.; Lee, Y.; Moon, G.-I.; Kim, M.-S.; Park, J.-H. Consumers’ knowledge and safety perceptions of food additives: Evaluation on the effectiveness of transmitting information on preservatives. Food Control 2011, 22, 1054–1060. [Google Scholar] [CrossRef]
- Franco-Arellano, B.; Vanderlee, L.; Ahmed, M.; Oh, A.; L’Abbé, M. Influence of front-of-pack labelling and regulated nutrition claims on consumers’ perceptions of product healthfulness and purchase intentions: A randomized controlled trial. Appetite 2020, 149, 104629. [Google Scholar] [CrossRef] [PubMed]
- Taillie, L.S.; Reyes, M.; Colchero, M.A.; Popkin, B.; Corvalán, C. An evaluation of Chile’s Law of Food Labeling and Advertising on sugar-sweetened beverage purchases from 2015 to 2017: A before-and-after study. PLOS Med. 2020, 17, e1003015. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Smith Taillie, L.; Popkin, B.; Kanter, R.; Vandevijvere, S.; Corvalán, C. Changes in the amount of nutrient of packaged foods and beverages after the initial implementation of the Chilean Law of Food Labelling and Advertising: A nonexperimental prospective study. PLoS Med. 2020, 17, e1003220. [Google Scholar] [CrossRef] [PubMed]
- El Financiero Menos Sodio y Menos Azúcar: Así Reformulan las Marcas sus Productos tras Nuevo Etiquetado. Available online: https://www.elfinanciero.com.mx/empresas/el-nuevo-etiquetado-de-advertencia-obliga-a-reformular-productos/ (accessed on 20 September 2021).
- INEGI Instituto Nacional de Estadística y Geografía. Available online: http://www.inegi.org.mx/ (accessed on 15 January 2018).

| n | % | |
|---|---|---|
| Age (years), mean ± SD | 36.5 | 15.35 |
| Age Tertiles (years) | ||
| 18–25 | 382 | 32.3 |
| 26–42 | 329 | 30.4 |
| 43–75 | 372 | 34.4 |
| Gender (Female) | 649 | 60% |
| Socio Economic Status | ||
| Low | 755 | 69.7 |
| Medium | 328 | 30.3 |
| Academic level | ||
| Secondary or lower | 169 | 15.6 |
| High school | 359 | 33.2 |
| Bachelor’s degree or above | 555 | 51.3 |
| BMI (kg/m2) a | ||
| Normal | 460 | 42.5 |
| Overweight | 407 | 37.6 |
| Obesity | 216 | 19.9 |
| Previous diagnosis | ||
| Hypertension | 151 | 13.9 |
| Diabetes | 79 | 7.3 |
| Overweight | 457 | 42.2 |
| Obesity | 173 | 16.0 |
| High cholesterol | 153 | 14.1 |
| High triglycerides | 147 | 13.6 |
| Nutrition knowledge b | ||
| Nothing knowledgeable | 145 | 13.4 |
| A little knowledgeable | 505 | 46.6 |
| Somewhat and very knowledgeable | 433 | 40.0 |
| Interest in own health c | ||
| Not or a little interested | 34 | 3.1 |
| Sufficiently interested | 337 | 31.1 |
| Very interested | 712 | 65.7 |
| GDA 1 | WL 1 | GDA-WL 2 | ||||
|---|---|---|---|---|---|---|
| September 2020 | October–November 2020 | |||||
| % | 95%CI | % | 95%CI | Diff pp | 95%CI | |
| Would you consume a food with this label more frequently? (Unlikely- very unlikely) 3 | 24.2 | 21.7, 26.7 | 81.5 | 79.2, 83.8 | 57.3 | 54.0, 60.6 |
| In your opinion, in what quantities should a product with this label be consumed? (Small or very small amounts) 3 | 41.6 | 38.7, 44.6 | 93.8 | 92.4, 95.5 | 52.2 | 48.9, 55.4 |
| What would you do if you saw this label on a product that you usually buy? (Probably or very probably stop buying it) 3 | 24.4 | 21.8, 26.9 | 72.8 | 70.1, 75.4 | 48.4 | 44.8, 51.9 |
| This label catches my attention (strongly agree- totally agree) 3 | 14.8 | 12.7, 16.9 | 51.6 | 48.7, 54.6 | 36.9 | 33.2, 40.5 |
| If a product had this label, you could easily identify and read it (strongly agree- totally agree) 3 | 19.8 | 17.5, 22.2 | 62.6 | 59.7, 65.5 | 42.8 | 39.2, 46.3 |
| I consider the information on this label to be credible and true (strongly agree- totally agree) 3 | 17.8 | 15.5, 20.1 | 53.3 | 50.3, 56.2 | 35.5 | 32.0, 38.9 |
