Effects of Diets Enriched with Conventional or High-Oleic Canola Oils on Vascular Endothelial Function: A Sub-Study of the Canola Oil Multi-Centre Intervention Trial 2 (COMIT-2), a Randomized Crossover Controlled Feeding Study
Abstract
:1. Introduction
2. Materials and Methods
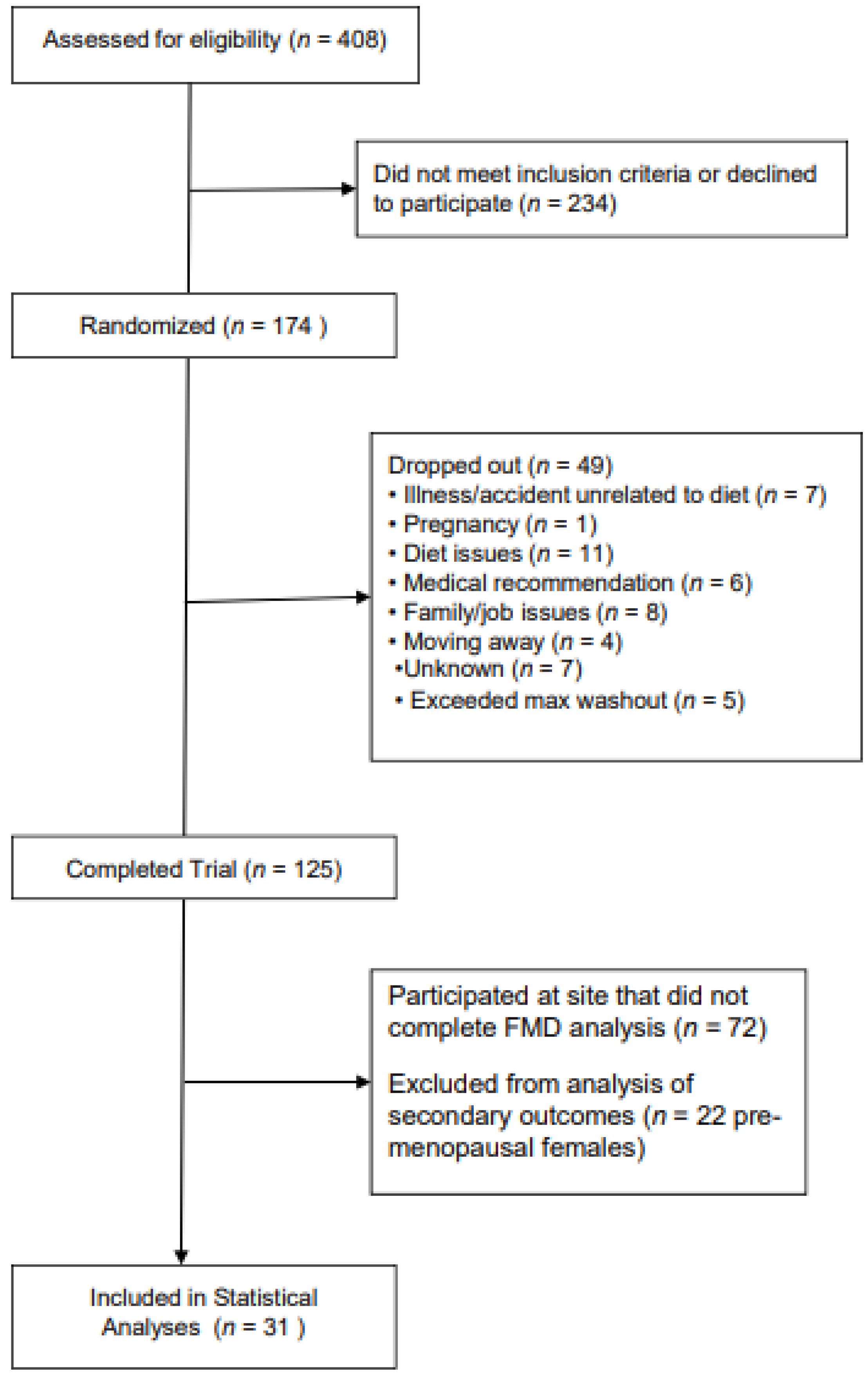
2.1. Participants
2.2. Intervention
2.3. Outcome Assessment
2.4. Endothelial Function
2.5. Blood Sample Collection and Analysis
2.6. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, D.; Kongpakpaisarn, K.; Bohra, C. Trends in the Prevalence of Metabolic Syndrome and Its Components in the United States 2007–2014. Int. J. Cardiol. 2018, 259, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Alshammary, A.F.; Alharbi, K.K.; Alshehri, N.J.; Vennu, V.; Khan, I.A. Metabolic Syndrome and Coronary Artery Disease Risk: A Meta-Analysis of Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 1773. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic Syndrome—A New World-Wide Definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Paoletti, R.; Bolego, C.; Poli, A.; Cignarella, A. Metabolic Syndrome, Inflammation and Atherosclerosis. Vasc. Health Risk Manag. 2006, 2, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Solymoss, B.C.; Bourassa, M.G.; Campeau, L.; Sniderman, A.; Marcil, M.; Lespérance, J.; Lévesque, S.; Varga, S. Effect of Increasing Metabolic Syndrome Score on Atherosclerotic Risk Profile and Coronary Artery Disease Angiographic Severity. Am. J. Cardiol. 2004, 93, 159–164. [Google Scholar] [CrossRef]
- Zhao, Y.; Evans, M.A.; Allison, M.A.; Bertoni, A.G.; Budoff, M.J.; Criqui, M.H.; Malik, S.; Ouyang, P.; Polak, J.F.; Wong, N.D. Multisite Atherosclerosis in Subjects with Metabolic Syndrome and Diabetes and Relation to Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2019, 282, 202–209. [Google Scholar] [CrossRef]
- Aboonabi, A.; Meyer, R.R.; Singh, I. The Association between Metabolic Syndrome Components and the Development of Atherosclerosis. J. Hum. Hypertens. 2019, 33, 844–855. [Google Scholar] [CrossRef]
- Varghese, J.F.; Patel, R.; Yadav, U.C.S. Novel Insights in the Metabolic Syndrome-Induced Oxidative Stress and Inflammation-Mediated Atherosclerosis. Curr. Cardiol. Rev. 2017, 14, 4–14. [Google Scholar] [CrossRef]
