Lower Non-Heme Iron Absorption in Healthy Females from Single Meals with Texturized Fava Bean Protein Compared to Beef and Cod Protein Meals: Two Single-Blinded Randomized Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design: Study 1 (Beef-Fava Bean) and Study 2 (Cod-Fava Bean)
2.3. Preparation and Serving of Test Meals
2.4. Radioiron Labeling of Meals
2.5. Iron Absorption Measurements
2.6. Oral Reference Dose
2.7. Statistical Analyses
2.8. Hematological Analysis
2.9. Extraction of Beef- and Cod Protein
2.10. Analysis of Food Composition
2.11. Ethics
3. Results
3.1. Chemical Composition of Meals
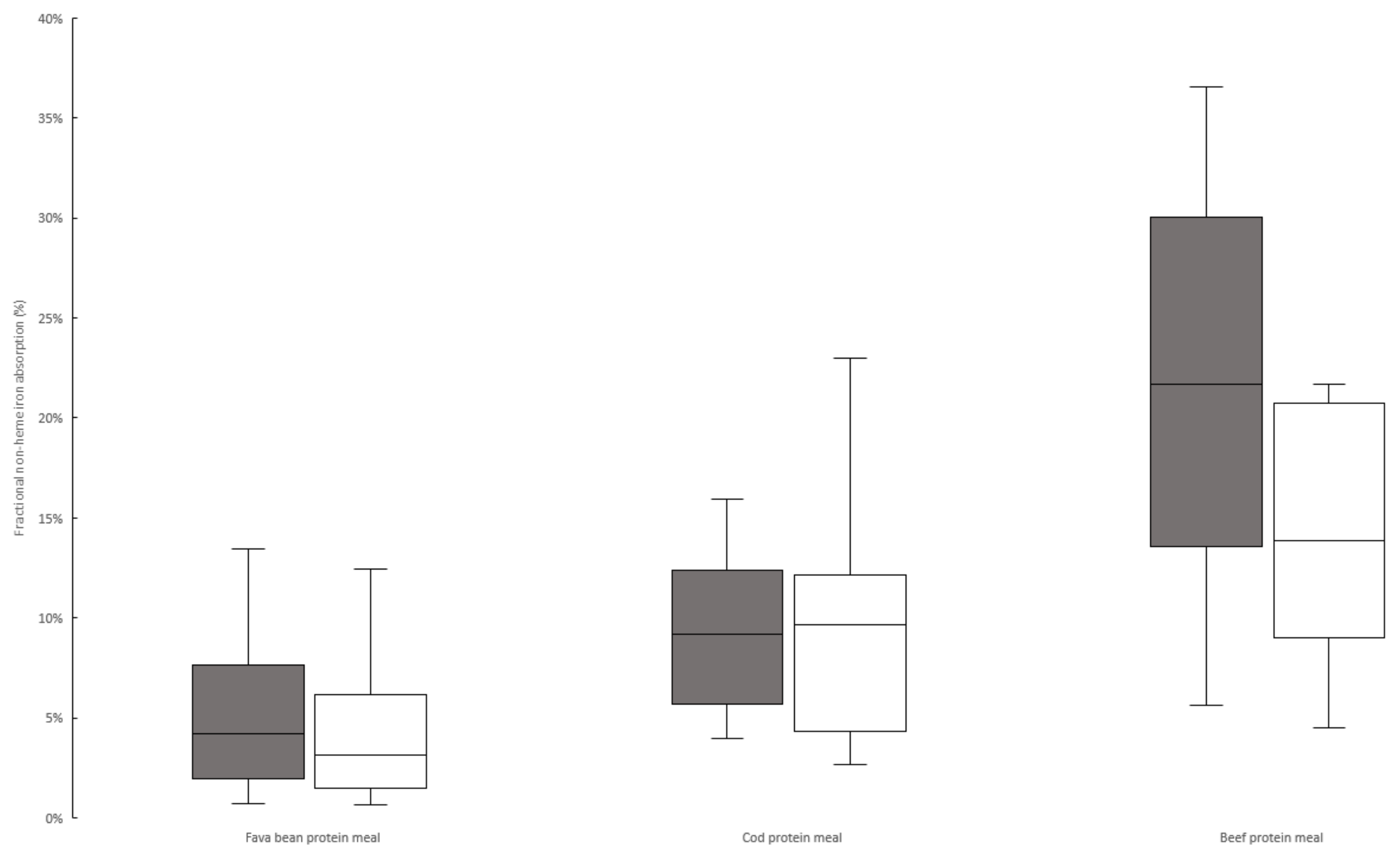
3.2. Iron Absorption from Meals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; de Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO) of the United Nations. The State of Food Insecurity in the World, Addressing Food Insecurity in Protracted Crises; FAO, Ed.; FAO: Rome, Italy, 2010. [Google Scholar]
- Micha, R.; Shulkin, M.L.; Peñalvo, J.L.; Khatibzadeh, S.; Singh, G.M.; Rao, M.; Fahimi, S.; Powles, J.; Mozaffarian, D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS ONE 2017, 12, e0175149. [Google Scholar]
- Hemler, C.E.; Hu, F.B. Plant-Based Diets for Cardiovascular Disease Prevention: All Plant Foods Are Not Created Equal. Curr. Atheroscler. Rep. 2019, 21, 18. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2022, 14, 29. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Van Der Poel, T.F.B.; Aarts, H.L.M.; Kik, M.J.L. Air classification of bean flour—Effects on protein, antinutritional factors and the effect of a fines fraction on cultured explants of small intestinal mucosa. J. Sci. Food Agric. 1990, 53, 143–157. [Google Scholar] [CrossRef]
- Gamel, T.H.; Linssen, J.P.; Mesallam, A.S.; Damir, A.A.; Shekib, L.A. Seed treatments affect functional and antinutritional properties of amaranth flours. J. Sci. Food Agric. 2006, 86, 1095–1102. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2014, 193, 34–42. [Google Scholar] [CrossRef]
- Sandberg, A.-S. Developing functional ingredient. A case study of pea protein. In Functional Foods. Concept to Product, 2nd ed.; Saarela, M., Ed.; Woodhead Publishing Ltd.: Abington, PA, USA; CRC Press LLC: Boca Raton, FL, USA, 2011; Chapter 15; pp. 358–383. [Google Scholar]
- El-Hady, E.-S.A.A.; Habiba, R.A.A. Effect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. LWT Food Sci. Technol. 2003, 36, 285–293. [Google Scholar] [CrossRef]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Batista, K.A.; Prudêncio, S.H.; Fernandes, K.F. Changes in the functional properties and antinutritional factors of extruded hard-to-cook common beans (Phaseolus vulgaris L.). J. Food Sci. 2010, 75, C286–C290. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Moshtaghian, H.; Bianchi, M.; Hallström, E. Nutritional assessment of plant-based meat analogues on the Swedish market. Int. J. Food Sci. Nutr. 2022, 6, 1–13. [Google Scholar] [CrossRef]
- Fredrikson, M.; Biot, P.; Alminger, M.L.; Carlsson, N.-G.; Sandberg, A.-S. Production Process for High-Quality Pea-Protein Isolate with Low Content of Oligosaccharides and Phytate. J. Agric. Food Chem. 2001, 49, 1208–1212. [Google Scholar] [CrossRef]
- Harnack, L.; Mork, S.; Valluri, S.; Weber, C.; Schmitz, K.; Stevenson, J.; Pettit, J. Nutrient Composition of a Selection of Plant-Based Ground Beef Alternative Products Available in the United States. J. Acad. Nutr. Diet. 2021, 121, 2401–2408.e12. [Google Scholar] [CrossRef]
