Heat-Killed Lactococcus lactis subsp. cremoris H61 Altered the Iron Status of Young Women: A Randomized, Double-Blinded, Placebo-Controlled, Parallel-Group Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
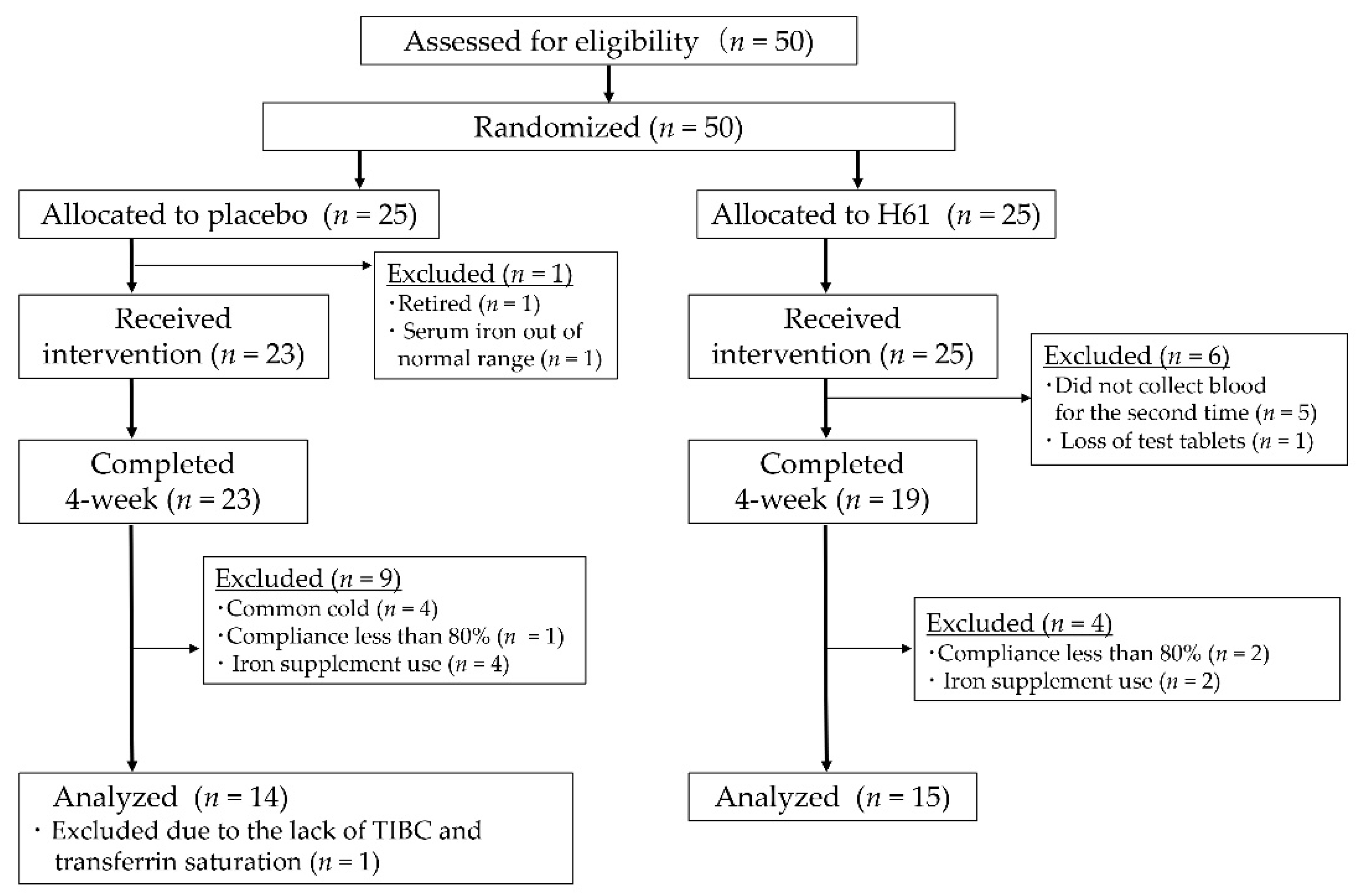
2.1. Participants
2.2. Experimental Design
2.3. Test Supplement
2.4. Blood Collection and Measurements
2.5. Dietary Survey
2.6. Menstrual Cycle
2.7. Statistical Analyses
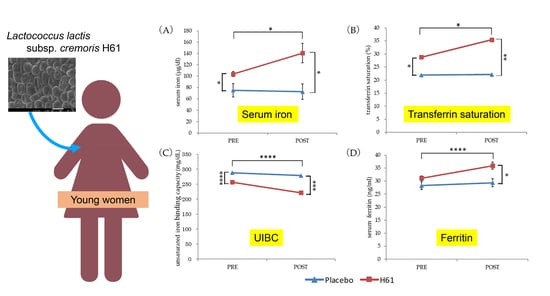
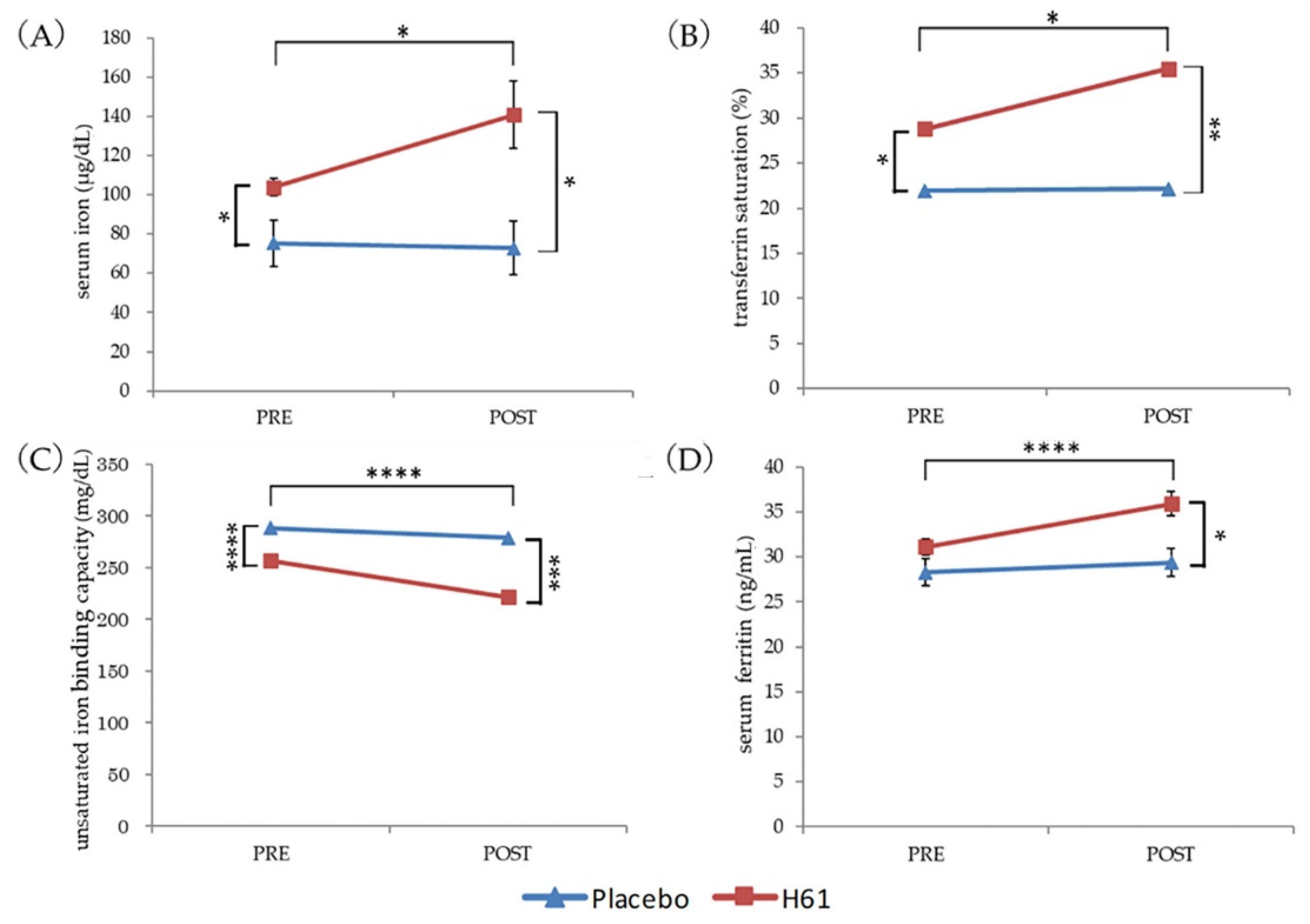
3. Results
3.1. Iron Status
3.2. Iron and Vitamin C Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bjorn-Rasmussen, E.; Hallberg, L.; Isaksson, B.; Arvidsson, B. Food iron absorption in man. Applications of the two-pool extrinsic tag method to measure heme and nonheme iron absorption from the whole diet. J. Clin. Investig. 1974, 53, 247–255. [Google Scholar] [CrossRef]
- Hurrell, R. How to ensure adequate iron absorption from iron-fortified food. Nutr. Rev. 2002, 60, S7–S15, discussion S43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoltzfus, R.J.; Dreyfuss, M.L. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anaemia. World Health Organization (WHO). 1998. Available online: https://motherchildnutrition.org/nutrition-protection-promotion/pdf/mcn-guidelines-for-iron-supplementation.pdf (accessed on 12 March 2022).