- Huang, P.L. ENOS, Metabolic Syndrome and Cardiovascular Disease. Trends Endocrinol. Metab. 2009, 20, 295–302. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic Syndrome: A Multiplex Cardiovascular Risk Factor. J. Clin. Endocrinol. Metab. 2007, 92, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Reynolds, K.; He, J. Metabolic Syndrome and Risk of Cardiovascular Disease: A Meta-Analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic Syndrome and Cardiovascular Diseases: Going beyond Traditional Risk Factors. Diabetes. Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; Topper, J.N.; Nagel, T.; Anderson, K.R.; Garcia-Cardeña, G. Endothelial Dysfunction, Hemodynamic Forces, and Atherogenesis. Ann. N. Y. Acad. Sci. 2000, 902, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial Dysfunction: A Marker of Atherosclerotic Risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef]
- Chien, K.R. Molecular Basis of Cardiovascular Disease: A Companion to Braunwald’s Heart Disease. J. Vasc. Surg. 1999, 30, 361–362. [Google Scholar]
- Fornoni, A.; Raij, L. Metabolic Syndrome and Endothelial Dysfunction. Curr. Hypertens. Rep. 2005, 7, 88–95. [Google Scholar] [CrossRef]
- Hammad, S.; Pu, S.; Jones, P.J. Current Evidence Supporting the Link Between Dietary Fatty Acids and Cardiovascular Disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef]
- Huth, P.J.; Fulgoni, V.L.; Larson, B.T. A Systematic Review of High-Oleic Vegetable Oil Substitutions for Other Fats and Oils on Cardiovascular Disease Risk Factors: Implications for Novel High-Oleic Soybean Oils. Adv. Nutr. 2015, 6, 674–693. [Google Scholar] [CrossRef]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated Fats Compared with Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease: A Prospective Cohort Study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Li, Y.; Sampson, L.; Dougherty, L.W.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Monounsaturated Fats from Plant and Animal Sources in Relation to Risk of Coronary Heart Disease among US Men and Women. Am. J. Clin. Nutr. 2018, 107, 445–453. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration Qualified Health Claims: Letter of Enforcement Discretion—Unsaturated Fatty Acids from Canola Oil and Reduced Risk of Coronary Heart Disease (Docket No. 2006Q-0091). 2006. Available online: http://wayback.archive-it.org/7993/20171114183734/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072958.htm (accessed on 22 June 2021).