- Sandstrom, B. Bioavailability of Zinc. Eur. J. Clin. Nutr. 1997, 51, 17–19. [Google Scholar]
- Zhou, J.R.; Erdman, J.W. Phytic acid in health and disease. Crit. Rev. Food Sci. Nutr. 1995, 35, 495–508. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chaouki, N.; Hurrell, R.F. Iron deficiency due to consumption of a habitual diet low in bioavailable iron: A longitudinal cohort study in Moroccan children. Am. J. Clin. Nutr. 2005, 81, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Haider, L.M.; Schwingshackl, L.; Hoffmann, G.; Ekmekcioglu, C. The effect of vegetarian diets on iron status in adults: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 1359–1374. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Berger, J.; Hines, I. Iron Status of Vegetarian Adults: A Review of Literature. Am. J. Lifestyle Med. 2016, 12, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Umbreit, J. Iron deficiency: A concise review. Am. J. Hematol. 2005, 78, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Greene-Finestone, L.; Lowell, H.; Levesque, J.; Robinson, S. Iron sufficiency of Canadians. Health Rep. 2012, 23, 41–48. [Google Scholar] [PubMed]
- Hercberg, S.; Preziosi, P.; Galan, P. Iron deficiency in Europe. Public Health Nutr. 2001, 4, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Lahti-Koski, M.; Valsta, L.M.; Alfthan, G.; Tapanainen, H.; Aro, A. Iron status of adults in the capital area of Finland. Eur. J. Nutr. 2003, 42, 287–292. [Google Scholar] [CrossRef]
- Modlinska, K.; Adamczyk, D.; Maison, D.; Pisula, W. Gender Differences in Attitudes to Vegans/Vegetarians and Their Food Preferences, and Their Implications for Promoting Sustainable Dietary Patterns—A Systematic Review. Sustainability 2020, 12, 6292. [Google Scholar] [CrossRef]
- Stoddard, F.; Hämäläinen, K. Towards the World’s earliest maturing faba beans. Grain Legumes 2011, 56, 9–10. [Google Scholar]
- Röös, E.; Carlsson, G.; Ferawati, F.; Hefni, M.; Stephan, A.; Tidåker, P.; Witthöft, C. Less meat, more legumes: Prospects and challenges in the transition toward sustainable diets in Sweden. Renew. Agric. Food Syst. 2020, 35, 192–205. [Google Scholar] [CrossRef] [Green Version]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.; Cook, J.D. Meat Protein Fractions Enhance Nonheme Iron Absorption in Humans. J. Nutr. 2006, 136, 2808–2812. [Google Scholar] [CrossRef]
- Weinborn, V.; Pizarro, F.; Olivares, M.; Brito, A.; Arredondo, M.; Flores, S.; Valenzuela, C. The Effect of Plant Proteins Derived from Cereals and Legumes on Heme Iron Absorption. Nutrients 2015, 7, 8977–8986. [Google Scholar] [CrossRef]
- Hallberg, L.; Rossander, L. Effect of soy protein on nonheme iron absorption in man. Am. J. Clin. Nutr. 1982, 36, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S.R.; Dassenko, S.A.; Cook, J.D.; Juillerat, M.A.; Hurrell, R.F. Inhibitory effect of a soybean-protein--related moiety on iron absorption in humans. Am. J. Clin. Nutr. 1994, 60, 567–572. [Google Scholar] [CrossRef]
- Warsame, A.O.; Michael, N.; O’Sullivan, D.M.; Tosi, P. Identification and Quantification of Major Faba Bean Seed Proteins. J. Agric. Food Chem. 2020, 68, 8535–8544. [Google Scholar] [CrossRef]