| I think this label will not help me identify healthier product (strongly disagree- totally disagree) 3 | 24.6 | 22.0, 27.1 | 47.1 | 44.1, 50.1 | 22.5 | 18.8, 26.3 |
| I think this label is easy to understand (strongly agree- totally agree) 3 | 20.9 | 18.5, 23.3 | 60.4 | 57.5, 63.3 | 39.5 | 36.0, 43.1 |
| This label will help me to decide quickly what products to buy (strongly agree- totally agree) 3 | 19.1 | 16.8, 21.4 | 53.1 | 50.2, 56.0 | 34.0 | 30.4, 37.6 |
| This label will help me decide whether or not to buy a product (strongly agree- totally agree) 3 | 20.8 | 18.4, 23.2 | 54.3 | 51.4, 57.2 | 33.5 | 29.9, 37.2 |
| This label will not change my decision about which products to buy (strongly disagree- totally disagree) 3 | 17.1 | 14.8, 19.3 | 38.8 | 35.9, 41.7 | 21.7 | 18.1, 25.3 |
| Reference: GDA | Dairy | RTEC | Salty Snack | SSB | RTEF |
|---|---|---|---|---|---|
| Perception | Diff. PP ± SE | Diff. PP ± SE | Diff. PP ± SE | Diff. PP ± SE | Diff. PP ± SE |
| How attractive is the product for consumption? 2 (Attractive or very attractive) | −4.4 ± 1.39 | −21.1 ± 1.67 | −14.5 ± 1.70 | −19.0 ± 1.71 | −4.5 ± 1.09 |
| How healthy is the product? 3 (Healthy or very healthy) | −8.4 ± 1.11 | −20.7 ± 1.38 | −8.1 ± 1.18 | −12.9 ± 1.38 | −5.2 ± 1.00 |
| Would you buy this product for yourself or your family? 2 (Unlikely- very unlikely) | −5.0 ± 1.11 | −20.0 ± 1.56 | −7.1 ± 1.43 | −11.1 ± 1.57 | −4.2 ± 0.95 |
| How often would you buy this product for yourself? 2 (once or twice per month or never) | 4.0 ± 1.06 | 5.8 ± 1.07 | 3.2 ± 1.23 | 6.0 ± 1.50 | 2.2 ± 0.86 |
| Does the label of this product provide enough information to determine if it’s healthy? 4 (Is not informative enough) | −22.2 ± 1.76 | −22.4 ± 1.71 | −15.2 ± 1.62 | −14.8 ± 1.67 | −17.6 ± 1.65 |
| Front of pack labeling makes you feel? 4 (Safer to decide if the product is healthy) | 38.1 ± 1.93 | 37.4 ± 1.87 | 34.9 ± 1.90 | 34.2 ± 1.89 | 37.0 ± 1.85 |
| Subjective Understanding | |||||
| Correct identification of total number of high critical nutrients 5 | 39.1 ± 1.71 | 74.9 ± 1.41 | 54.8 ± 1.68 | 27.7 ± 1.53 | 35.1 ± 1.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Nonato, I.; Cervantes-Armenta, M.A.; Pacheco-Miranda, S.; Quezada-Sánchez, A.D.; Contreras-Manzano, A.; Barquera, S.; Vargas-Meza, J. Perception and Understanding of Guideline Daily Amount and Warning Labeling among Mexican Adults during the Law Modification Period. Nutrients 2022, 14, 3403. https://doi.org/10.3390/nu14163403
Campos-Nonato I, Cervantes-Armenta MA, Pacheco-Miranda S, Quezada-Sánchez AD, Contreras-Manzano A, Barquera S, Vargas-Meza J. Perception and Understanding of Guideline Daily Amount and Warning Labeling among Mexican Adults during the Law Modification Period. Nutrients. 2022; 14(16):3403. https://doi.org/10.3390/nu14163403
Chicago/Turabian StyleCampos-Nonato, Ismael, Manuel A. Cervantes-Armenta, Selene Pacheco-Miranda, Amado D. Quezada-Sánchez, Alejandra Contreras-Manzano, Simón Barquera, and Jorge Vargas-Meza. 2022. "Perception and Understanding of Guideline Daily Amount and Warning Labeling among Mexican Adults during the Law Modification Period" Nutrients 14, no. 16: 3403. https://doi.org/10.3390/nu14163403
APA StyleCampos-Nonato, I., Cervantes-Armenta, M. A., Pacheco-Miranda, S., Quezada-Sánchez, A. D., Contreras-Manzano, A., Barquera, S., & Vargas-Meza, J. (2022). Perception and Understanding of Guideline Daily Amount and Warning Labeling among Mexican Adults during the Law Modification Period. Nutrients, 14(16), 3403. https://doi.org/10.3390/nu14163403







