The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia—A Narrative Review
Abstract
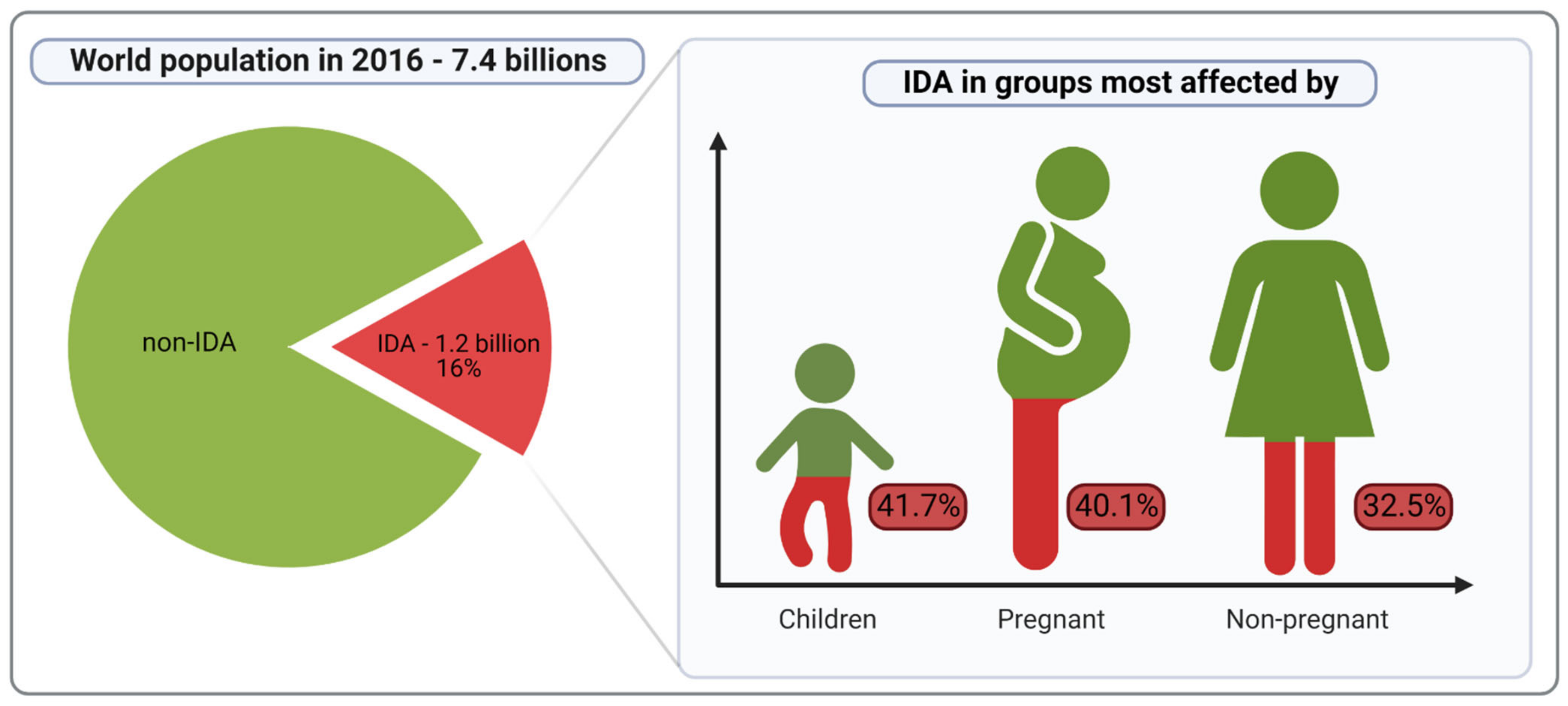
:1. Introduction
2. Search Strategy and Selection Criteria
2.1. Iron in the Human Body
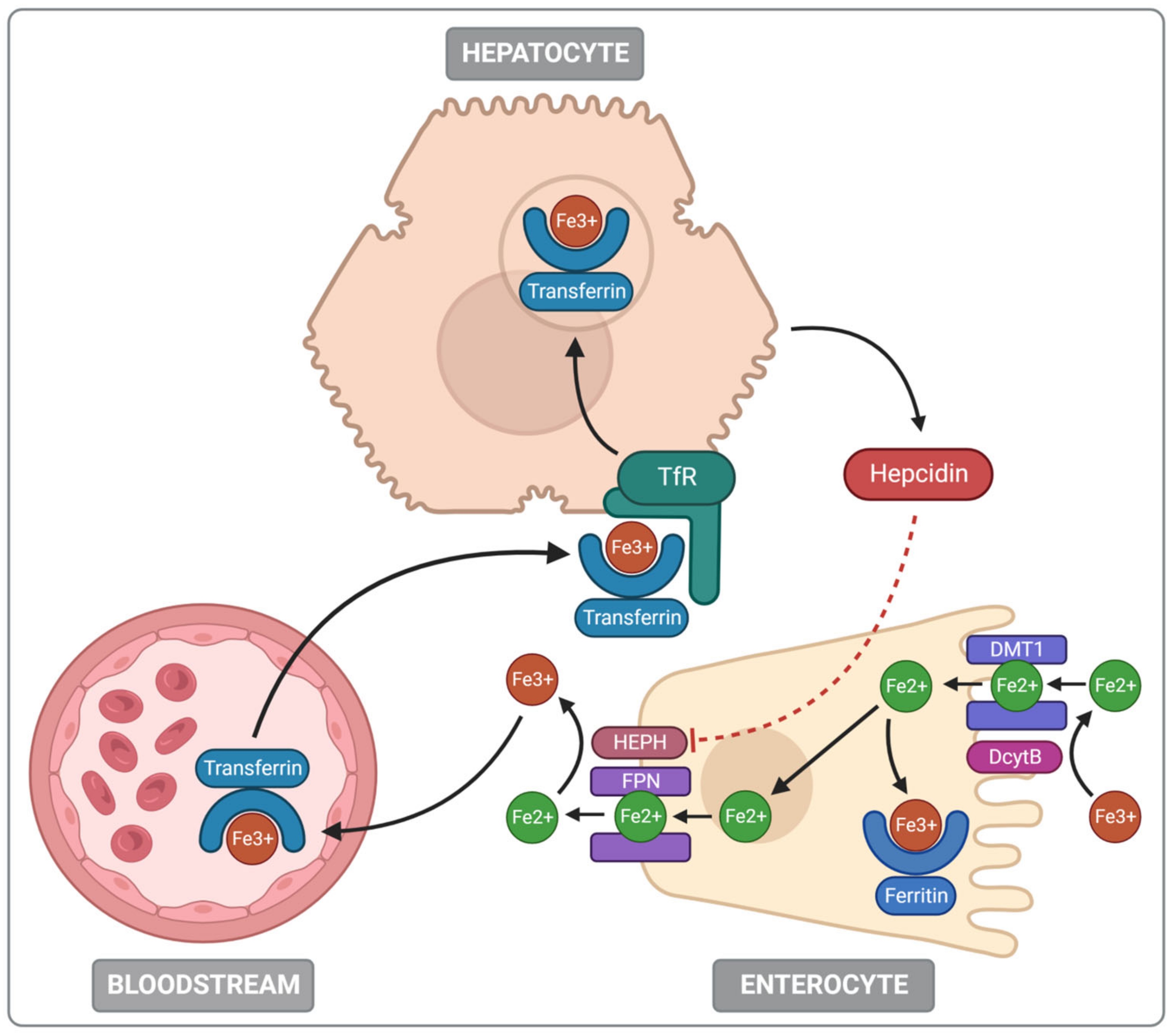
2.2. Iron Absorption and Metabolism
2.3. Iron Toxicity
2.4. Iron and Oxidative Stress
2.5. Iron and Microbiota
3. Inflammatory Bowel Diseases (IBDs)
3.1. Anaemia as a Complication of IBD
3.2. Iron Replacement Therapy in IBD
3.3. Negative Consequences of Oral Iron
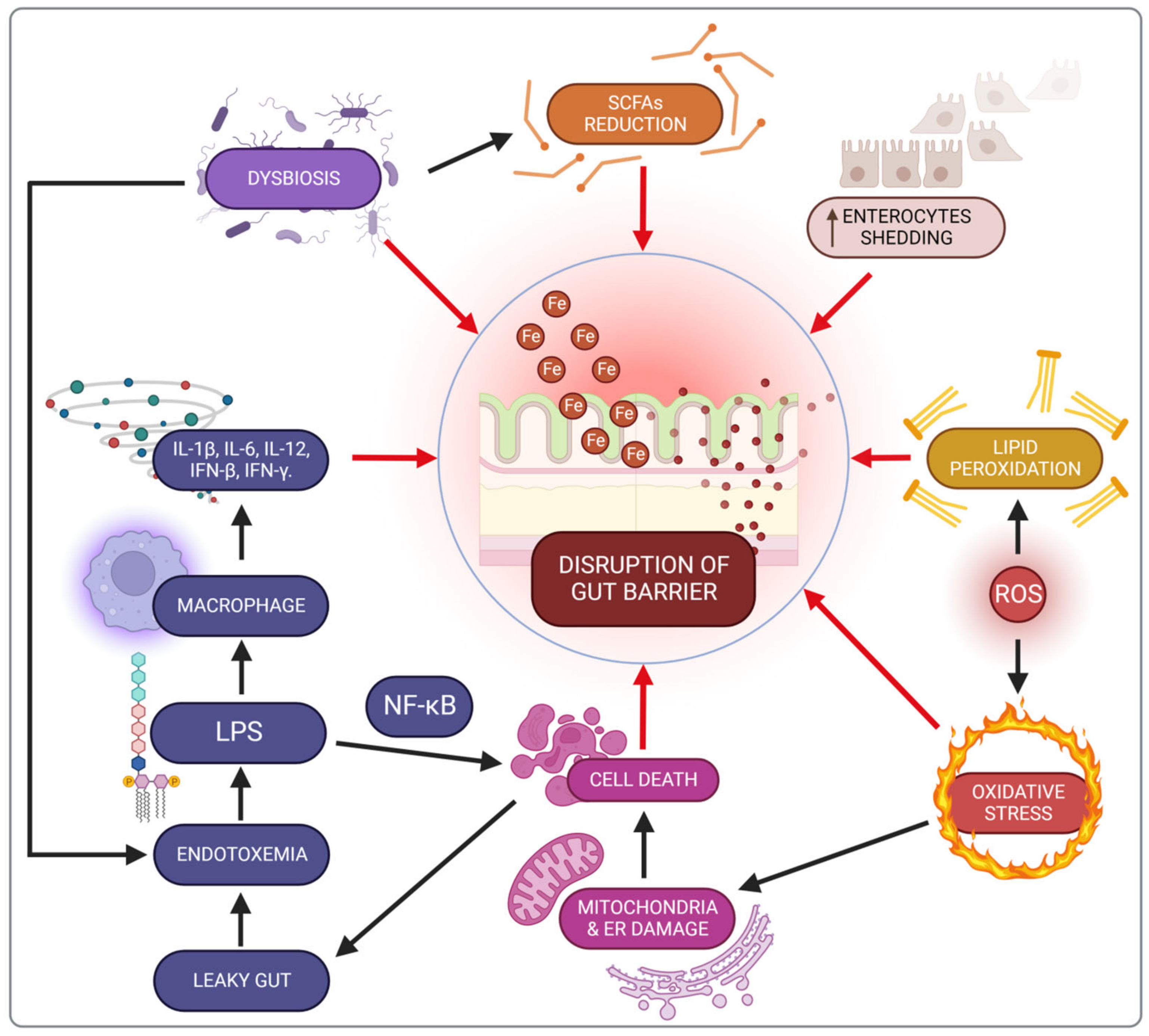
3.4. Impact of Iron on the Intestine
3.5. Impact of Iron on the Microbiota in IBD Patients
4. Colorectal Cancer
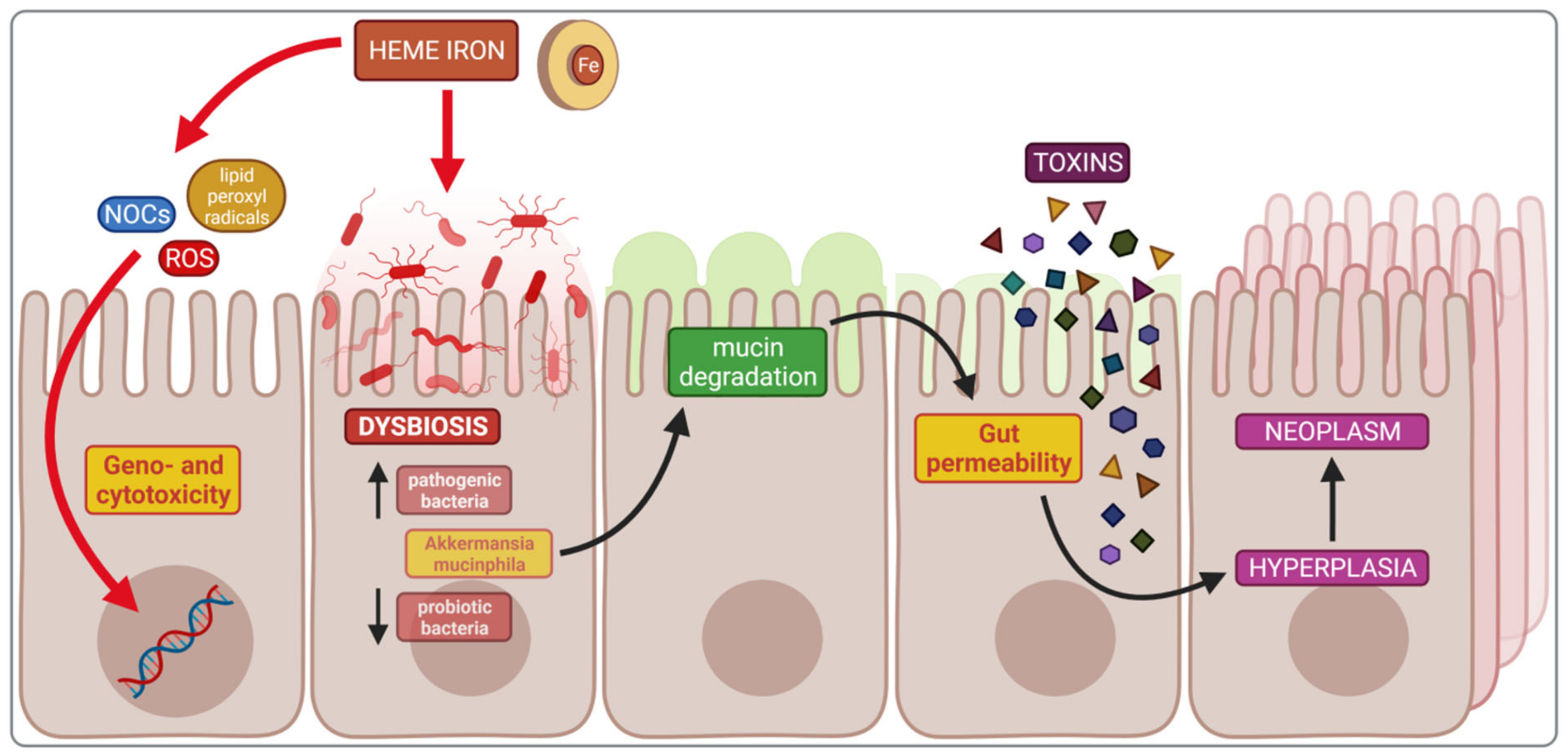
4.1. Impact of Heme Iron on Cancerogenesis
4.2. Dysbiosis and Cancerogenesis
4.3. Microbiota Composition and Bacterial Metabolites
4.4. Comparison of the Intestinal Microflora of Healthy Subjects and CRC Patients
4.5. Driver–Passenger Model
4.6. Probiotic Bacteria and Butyrate
4.7. Iron Deficiency (ID) and Supplementation
5. Obesity
5.1. Adipose Tissue as an Endocrine Organ
5.2. Chronic Low-Grade Inflammation: An Inherent Consequence of Obesity
5.3. Iron Deficiency (ID) Is Common in Obese Individuals
5.4. Dysmetabolic Iron Overload Syndrome (DIOS)
5.5. Treatment of Iron Deficiency (ID) in Obesity
6. Summary
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- GHO|By Category|Anaemia in Children <5 Years—Estimates by WHO Region. Available online: https://apps.who.int/gho/data/view.main.ANEMIACHILDRENREGv?lang=en (accessed on 20 July 2022).
- GHO|By Category|Prevalence of Anaemia in Women. Available online: https://apps.who.int/gho/data/node.main.ANAEMIAWOMEN?lang=en (accessed on 20 July 2022).
- Global Nutrition Targets 2025: Policy Brief Series. Available online: https://www.who.int/publications-detail-redirect/WHO-NMH-NHD-14.2 (accessed on 20 July 2022).
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron Deficiency Anaemia Revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Aspuru, K.; Villa, C.; Bermejo, F.; Herrero, P.; López, S.G. Optimal Management of Iron Deficiency Anemia Due to Poor Dietary Intake. Int. J. Gen. Med. 2011, 4, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef]
- Duck, K.A.; Connor, J.R. Iron Uptake and Transport across Physiological Barriers. Biometals 2016, 29, 573–591. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and Regulatory Aspects of Intestinal Iron Absorption. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron Bioavailability and Dietary Reference Values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Lane, D.J.R.; Bae, D.-H.; Merlot, A.M.; Sahni, S.; Richardson, D.R. Duodenal Cytochrome b (DCYTB) in Iron Metabolism: An Update on Function and Regulation. Nutrients 2015, 7, 2274–2296. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.J.; Frazer, D.M. Current Understanding of Iron Homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef]
- Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J. Intestinal Iron Absorption. J. Trace Elem. Med. Biol. 2012, 26, 115–119. [Google Scholar] [CrossRef]