- Bowen, K.J.; Kris-Etherton, P.M.; West, S.G.; Fleming, J.A.; Connelly, P.W.; Lamarche, B.; Couture, P.; Jenkins, D.J.A.; Taylor, C.G.; Zahradka, P.; et al. Diets Enriched with Conventional or High-Oleic Acid Canola Oils Lower Atherogenic Lipids and Lipoproteins Compared to a Diet with a Western Fatty Acid Profile in Adults with Central Adiposity. J. Nutr. 2019, 149, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J.H. Dietary Monounsaturated Fatty Acids Are Protective against Metabolic Syndrome and Cardiovascular Disease Risk Factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef]
- Vafeiadou, K.; Weech, M.; Sharma, V.; Yaqoob, P.; Todd, S.; Williams, C.M.; Jackson, K.G.; Lovegrove, J.A. A Review of the Evidence for the Effects of Total Dietary Fat, Saturated, Monounsaturated and n-6 Polyunsaturated Fatty Acids on Vascular Function, Endothelial Progenitor Cells and Microparticles. Br. J. Nutr. 2012, 107, 303–324. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Massaro, M.; Bonfrate, C.; Siculella, L.; Maffia, M.; Nicolardi, G.; Distante, A.; Storelli, C.; De Caterina, R. Oleic Acid Inhibits Endothelial Activation: A Direct Vascular Antiatherogenic Mechanism of a Nutritional Component in the Mediterranean Diet. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 220–228. [Google Scholar] [CrossRef]
- Khan, F.; Elherik, K.; Bolton-Smith, C.; Barr, R.; Hill, A.; Murrie, I.; Belch, J.J.F. The Effects of Dietary Fatty Acid Supplementation on Endothelial Function and Vascular Tone in Healthy Subjects. Cardiovasc. Res. 2003, 59, 955–962. [Google Scholar] [CrossRef]
- Davda, R.K.; Stepniakowski, K.T.; Lu, G.; Ullian, M.E.; Goodfriend, T.L.; Egan, B.M. Oleic Acid Inhibits Endothelial Nitric Oxide Synthase by a Protein Kinase C-Independent Mechanism. Hypertension 1995, 26, 764–770. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Vascular Endothelium—Gatekeeper of Vessel Health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The Assessment of Endothelial Function: From Research into Clinical Practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Bull, C.; Robinson, J.; Deanfield, J.E. Endothelium-Dependent Dilation in the Systemic Arteries of Asymptomatic Subjects Relates to Coronary Risk Factors and Their Interaction. J. Am. Coll. Cardiol. 1994, 24, 1468–1474. [Google Scholar] [CrossRef]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Hidaka, T.; Kihara, Y.; et al. Relationship between Flow-Mediated Vasodilation and Cardiovascular Risk Factors in a Large Community-Based Study. Heart 2013, 99, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Streppel, M.T.; Draijer, R.; Zock, P.L. Flow-Mediated Dilation and Cardiovascular Risk Prediction: A Systematic Review with Meta-Analysis. Int. J. Cardiol. 2013, 168, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M.; Shechter, A.; Koren-Morag, N.; Feinberg, M.S.; Hiersch, L. Usefulness of Brachial Artery Flow-Mediated Dilation to Predict Long-Term Cardiovascular Events in Subjects without Heart Disease. Am. J. Cardiol. 2014, 113, 162–167. [Google Scholar] [CrossRef]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of Future Cardiovascular Outcomes by Flow-Mediated Vasodilatation of Brachial Artery: A Meta-Analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; Lima, J.A.; Crouse, J.R.; Herrington, D.M. Predictive Value of Brachial Flow-Mediated Dilation for Incident Cardiovascular Events in a Population-Based Study: The Multi-Ethnic Study of Atherosclerosis. Circulation 2009, 120, 502–509. [Google Scholar] [CrossRef]