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Core, A.B.; Canali, S.; Babitt, J.L. Hemojuvelin and bone morphogenetic protein (BMP) signaling in iron homeostasis. Front. Pharmacol. 2014, 5, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziemann, E.; Kasprowicz, K.; Kasperska, A.; Zembron-Lacny, A.; Antosiewicz, J.; Laskowski, R. Do high blood hepcidin concentrations contribute to low ferritin levels in young tennis players at the end of tournament season? J. Sports Sci. Med. 2013, 12, 249–258. [Google Scholar] [PubMed]
- Ishibashi, A.; Maeda, N.; Sumi, D.; Goto, K. Elevated Serum Hepcidin Levels during an Intensified Training Period in Well-Trained Female Long-Distance Runners. Nutrients 2017, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Tomosugi, N.; Kawabata, H.; Wakatabe, R.; Higuchi, M.; Yamaya, H.; Umehara, H.; Ishikawa, I. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood 2006, 108, 1381–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimon, E.; Kagansky, N.; Kagansky, M.; Mechnick, L.; Mashiah, T.; Namir, M.; Levy, S. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am. J. Med. 2005, 118, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Kimoto-Nira, H.; Nagakura, Y.; Kodama, C.; Shimizu, T.; Okuta, M.; Sasaki, K.; Koikawa, N.; Sakuraba, K.; Suzuki, C.; Suzuki, Y. Effects of ingesting milk fermented by Lactococcus lactis H61 on skin health in young women: A randomized double-blind study. J. Dairy Sci. 2014, 97, 5898–5903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Takaragawa, M.; Miyahara, T.; Sakuraba, K.; Nagato, S.; Matsumoto, N.; Misawa, Y.; Minami, S.; Morio, K. Lactococcus lactis subsp. cremoris H61 improved iron status in male distance runners. Int. J. Anal. Bio-Sci. 2022, 10, 33–41. [Google Scholar]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.G.; Carr, B.R. The Normal Menstrual Cycle and the Control of Ovulation. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText. com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bland, J.M.; Altman, D.G. Some examples of regression towards the mean. BMJ 1994, 309, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health, Labour and Welfare, Japan. The National Health and Nutrition Survey in Japan, 2017. 2018. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/h30-houkoku_00001.html (accessed on 22 May 2022).