- Fang, S.; Zhuo, Z.; Yu, X.; Wang, H.; Feng, J. Oral Administration of Liquid Iron Preparation Containing Excess Iron Induces Intestine and Liver Injury, Impairs Intestinal Barrier Function and Alters the Gut Microbiota in Rats. J. Trace Elem. Med. Biol. 2018, 47, 12–20. [Google Scholar] [CrossRef]
- Galaris, D.; Pantopoulos, K. Oxidative Stress and Iron Homeostasis: Mechanistic and Health Aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef]
- Mackenzie, B.; Garrick, M.D. Iron Imports. II. Iron Uptake at the Apical Membrane in the Intestine. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 289, G981–G986. [Google Scholar] [CrossRef]
- West, A.R.; Oates, P.S. Mechanisms of Heme Iron Absorption: Current Questions and Controversies. World J. Gastroenterol. WJG 2008, 14, 4101–4110. [Google Scholar] [CrossRef]
- Shawki, A.; Knight, P.B.; Maliken, B.D.; Niespodzany, E.J.; Mackenzie, B. H+-Coupled Divalent Metal-Ion Transporter-1: Functional Properties, Physiological Roles and Therapeutics. Curr. Top. Membr. 2012, 70, 169–214. [Google Scholar] [CrossRef]
- Collins, J.F.; Prohaska, J.R.; Knutson, M.D. Metabolic Crossroads of Iron and Copper. Nutr. Rev. 2010, 68, 133–147. [Google Scholar] [CrossRef]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Öhrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 Is Critical for Iron Absorption in the Mouse but Is Not Required for the Absorption of Copper or Manganese. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef]
- Mims, M.P.; Guan, Y.; Pospisilova, D.; Priwitzerova, M.; Indrak, K.; Ponka, P.; Divoky, V.; Prchal, J.T. Identification of a Human Mutation of DMT1 in a Patient with Microcytic Anemia and Iron Overload. Blood 2005, 105, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Ems, T.; St Lucia, K.; Huecker, M.R. Biochemistry, Iron Absorption. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- McKie, A.T. The Role of Dcytb in Iron Metabolism: An Update. Biochem. Soc. Trans. 2008, 36, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.C.; Mesquita, G.; Gomes, M.S. Ferritin: An Inflammatory Player Keeping Iron at the Core of Pathogen-Host Interactions. Microorganisms 2020, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Avila, D.S.; Da Rocha, J.B.T.; Aschner, M. Metals, Oxidative Stress and Neurodegeneration: A Focus on Iron, Manganese and Mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin and Iron Regulation, 10 Years Later. Blood 2011, 117, 4425–4433. [Google Scholar] [CrossRef]
- Wilkinson, N.; Pantopoulos, K. The IRP/IRE System in Vivo: Insights from Mouse Models. Front. Pharmacol. 2014, 5, 176. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Fleming, R.E.; Ponka, P. Iron Overload in Human Disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in Metal-Induced Oxidative Stress and Human Disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Lee, J.K.; Ha, J.-H.; Collins, J.F. Dietary Iron Intake in Excess of Requirements Impairs Intestinal Copper Absorption in Sprague Dawley Rat Dams, Causing Copper Deficiency in Suckling Pups. Biomedicines 2021, 9, 338. [Google Scholar] [CrossRef]