- Tziomalos, K.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Endothelial Dysfunction in Metabolic Syndrome: Prevalence, Pathogenesis and Management. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 140–146. [Google Scholar] [CrossRef]
- Berry, S.E.E.; Tucker, S.; Banerji, R.; Jiang, B.; Chowienczyk, P.J.; Charles, S.M.; Sanders, T.A.B. Impaired Postprandial Endothelial Function Depends on the Type of Fat Consumed by Healthy Men. J. Nutr. 2008, 138, 1910–1914. [Google Scholar] [CrossRef]
- Pacheco, Y.M.; López, S.; Bermúdez, B.; Abia, R.; Villar, J.; Muriana, F.J.G. A Meal Rich in Oleic Acid Beneficially Modulates Postprandial SICAM-1 and SVCAM-1 in Normotensive and Hypertensive Hypertriglyceridemic Subjects. J. Nutr. Biochem. 2008, 19, 200–205. [Google Scholar] [CrossRef]
- Ryan, M.; Mcinerney, D.; Owens, D.; Collins, P.; Johnson, A.; Tomkin, G.H. Diabetes and the Mediterranean Diet: A Beneficial Effect of Oleic Acid on Insulin Sensitivity, Adipocyte Glucose Transport and Endothelium-Dependent Vasoreactivity. QJM—Mon. J. Assoc. Phys. 2000, 93, 85–91. [Google Scholar] [CrossRef]
- Sanders, T.A.B.; Lewis, F.J.; Goff, L.M.; Chowienczyk, P.J. SFAs Do Not Impair Endothelial Function and Arterial Stiffness. Am. J. Clin. Nutr. 2013, 98, 677–683. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration Final Determination Regarding Partially Hydrogenated Oils. (Docket No. FDA–2013–N–1317). Available online: https://www.govinfo.gov/content/pkg/FR-2018-05-21/pdf/2018-10714.pdf (accessed on 28 June 2021).
- Raatz, S.K.; Conrad, Z.; Jahns, L.; Belury, M.A.; Picklo, M.J. Modeled Replacement of Traditional Soybean and Canola Oil with High-Oleic Varieties Increases Monounsaturated Fatty Acid and Reduces Both Saturated Fatty Acid and Polyunsaturated Fatty Acid Intake in the US Adult Population. Am. J. Clin. Nutr. 2018, 108, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kris-Etherton, P.M.; West, S.G.; Lamarche, B.; Jenkins, D.J.A.; Fleming, J.A.; McCrea, C.E.; Pu, S.; Couture, P.; Connelly, P.W.; et al. Effects of Canola and High-Oleic-Acid Canola Oils on Abdominal Fat Mass in Individuals with Central Obesity. Obesity 2016, 24, 2261–2268. [Google Scholar] [CrossRef]
- Hammad, S.; Eck, P.; Sihag, J.; Chen, X.; Connelly, P.W.; Lamarche, B.; Couture, P.; Guay, V.; Maltais-Giguère, J.; West, S.G.; et al. Common Variants in Lipid Metabolism-Related Genes Associate with Fat Mass Changes in Response to Dietary Monounsaturated Fatty Acids in Adults with Abdominal Obesity. J. Nutr. 2019, 149, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Akishita, M.; Eto, M.; Ishikawa, M.; Kozaki, K.; Toba, K.; Sagara, Y.; Taketani, Y.; Orimo, H.; Ouchi, Y. Modulation of Endothelium-Dependent, Flow-Mediated Dilatation of the Brachial Artery by Sex and Menstrual Cycle. Circulation 1995, 12, 3431–3435. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.I.; Westerman, R.A.; Kingwell, B.A.; Paige, J.; Blombery, P.A.; Sudhir, K.; Komesaroff, A.P.A. Variations in Endothelial Function and Arterial Compliance during the Menstrual Cycle. J. Clin. Endocrinol. Metab. 2001, 86, 5389–5395. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef]
- US Department of Agriculture Agricultural Research Service Energy Intakes: Percentages of Energy from Protein, Carbohydrate, Fat, and Alcohol, by Gender and Age, What We Eat in America, NHANES 2015–2016. 2018. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1718/Table_5_EIN_GEN_17.pdf (accessed on 13 August 2021).