- Lane, D.J.; Richardson, D.R. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption! Free. Radic. Biol. Med. 2014, 75, 69–83. [Google Scholar] [CrossRef]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, A.; Le Blanc, S.; Olivares, M.; Pizarro, F.; Ruz, M.; Arredondo, M. Iron, copper, and zinc transport: Inhibition of divalent metal transporter 1 (DMT1) and human copper transporter 1 (hCTR1) by shRNA. Biol. Trace Elem. Res. 2012, 146, 281–286. [Google Scholar] [CrossRef]
- Arredondo, M.; Martinez, R.; Nunez, M.T.; Ruz, M.; Olivares, M. Inhibition of iron and copper uptake by iron, copper and zinc. Biol. Res. 2006, 39, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Et Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallberg, L.; Rossander-Hulten, L. Iron requirements in menstruating women. Am. J. Clin. Nutr. 1991, 54, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef]
- Gillooly, M.; Bothwell, T.H.; Torrance, J.D.; MacPhail, A.P.; Derman, D.P.; Bezwoda, W.R.; Mills, W.; Charlton, R.W.; Mayet, F. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br. J. Nutr. 1983, 49, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Aoki-Yoshida, A.; Kimoto-Nira, H.; Yamagishi, N.; Tomita, S.; Sekiyama, Y.; Wakagi, M.; Sakurai, M.; Ippoushi, K.; Suzuki, C.; et al. Dietary intake of heat-killed Lactococcus lactis H61 delays age-related hearing loss in C57BL/6J mice. Sci. Rep. 2016, 6, 23556. [Google Scholar] [CrossRef] [Green Version]
- Vonderheid, S.C.; Tussing-Humphreys, L.; Park, C.; Pauls, H.; OjiNjideka Hemphill, N.; LaBomascus, B.; McLeod, A.; Koenig, M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients 2019, 11, 2938. [Google Scholar] [CrossRef] [Green Version]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Laine, F.; Angeli, A.; Ropert, M.; Jezequel, C.; Bardou-Jacquet, E.; Deugnier, Y.; Gissot, V.; Lacut, K.; Sacher-Huvelin, S.; Lavenu, A.; et al. Variations of hepcidin and iron-status parameters during the menstrual cycle in healthy women. Br. J. Haematol. 2016, 175, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sakuraba, K.; Sunohara, M.; Takaragawa, M. Variations in iron status linked to menstrual cycles among Japanese female athletes. Int. J. Anal. Bio-Sci. 2018, 6, 45–50. [Google Scholar]
| Placebo (n = 15) | H61 (n = 14) | p | |
|---|---|---|---|
| Age (years) | 19.9 ± 1.5 | 19.9 ± 1.1 | 0.772 |
| Height (cm) | 163.0 ± 6.4 | 161.7 ± 6.7 | 0.603 |
| Body weight (kg) | 58.5 ± 5.9 | 56.6 ± 5.5 | 0.381 |
| EAR | Group | PRE | POST | p b | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | pa | Mean | SD | p a | ||||
| Iron (mg/day) | 8.5 # | H61 | 8.2 | 1.6 | 0.215 | 7.8 | 2.4 | 0.766 | 0.361 |
| placebo | 7.5 | 1.3 | 7.6 | 1.9 | 0.900 | ||||
| Vitamin C (mg/day) | 85 | H61 | 126.5 | 44.4 | 0.234 | 110.8 | 51.8 | 0.779 | 0.121 |
| placebo | 107.6 | 38.5 | 105.9 | 40.6 | 0.891 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaragawa, M.; Sakuraba, K.; Suzuki, Y. Heat-Killed Lactococcus lactis subsp. cremoris H61 Altered the Iron Status of Young Women: A Randomized, Double-Blinded, Placebo-Controlled, Parallel-Group Comparative Study. Nutrients 2022, 14, 3144. https://doi.org/10.3390/nu14153144
Takaragawa M, Sakuraba K, Suzuki Y. Heat-Killed Lactococcus lactis subsp. cremoris H61 Altered the Iron Status of Young Women: A Randomized, Double-Blinded, Placebo-Controlled, Parallel-Group Comparative Study. Nutrients. 2022; 14(15):3144. https://doi.org/10.3390/nu14153144
Chicago/Turabian StyleTakaragawa, Mizuki, Keishoku Sakuraba, and Yoshio Suzuki. 2022. "Heat-Killed Lactococcus lactis subsp. cremoris H61 Altered the Iron Status of Young Women: A Randomized, Double-Blinded, Placebo-Controlled, Parallel-Group Comparative Study" Nutrients 14, no. 15: 3144. https://doi.org/10.3390/nu14153144
APA StyleTakaragawa, M., Sakuraba, K., & Suzuki, Y. (2022). Heat-Killed Lactococcus lactis subsp. cremoris H61 Altered the Iron Status of Young Women: A Randomized, Double-Blinded, Placebo-Controlled, Parallel-Group Comparative Study. Nutrients, 14(15), 3144. https://doi.org/10.3390/nu14153144