- Ha, J.-H.; Doguer, C.; Flores, S.R.L.; Wang, T.; Collins, J.F. Progressive Increases in Dietary Iron Are Associated with the Emergence of Pathologic Disturbances of Copper Homeostasis in Growing Rats. J. Nutr. 2018, 148, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.-H.; Doguer, C.; Collins, J.F. Consumption of a High-Iron Diet Disrupts Homeostatic Regulation of Intestinal Copper Absorption in Adolescent Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 313, G353–G360. [Google Scholar] [CrossRef]
- Ha, J.-H.; Doguer, C.; Wang, X.; Flores, S.R.; Collins, J.F. High-Iron Consumption Impairs Growth and Causes Copper-Deficiency Anemia in Weanling Sprague-Dawley Rats. PLoS ONE 2016, 11, e0161033. [Google Scholar] [CrossRef]
- Pouillevet, H.; Soetart, N.; Boucher, D.; Wedlarski, R.; Jaillardon, L. Inflammatory and Oxidative Status in European Captive Black Rhinoceroses: A Link with Iron Overload Disorder? PLoS ONE 2020, 15, e0231514. [Google Scholar] [CrossRef]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron Homeostasis and Iron-Regulated ROS in Cell Death, Senescence and Human Diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef]
- Patel, M.; Ramavataram, D.V.S.S. Non Transferrin Bound Iron: Nature, Manifestations and Analytical Approaches for Estimation. Indian J. Clin. Biochem. 2012, 27, 322–332. [Google Scholar] [CrossRef]
- Cabantchik, Z.I.; Sohn, Y.S.; Breuer, W.; Espósito, B.P. The Molecular and Cellular Basis of Iron Toxicity in Iron Overload (IO) Disorders. Diagnostic and Therapeutic Approaches. Thalass. Rep. 2013, 3, e3. [Google Scholar] [CrossRef]
- Pietrangelo, A. Iron and the Liver. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36 (Suppl 1), 116–123. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Kindy, M.C. Overdosing on Iron: Elevated Iron and Degenerative Brain Disorders. Exp. Biol. Med. Maywood NJ 2020, 245, 1444–1473. [Google Scholar] [CrossRef]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral Iron Supplements Increase Hepcidin and Decrease Iron Absorption from Daily or Twice-Daily Doses in Iron-Depleted Young Women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron Absorption from Oral Iron Supplements given on Consecutive versus Alternate Days and as Single Morning Doses versus Twice-Daily Split Dosing in Iron-Depleted Women: Two Open-Label, Randomised Controlled Trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The Effects of Iron Fortification on the Gut Microbiota in African Children: A Randomized Controlled Trial in Côte d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, Y.; Guo, H.; Hai, Y.; Luo, Y.; Yue, T. Mechanism and Intervention Measures of Iron Side Effects on the Intestine. Crit. Rev. Food Sci. Nutr. 2020, 60, 2113–2125. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Gou, Z.Y.; Li, L.; Fan, Q.L.; Lin, X.J.; Jiang, Z.Y.; Zheng, C.T.; Ding, F.Y.; Jiang, S.Q. Effects of Oxidative Stress Induced by High Dosage of Dietary Iron Ingested on Intestinal Damage and Caecal Microbiota in Chinese Yellow Broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 924–932. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef]
- Seyoum, Y.; Baye, K.; Humblot, C. Iron Homeostasis in Host and Gut Bacteria—A Complex Interrelationship. Gut Microbes 2021, 13, 1874855. [Google Scholar] [CrossRef]