- West, S.G.; Wagner, P.; Schoemer, S.L.; Hecker, K.D.; Hurston, K.L.; Krick, A.L.; Boseska, L.; Ulbrecht, J.; Hinderliter, A.L. Biological Correlates of Day-to-Day Variation in Flow-Mediated Dilation in Individuals with Type 2 Diabetes: A Study of Test-Retest Reliability. Diabetologia 2004, 47, 1625–1631. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function—A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef]
- Perona, J.S.; Cabello-Moruno, R.; Ruiz-Gutierrez, V. The Role of Virgin Olive Oil Components in the Modulation of Endothelial Function. J. Nutr. Biochem. 2006, 17, 429–445. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Rowley, N.; Padilla, J.; Simmons, G.H.; Harold Laughlin, M.; Whyte, G.; Timothy Cable, N.; Green, D.J. Relationship between Upper and Lower Limb Conduit Artery Vasodilator Function in Humans. J. Appl. Physiol. 2011, 111, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. The Postprandial Effect of Components of the Mediterranean Diet on Endothelial Function. J. Am. Coll. Cardiol. 2000, 36, 1455–1460. [Google Scholar] [CrossRef]
- Jackson, K.G.; Armah, C.K.; Minihane, A.M. Meal Fatty Acids and Postprandial Vascular Reactivity. Biochem. Soc. Trans. 2007, 35, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.Y.; Lavori, P.W.; Cohen, H.J.; Feussner, J.R. An Overview of Variance Inflation Factors for Sample-Size Calculation. Eval. Health Prof. 2003, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Engan, H.; Patrician, A.; Schagatay, E.; Karlsen, T.; Wisløff, U.; Gaustad, S.E. Acute Dietary Nitrate Supplementation Improves Arterial Endothelial Function at High Altitude: A Double-Blinded Randomized Controlled Cross over Study. Nitric Oxide—Biol. Chem. 2015, 50, 58–64. [Google Scholar] [CrossRef]
- Xaplanteris, P.; Vlachopoulos, C.; Pietri, P.; Terentes-Printzios, D.; Kardara, D.; Alexopoulos, N.; Aznaouridis, K.; Miliou, A.; Stefanadis, C. Tomato Paste Supplementation Improves Endothelial Dynamics and Reduces Plasma Total Oxidative Status in Healthy Subjects. Nutr. Res. 2012, 32, 390–394. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Oikonomou, E.; Zaromitidou, M.; Verveniotis, A.; Plastiras, A.; Kioufis, S.; Maniatis, K.; Miliou, A.; Siasou, Z.; et al. Effects of Omega-3 Fatty Acids on Endothelial Function, Arterial Wall Properties, Inflammatory and Fibrinolytic Status in Smokers: A Cross over Study. Int. J. Cardiol. 2013, 166, 340–346. [Google Scholar] [CrossRef]
| Canola | HOCO | Control | |
|---|---|---|---|
| Carbohydrate | 50.79 | 50.79 | 50.75 |
| Protein | 15.87 | 15.87 | 15.71 |
| Fat | 35.26 | 35.26 | 35.21 |
| MUFA | 17.45 | 19.11 | 10.50 |
| Oleic Acid | 15.55 | 17.86 | 5.92 |
| PUFA | 9.21 | 7.02 | 9.96 |
| α-linolenic acid | 2.10 | 0.76 | 1.73 |
| Linoleic Acid | 6.42 | 5.56 | 7.28 |
| SFA | 6.56 | 6.43 | 12.26 |
| Starting Diet | ||||
|---|---|---|---|---|
| Variable | Overall n = 31 | Canola n = 11 | HOCO n = 11 | Control n = 9 |
| Sex | n (%) | |||
| Male | 20 (67) | 9 (82) | 7 (64) | 4 (44) |
| Female | 11 (33) | 2 (18) | 4 (36) | 5 (56) |
| Anthropometric Measures | Mean ± SD | |||
| Age (years) | 43 ± 14 | 39 ± 14 | 43 ± 13 | 45 ± 14 |
| BMI (kg/m2) | 32.1 ± 5.6 | 35.4 ± 7.0 | 30.0 ± 2.2 | 31.5 ± 5.5 |
| MetS Criteria | ||||