- Sousa Gerós, A.; Simmons, A.; Drakesmith, H.; Aulicino, A.; Frost, J.N. The Battle for Iron in Enteric Infections. Immunology 2020, 161, 186–199. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Richard, K.L.; Kelley, B.R.; Johnson, J.G. Heme Uptake and Utilization by Gram-Negative Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Ferring-Appel, D.; Casarrubea, D.; Sonnweber, T.; Viatte, L.; Schroll, A.; Haschka, D.; Fang, F.C.; Hentze, M.W.; Weiss, G.; et al. Iron Regulatory Proteins Mediate Host Resistance to Salmonella Infection. Cell Host Microbe 2015, 18, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drakesmith, H.; Prentice, A.M. Hepcidin and the Iron-Infection Axis. Science 2012, 338, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, W.H.; Hall, P.; Briles, D.E. A HemA Mutation Renders Salmonella Typhimurium Avirulent in Mice, yet Capable of Eliciting Protection against Intravenous Infection with S. Typhimurium. Microb. Pathog. 1991, 11, 289–295. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence Factors, Prevalence and Potential Transmission of Extraintestinal Pathogenic Escherichia Coli Isolated from Different Sources: Recent Reports—PMC. Gut Pathog. 2019, 11, 10. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6383261/ (accessed on 21 July 2022). [CrossRef]
- Paganini, D.; Zimmermann, M.B. The Effects of Iron Fortification and Supplementation on the Gut Microbiome and Diarrhea in Infants and Children: A Review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef]
- Schwartz, A.J.; Das, N.K.; Ramakrishnan, S.K.; Jain, C.; Jurkovic, M.T.; Wu, J.; Nemeth, E.; Lakhal-Littleton, S.; Colacino, J.A.; Shah, Y.M. Hepatic Hepcidin/Intestinal HIF-2α Axis Maintains Iron Absorption during Iron Deficiency and Overload. J. Clin. Investig. 2018, 129, 336–348. [Google Scholar] [CrossRef]
- Xu, M.-M.; Wang, J.; Xie, J.-X. Regulation of Iron Metabolism by Hypoxia-Inducible Factors. Sheng Li Xue Bao 2017, 69, 598–610. [Google Scholar]
- Mastrogiannaki, M.; Matak, P.; Peyssonnaux, C. The Gut in Iron Homeostasis: Role of HIF-2 under Normal and Pathological Conditions. Blood 2013, 122, 885–892. [Google Scholar] [CrossRef]
- Das, N.K.; Schwartz, A.J.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A.; Hill, D.; Ma, X.; Lamberg, O.; Schnizlein, M.K.; et al. Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis. Cell Metab. 2020, 31, 115–130.e6. [Google Scholar] [CrossRef]
- Axling, U.; Önning, G.; Combs, M.A.; Bogale, A.; Högström, M.; Svensson, M. The Effect of Lactobacillus Plantarum 299v on Iron Status and Physical Performance in Female Iron-Deficient Athletes: A Randomized Controlled Trial. Nutrients 2020, 12, 1279. [Google Scholar] [CrossRef]
- Sandberg, A.-S.; Önning, G.; Engström, N.; Scheers, N. Iron Supplements Containing Lactobacillus Plantarum 299v Increase Ferric Iron and Up-Regulate the Ferric Reductase DCYTB in Human Caco-2/HT29 MTX Co-Cultures. Nutrients 2018, 10, 1949. [Google Scholar] [CrossRef]
- Yilmaz, B.; Li, H. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals 2018, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; An, S.; Park, D.K.; Kwon, K.A.; Kim, K.O.; Chung, J.-W.; Kim, J.H.; Kim, Y.J. Efficacy of Iron Supplementation in Patients with Inflammatory Bowel Disease Treated with Anti-Tumor Necrosis Factor-Alpha Agents. Ther. Adv. Gastroenterol. 2020, 13, 1756284820961302. [Google Scholar] [CrossRef]
- Tang, M.; Frank, D.N.; Hendricks, A.E.; Ir, D.; Esamai, F.; Liechty, E.; Hambidge, K.M.; Krebs, N.F. Iron in Micronutrient Powder Promotes an Unfavorable Gut Microbiota in Kenyan Infants. Nutrients 2017, 9, 776. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.M.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron Fortification Adversely Affects the Gut Microbiome, Increases Pathogen Abundance and Induces Intestinal Inflammation in Kenyan Infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Dostal, A.; Lacroix, C.; Pham, V.T.; Zimmermann, M.B.; Del’homme, C.; Bernalier-Donadille, A.; Chassard, C. Iron Supplementation Promotes Gut Microbiota Metabolic Activity but Not Colitis Markers in Human Gut Microbiota-Associated Rats. Br. J. Nutr. 2014, 111, 2135–2145. [Google Scholar] [CrossRef]
- Dostal, A.; Baumgartner, J.; Riesen, N.; Chassard, C.; Smuts, C.M.; Zimmermann, M.B.; Lacroix, C. Effects of Iron Supplementation on Dominant Bacterial Groups in the Gut, Faecal SCFA and Gut Inflammation: A Randomised, Placebo-Controlled Intervention Trial in South African Children. Br. J. Nutr. 2014, 112, 547–556. [Google Scholar] [CrossRef]
- Fischer, J.A.; Pei, L.X.; Goldfarb, D.M.; Albert, A.; Elango, R.; Kroeun, H.; Karakochuk, C.D. Is Untargeted Iron Supplementation Harmful When Iron Deficiency Is Not the Major Cause of Anaemia? Study Protocol for a Double-Blind, Randomised Controlled Trial among Non-Pregnant Cambodian Women. BMJ Open 2020, 10, e037232. [Google Scholar] [CrossRef]
- Younis, N.; Zarif, R.; Mahfouz, R. Inflammatory Bowel Disease: Between Genetics and Microbiota. Mol. Biol. Rep. 2020, 47, 3053–3063. [Google Scholar] [CrossRef]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of Surgery for Inflammatory Bowel Diseases Has Decreased over Time: A Systematic Review and Meta-Analysis of Population-Based Studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Soendergaard, C.; Vikner, M.E.; Weiss, G. Rational Management of Iron-Deficiency Anaemia in Inflammatory Bowel Disease. Nutrients 2018, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Mahadea, D.; Adamczewska, E.; Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Eder, P.; Dobrowolska, A.; Krela-Kaźmierczak, I. Iron Deficiency Anemia in Inflammatory Bowel Diseases-A Narrative Review. Nutrients 2021, 13, 4008. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of Chronic Disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Stein, J.; Sharma, N.; Kulnigg-Dabsch, S.; Vel, S.; Gasche, C. Clinical Significance of C-Reactive Protein Levels in Predicting Responsiveness to Iron Therapy in Patients with Inflammatory Bowel Disease and Iron Deficiency Anemia. Dig. Dis. Sci. 2015, 60, 1375–1381. [Google Scholar] [CrossRef]
- European Crohn’s and Colitis Organisatio—ECCO—Industry Exhibition. Available online: https://www.ecco-ibd.eu/exhibit-sponsor-2015/industry-exhibition-2015.html (accessed on 21 July 2022).