| Waist Circumference (cm) | Male: 106.9 ± 1.1 1 Female: 103.5 ± 1.1 1 | 115.2 ± 1.1 1 111.2 ± 1.0 1 | 99.9 ± 1.1 1 97.4 ± 1.1 1 | 101.5 ± 1.1 1 105.6 ± 1.0 1 |
| TG (mmol/L) | 1.6 ± 0.8 | 1.7 ± 0.7 | 1.7 ± 1.1 | 1.2 ± 0.6 |
| HDL-C (mmol/L) | Male 1.1 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.3 | 1.2 ± 0.4 |
| Female 1.6 ± 0.4 | 1.4 ± 0.2 | 1.6 ± 0.6 | 1.7 ± 0.5 | |
| SBP (mmHg) | 125 ± 13 | 125 ± 7 | 120 ± 15 | 128 ± 16 |
| DBP (mmHg) | 86 ± 9 | 86 ± 8 | 84 ± 11 | 86 ± 9 |
| Fasting Glucose (mmol/L) | 5.3 ± 0.5 | 5.4 ± 0.5 | 5.2 ± 0.5 | 5.4 ± 0.5 |
| Met full MetS criteria * | n = 21 (72.4%) | 9 (81.8%) | 5 (50%) | 7 (77.8%) |
| Additional CVD risk factors | ||||
| Total Cholesterol (mmol/L) | 5.1 ± 1.0 | 5.1 ± 1.2 | 5.4 ± 0.9 | 5.0 ± 0.7 |
| LDL-C (mmol/L) | 3.2 ± 0.8 | 3.1 ± 0.9 | 3.4 ± 0.9 | 3.0 ± 0.6 |
| Baseline | Control | Canola | HOCO | p | |
|---|---|---|---|---|---|
| Avg BAD (mm) | 6.55 ± 0.14 | 6.73 ± 0.14 | 6.70 ± 0.15 | 6.57 ± 0.15 | 0.72 |
| Peak BAD (mm) | 6.97 ± 0.14 | 7.14 ± 0.14 | 7.11 ± 0.15 | 7.02 ± 0.15 | 0.80 |
| FMD (% change) | 6.48 ± 0.49 | 6.41 ± 0.48 | 6.32 ± 0.51 | 6.96 ± 0.49 | 0.81 |
| Baseline Flow # | 176.7 ± 19.1 | 241.0 ± 24.9 | 246.5 ± 26.4 | 246.5 ± 26.4 | 0.72 |
| Peak Flow | 1067.8 ± 93.0 | 1168.4 ± 89.2 | 1376.6 ± 99.7 | 1307.9 ± 92.3 | 0.29 |
| RH | 503.9 ± 47.8 | 393.53 ± 46.1 | 351.1 ± 47.9 | 376.4 ± 47.9 | 0.41 |
| FMD Change from Baseline (mm) | N/A | −0.2 ± 0.6 | 0.1 ± 0.7 | 0.7 ± 0.6 | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, K.M.; Petersen, K.S.; Bowen, K.J.; Jones, P.J.H.; Taylor, C.G.; Zahradka, P.; Letourneau, K.; Perera, D.; Wilson, A.; Wagner, P.R.; et al. Effects of Diets Enriched with Conventional or High-Oleic Canola Oils on Vascular Endothelial Function: A Sub-Study of the Canola Oil Multi-Centre Intervention Trial 2 (COMIT-2), a Randomized Crossover Controlled Feeding Study. Nutrients 2022, 14, 3404. https://doi.org/10.3390/nu14163404
Davis KM, Petersen KS, Bowen KJ, Jones PJH, Taylor CG, Zahradka P, Letourneau K, Perera D, Wilson A, Wagner PR, et al. Effects of Diets Enriched with Conventional or High-Oleic Canola Oils on Vascular Endothelial Function: A Sub-Study of the Canola Oil Multi-Centre Intervention Trial 2 (COMIT-2), a Randomized Crossover Controlled Feeding Study. Nutrients. 2022; 14(16):3404. https://doi.org/10.3390/nu14163404
Chicago/Turabian StyleDavis, Kristin M., Kristina S. Petersen, Kate J. Bowen, Peter J. H. Jones, Carla G. Taylor, Peter Zahradka, Karen Letourneau, Danielle Perera, Angela Wilson, Paul R. Wagner, and et al. 2022. "Effects of Diets Enriched with Conventional or High-Oleic Canola Oils on Vascular Endothelial Function: A Sub-Study of the Canola Oil Multi-Centre Intervention Trial 2 (COMIT-2), a Randomized Crossover Controlled Feeding Study" Nutrients 14, no. 16: 3404. https://doi.org/10.3390/nu14163404
APA StyleDavis, K. M., Petersen, K. S., Bowen, K. J., Jones, P. J. H., Taylor, C. G., Zahradka, P., Letourneau, K., Perera, D., Wilson, A., Wagner, P. R., Kris-Etherton, P. M., & West, S. G. (2022). Effects of Diets Enriched with Conventional or High-Oleic Canola Oils on Vascular Endothelial Function: A Sub-Study of the Canola Oil Multi-Centre Intervention Trial 2 (COMIT-2), a Randomized Crossover Controlled Feeding Study. Nutrients, 14(16), 3404. https://doi.org/10.3390/nu14163404