- D’Amico, F.; Peyrin-Biroulet, L.; Danese, S. Oral Iron for IBD Patients: Lessons Learned at Time of COVID-19 Pandemic. J. Clin. Med. 2020, 9, E1536. [Google Scholar] [CrossRef]
- Niepel, D.; Klag, T.; Malek, N.P.; Wehkamp, J. Practical Guidance for the Management of Iron Deficiency in Patients with Inflammatory Bowel Disease. Ther. Adv. Gastroenterol. 2018, 11, 1756284818769074. [Google Scholar] [CrossRef]
- Stein, J.; Walper, A.; Klemm, W.; Farrag, K.; Aksan, A.; Dignass, A. Safety and Efficacy of Intravenous Iron Isomaltoside for Correction of Anaemia in Patients with Inflammatory Bowel Disease in Everyday Clinical Practice. Scand. J. Gastroenterol. 2018, 53, 1059–1065. [Google Scholar] [CrossRef]
- Stein, J.; Dignass, A.U. Management of Iron Deficiency Anemia in Inflammatory Bowel Disease—A Practical Approach. Ann. Gastroenterol. 2013, 26, 104–113. [Google Scholar]
- Carrier, J.C.; Aghdassi, E.; Jeejeebhoy, K.; Allard, J.P. Exacerbation of Dextran Sulfate Sodium-Induced Colitis by Dietary Iron Supplementation: Role of NF-KappaB. Int. J. Colorectal Dis. 2006, 21, 381–387. [Google Scholar] [CrossRef]
- Werner, T.; Wagner, S.J.; Martínez, I.; Walter, J.; Chang, J.-S.; Clavel, T.; Kisling, S.; Schuemann, K.; Haller, D. Depletion of Luminal Iron Alters the Gut Microbiota and Prevents Crohn’s Disease-like Ileitis. Gut 2011, 60, 325–333. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. Review Article: The Role of Oxidative Stress in Pathogenesis and Treatment of Inflammatory Bowel Diseases. Naunyn. Schmiedebergs Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef]
- Yu, L.C.-H.; Wei, S.-C.; Ni, Y.-H. Impact of Microbiota in Colorectal Carcinogenesis: Lessons from Experimental Models. Intest. Res. 2018, 16, 346–357. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the Intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. Nihon Chikusan Gakkaiho 2020, 91, e13357. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hansen, S.L.; Borst, L.B.; Spears, J.W.; Moeser, A.J. Dietary Iron Deficiency and Oversupplementation Increase Intestinal Permeability, Ion Transport, and Inflammation in Pigs. J. Nutr. 2016, 146, 1499–1505. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Ferruzza, S.; Scarino, M.L.; Gambling, L.; Natella, F.; Sambuy, Y. Biphasic Effect of Iron on Human Intestinal Caco-2 Cells: Early Effect on Tight Junction Permeability with Delayed Onset of Oxidative Cytotoxic Damage. Cell. Mol. Biol. Noisy-Gd. Fr. 2003, 49, 89–99. [Google Scholar]
- Constante, M.; Fragoso, G.; Calvé, A.; Samba-Mondonga, M.; Santos, M.M. Dietary Heme Induces Gut Dysbiosis, Aggravates Colitis, and Potentiates the Development of Adenomas in Mice. Front. Microbiol. 2017, 8, 1809. [Google Scholar] [CrossRef]
- Lewis, G.; Wang, B.; Shafiei Jahani, P.; Hurrell, B.P.; Banie, H.; Aleman Muench, G.R.; Maazi, H.; Helou, D.G.; Howard, E.; Galle-Treger, L.; et al. Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress ILC2-Dependent Airway Inflammation. Front. Immunol. 2019, 10, 2051. [Google Scholar] [CrossRef]
- Dostal, A.; Chassard, C.; Hilty, F.M.; Zimmermann, M.B.; Jaeggi, T.; Rossi, S.; Lacroix, C. Iron Depletion and Repletion with Ferrous Sulfate or Electrolytic Iron Modifies the Composition and Metabolic Activity of the Gut Microbiota in Rats. J. Nutr. 2012, 142, 271–277. [Google Scholar] [CrossRef]
- Botta, A.; Barra, N.G.; Lam, N.H.; Chow, S.; Pantopoulos, K.; Schertzer, J.D.; Sweeney, G. Iron Reshapes the Gut Microbiome and Host Metabolism. J. Lipid Atheroscler. 2021, 10, 160–183. [Google Scholar] [CrossRef]
- Dostal, A.; Fehlbaum, S.; Chassard, C.; Zimmermann, M.B.; Lacroix, C. Low Iron Availability in Continuous in Vitro Colonic Fermentations Induces Strong Dysbiosis of the Child Gut Microbial Consortium and a Decrease in Main Metabolites. FEMS Microbiol. Ecol. 2013, 83, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Mahalhal, A.; Williams, J.M.; Johnson, S.; Ellaby, N.; Duckworth, C.A.; Burkitt, M.D.; Liu, X.; Hold, G.L.; Campbell, B.J.; Pritchard, D.M.; et al. Oral Iron Exacerbates Colitis and Influences the Intestinal Microbiome. PLoS ONE 2018, 13, e0202460. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G.; Motta, J.-P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont Release from Dysbiotic Gut Microbiota Biofilms in Intestinal Inflammatory Diseases: A Role for Iron? J. Biomed. Sci. 2019, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Rausch, P.; Wang, J.; Skieceviciene, J.; Kiudelis, G.; Bhagalia, K.; Amarapurkar, D.; Kupcinskas, L.; Schreiber, S.; Rosenstiel, P.; et al. Geographical Patterns of the Standing and Active Human Gut Microbiome in Health and IBD. Gut 2016, 65, 238–248. [Google Scholar] [CrossRef]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the Human Gut Microbiome in Inflammatory Bowel Disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Cevallos, S.A.; Byndloss, M.X.; Tiffany, C.R.; Olsan, E.E.; Butler, B.P.; Young, B.M.; Rogers, A.W.L.; Nguyen, H.; Kim, K.; et al. High-Fat Diet and Antibiotics Cooperatively Impair Mitochondrial Bioenergetics to Trigger Dysbiosis That Exacerbates Pre-Inflammatory Bowel Disease. Cell Host Microbe 2020, 28, 273–284.e6. [Google Scholar] [CrossRef]
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries—Sung—2021—CA: A Cancer Journal for Clinicians—Wiley Online Library. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660 (accessed on 21 July 2022).
- Phipps, O.; Brookes, M.J.; Al-Hassi, H.O. Iron Deficiency, Immunology, and Colorectal Cancer. Nutr. Rev. 2021, 79, 88–97. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; Van Mil, S.W.C.; Müller, M.; Kleerebezem, M.; Van der Meer, R. Gut Microbiota Facilitates Dietary Heme-Induced Epithelial Hyperproliferation by Opening the Mucus Barrier in Colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef]
- Seiwert, N.; Heylmann, D.; Hasselwander, S.; Fahrer, J. Mechanism of Colorectal Carcinogenesis Triggered by Heme Iron from Red Meat. Biochim. Biophys. Acta BBA-Rev. Cancer 2020, 1873, 188334. [Google Scholar] [CrossRef]
- Bastide, N.M.; Chenni, F.; Audebert, M.; Santarelli, R.L.; Taché, S.; Naud, N.; Baradat, M.; Jouanin, I.; Surya, R.; Hobbs, D.A.; et al. A Central Role for Heme Iron in Colon Carcinogenesis Associated with Red Meat Intake. Cancer Res. 2015, 75, 870–879. [Google Scholar] [CrossRef]
- Wandersman, C.; Stojiljkovic, I. Bacterial Heme Sources: The Role of Heme, Hemoprotein Receptors and Hemophores. Curr. Opin. Microbiol. 2000, 3, 215–220. [Google Scholar] [CrossRef]
- Candela, M.; Turroni, S.; Biagi, E.; Carbonero, F.; Rampelli, S.; Fiorentini, C.; Brigidi, P. Inflammation and Colorectal Cancer, When Microbiota-Host Mutualism Breaks. World J. Gastroenterol. 2014, 20, 908–922. [Google Scholar] [CrossRef]
- Park, C.H.; Eun, C.S.; Han, D.S. Intestinal Microbiota, Chronic Inflammation, and Colorectal Cancer. Intest. Res. 2018, 16, 338–345. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A Bacterial Driver-Passenger Model for Colorectal Cancer: Beyond the Usual Suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef]
- Chen, J.; Pitmon, E.; Wang, K. Microbiome, Inflammation and Colorectal Cancer. Semin. Immunol. 2017, 32, 43–53. [Google Scholar] [CrossRef]
- Reis, S.A.D.; Da Conceição, L.L.; Peluzio, M.D.C.G. Intestinal Microbiota and Colorectal Cancer: Changes in the Intestinal Microenvironment and Their Relation to the Disease. J. Med. Microbiol. 2019, 68, 1391–1407. [Google Scholar] [CrossRef]
- Faïs, T.; Delmas, J.; Barnich, N.; Bonnet, R.; Dalmasso, G. Colibactin: More Than a New Bacterial Toxin. Toxins 2018, 10, 151. [Google Scholar] [CrossRef]
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. Evidence That Hydrogen Sulfide Is a Genotoxic Agent. Mol. Cancer Res. MCR 2006, 4, 9–14. [Google Scholar] [CrossRef]
- Warren, R.L.; Freeman, D.J.; Pleasance, S.; Watson, P.; Moore, R.A.; Cochrane, K.; Allen-Vercoe, E.; Holt, R.A. Co-Occurrence of Anaerobic Bacteria in Colorectal Carcinomas. Microbiome 2013, 1, 16. [Google Scholar] [CrossRef]
- Garza, D.R.; Taddese, R.; Wirbel, J.; Zeller, G.; Boleij, A.; Huynen, M.A.; Dutilh, B.E. Metabolic Models Predict Bacterial Passengers in Colorectal Cancer. Cancer Metab. 2020, 8, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Hou, S.; Wu, X.; Liu, J.; Wan, X. Analyses of Potential Driver and Passenger Bacteria in Human Colorectal Cancer. Cancer Manag. Res. 2020, 12, 11553–11561. [Google Scholar] [CrossRef]
- Pitsillides, L.; Pellino, G.; Tekkis, P.; Kontovounisios, C. The Effect of Perioperative Administration of Probiotics on Colorectal Cancer Surgery Outcomes. Nutrients 2021, 13, 1451. [Google Scholar] [CrossRef] [PubMed]
- Faghfoori, Z.; Faghfoori, M.H.; Saber, A.; Izadi, A.; Yari Khosroushahi, A. Anticancer Effects of Bifidobacteria on Colon Cancer Cell Lines. Cancer Cell Int. 2021, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; He, L.; Wu, L.; Wang, X.; Liu, Z. Effects of the Intestinal Microbial Metabolite Butyrate on the Development of Colorectal Cancer. J. Cancer 2018, 9, 2510–2517. [Google Scholar] [CrossRef] [PubMed]
- Kam, P.M.-H.; Chu, C.W.-H.; Chan, E.M.-Y.; Liu, O.-L.; Kwok, K.-H. Use of Intravenous Iron Therapy in Colorectal Cancer Patient with Iron Deficiency Anemia: A Propensity-Score Matched Study. Int. J. Colorectal Dis. 2020, 35, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Laso-Morales, M.J.; Vives, R.; Gómez-Ramírez, S.; Pallisera-Lloveras, A.; Pontes, C. Intravenous Iron Administration for Post-Operative Anaemia Management after Colorectal Cancer Surgery in Clinical Practice: A Single-Centre, Retrospective Study. Blood Transfus. Trasfus. Sangue 2018, 16, 338–342. [Google Scholar] [CrossRef]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an Emerging Risk Factor for Iron Deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef]
- Mujica-Coopman, M.F.; Brito, A.; López de Romaña, D.; Pizarro, F.; Olivares, M. Body Mass Index, Iron Absorption and Iron Status in Childbearing Age Women. J. Trace Elem. Med. Biol. 2015, 30, 215–219. [Google Scholar] [CrossRef]
- Skrypnik, K.; Bogdański, P.; Sobieska, M.; Suliburska, J. The Effect of Multistrain Probiotic Supplementation in Two Doses on Iron Metabolism in Obese Postmenopausal Women: A Randomized Trial. Food Funct. 2019, 10, 5228–5238. [Google Scholar] [CrossRef]
- Coelho, R.; Viola, T.W.; Walss-Bass, C.; Brietzke, E.; Grassi-Oliveira, R. Childhood Maltreatment and Inflammatory Markers: A Systematic Review. Acta Psychiatr. Scand. 2014, 129, 180–192. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; Inês de Castro, R.R.; Cannon, G. A New Classification of Foods Based on the Extent and Purpose of Their Processing. Cad. Saude Publica 2010, 26, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S.H. Changes Seen in Gut Bacteria Content and Distribution with Obesity: Causation or Association? Postgrad. Med. 2015, 127, 863–868. [Google Scholar] [CrossRef]
- Singer-Englar, T.; Barlow, G.; Mathur, R. Obesity, Diabetes, and the Gut Microbiome: An Updated Review. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 3–15. [Google Scholar] [CrossRef]
- Alshwaiyat, N.M.; Ahmad, A.; Wan Hassan, W.M.R.; Al-Jamal, H.A.N. Association between Obesity and Iron Deficiency (Review). Exp. Ther. Med. 2021, 22, 1268. [Google Scholar] [CrossRef]
- Ortíz Pérez, M.; Vázquez López, M.A.; Ibáñez Alcalde, M.; Galera Martínez, R.; Martín González, M.; Lendínez Molinos, F.; Bonillo Perales, A. Relationship between Obesity and Iron Deficiency in Healthy Adolescents. Child. Obes. Print 2020, 16, 440–447. [Google Scholar] [CrossRef]
- Aeberli, I.; Hurrell, R.F.; Zimmermann, M.B. Overweight Children Have Higher Circulating Hepcidin Concentrations and Lower Iron Status but Have Dietary Iron Intakes and Bioavailability Comparable with Normal Weight Children. Int. J. Obes. 2009, 33, 1111–1117. [Google Scholar] [CrossRef]
- Gottardo, E.T.; Prokoski, K.; Horn, D.; Viott, A.D.; Santos, T.C.; Fernandes, J.I.M. Regeneration of the Intestinal Mucosa in Eimeria and E. Coli Challenged Broilers Supplemented with Amino Acids. Poult. Sci. 2016, 95, 1056–1065. [Google Scholar] [CrossRef]
- Citelli, M.; Fonte-Faria, T.; Nascimento-Silva, V.; Renovato-Martins, M.; Silva, R.; Luna, A.S.; Vargas da Silva, S.; Barja-Fidalgo, C. Obesity Promotes Alterations in Iron Recycling. Nutrients 2015, 7, 335–348. [Google Scholar] [CrossRef]
- Hurrell, R.F. Influence of Inflammatory Disorders and Infection on Iron Absorption and Efficacy of Iron-Fortified Foods. Nestle Nutr. Inst. Workshop Ser. 2012, 70, 107–116. [Google Scholar] [CrossRef]
- Gotardo, É.M.F.; Caria, C.R.E.P.; De Oliveira, C.C.; Rocha, T.; Ribeiro, M.L.; Gambero, A. Effects of Iron Supplementation in Mice with Hypoferremia Induced by Obesity. Exp. Biol. Med. 2016, 241, 2049–2055. [Google Scholar] [CrossRef]
- Sonnweber, T.; Ress, C.; Nairz, M.; Theurl, I.; Schroll, A.; Murphy, A.T.; Wroblewski, V.; Witcher, D.R.; Moser, P.; Ebenbichler, C.F.; et al. High-Fat Diet Causes Iron Deficiency via Hepcidin-Independent Reduction of Duodenal Iron Absorption. J. Nutr. Biochem. 2012, 23, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Deugnier, Y.; Bardou-Jacquet, É.; Lainé, F. Dysmetabolic Iron Overload Syndrome (DIOS). Presse Medicale 2017, 46, e306–e311. [Google Scholar] [CrossRef] [PubMed]
- Rabindrakumar, M.S.K.; Wickramasinghe, V.P.; Arambepola, C.; Senanayake, H.; Karunaratne, V.; Thoradeniya, T. Baseline Iron and Low-Grade Inflammation Modulate the Effectiveness of Iron Supplementation: Evidence from Follow-up of Pregnant Sri Lankan Women. Eur. J. Nutr. 2021, 60, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Luciani, N.; Brasse-Lagnel, C.; Poli, M.; Anty, R.; Lesueur, C.; Cormont, M.; Laquerriere, A.; Folope, V.; LeMarchand-Brustel, Y.; Gugenheim, J.; et al. Hemojuvelin: A New Link between Obesity and Iron Homeostasis. Obes. Silver 2011, 19, 1545–1551. [Google Scholar] [CrossRef]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef]



| Bacteria | Iron Supplementation |
|---|---|
| Phylum: Firmicutes | ↓ |
| Genus: Enterococcus | ↑ |
| Genus: Lactobacillus | ↓ |
| Genus: Roseburia | ↑ |
| Genus: Clostridium | ↑ |
| Phylum: Proteobacteria | ↑ |
| Family: Enterobacteriaceae | ↑ |
| Species: E. coli | ↑ |
| Genus: Salmonella | ↑ |
| Genus: Shigella | ↑ |
| Genus: Citrobacter | ↑ |
| Order: Bacteroidales | ↑ |
| Genus: Bacteroides | ↑ |
| Genus: Campylobacter | ↑ |
| Genus: Bifidobacterium | ↓ |
| Genus: Prevotella Genus: Rothia | ↓ ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malesza, I.J.; Bartkowiak-Wieczorek, J.; Winkler-Galicki, J.; Nowicka, A.; Dzięciołowska, D.; Błaszczyk, M.; Gajniak, P.; Słowińska, K.; Niepolski, L.; Walkowiak, J.; et al. The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia—A Narrative Review. Nutrients 2022, 14, 3478. https://doi.org/10.3390/nu14173478
Malesza IJ, Bartkowiak-Wieczorek J, Winkler-Galicki J, Nowicka A, Dzięciołowska D, Błaszczyk M, Gajniak P, Słowińska K, Niepolski L, Walkowiak J, et al. The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia—A Narrative Review. Nutrients. 2022; 14(17):3478. https://doi.org/10.3390/nu14173478
Chicago/Turabian StyleMalesza, Ida J., Joanna Bartkowiak-Wieczorek, Jakub Winkler-Galicki, Aleksandra Nowicka, Dominika Dzięciołowska, Marta Błaszczyk, Paulina Gajniak, Karolina Słowińska, Leszek Niepolski, Jarosław Walkowiak, and et al. 2022. "The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia—A Narrative Review" Nutrients 14, no. 17: 3478. https://doi.org/10.3390/nu14173478
APA StyleMalesza, I. J., Bartkowiak-Wieczorek, J., Winkler-Galicki, J., Nowicka, A., Dzięciołowska, D., Błaszczyk, M., Gajniak, P., Słowińska, K., Niepolski, L., Walkowiak, J., & Mądry, E. (2022). The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia—A Narrative Review. Nutrients, 14(17), 3478. https://doi.org/10.3390/nu14173478