- Mayer Labba, I.-C.; Frøkiær, H.; Sandberg, A.-S. Nutritional and antinutritional composition of fava bean (Vicia faba L., var. minor) cultivars. Food Res. Int. 2021, 140, 110038. [Google Scholar] [CrossRef]
- Lazarte, C.E.; Carlsson, N.-G.; Almgren, A.; Sandberg, A.-S.; Granfeldt, Y. Phytate, zinc, iron and calcium content of common Bolivian food, and implications for mineral bioavailability. J. Food Compos. Anal. 2015, 39, 111–119. [Google Scholar] [CrossRef]
- Bothwell, T.H.; Finch, C.A. Iron Metabolism; Little Brown: Boston, MA, USA, 1962. [Google Scholar]
- Reddy, M.B.; Hurrell, R.F.; Juillerat, M.A.; Cook, J.D. The influence of different protein sources on phytate inhibition of nonheme-iron absorption in humans. Am. J. Clin. Nutr. 1996, 63, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Skoldborm, H.; Arvidsson, B.; Andersson, M. A new whole body monitoring laboratory. Acta Radiol. 1972, 313, 233–241. [Google Scholar] [CrossRef]
- Fredlund, K.; Isaksson, M.; Rossander-Hulthén, L.; Almgren, A.; Sandberg, A.-S. Absorption of zinc and retention of calcium: Dose-dependent inhibition by phytate. J. Trace Elem. Med. Biol. 2006, 20, 49–57. [Google Scholar] [CrossRef]
- Hahn, P.F.; Bale, W.F.; Lawrence, E.O.; Whipple, G.H. Radioactive Iron and Its Metabolism in Anemia: Its Absorption, Transportation, and Utilization. J. Exp. Med. 1939, 69, 739–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.D.; Layrisse, M.; Martinez-Torres, C.; Walker, R.; Monsen, E.; Finch, C.A. Food iron absorption measured by an extrinsic tag. J. Clin. Investig. 1972, 51, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Björn-Rasmussen, E. Determination of iron absorption from whole diet. A new two-pool model using two radioiron isotopes given as haem and non-haem iron. Scand. J. Haematol. 1972, 9, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L. Bioavailability of dietary iron in man. Annu. Rev. Nutr. 1981, 1, 123–147. [Google Scholar] [CrossRef]
- Eakins, J.D.; Brown, D.A. An improved method for the simultaneous determination of iron-55 and iron-59 in blood by liquid scintillation counting. Int. J. Appl. Radiat. Isot. 1966, 17, 391–397. [Google Scholar] [CrossRef]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, B.; Björn-Rassmussen, E.; Hallberg, L.; Rossander, L. Iron absorption in relation to iron status. Model proposed to express results to food iron absorption measurements. Scand. J. Haematol. 1981, 27, 201–208. [Google Scholar] [CrossRef]
- Brise, H.; Hallberg, L. A method for comparative studies on iron absorption in man using two radioiron isotopes. Acta Med. Scand. 1962, 376, 7–22. [Google Scholar] [CrossRef]
- Björn-Rasmussen, E.; Halberg, L.; Magnusson, B.; Rossander, L.; Svanberg, B.; Arvidsson, B. Measurement of iron absorption from compositite meals. Am. J. Clin. Nutr. 1976, 29, 772–778. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, M.; Undeland, I. Physicochemical and gel-forming properties of protein isolated from salmon, cod and herring by-products using the pH-shift method. LWT 2019, 101, 678–684. [Google Scholar] [CrossRef]
- Fredriksson, M.; Carlsson, N.-G.; Almgren, A.; Sandberg, A.-S. Simultaneous and sensitive analysis of Cu, Ni, Zn, Co, Mn, and Fe in food and biological samples by ion chromatography. J. Agric. Food Chem. 2002, 50, 59–65. [Google Scholar] [CrossRef]
- Ryter, S.W.; Tyrrell, R.M. An HPLC method to detect heme oxygenase activity. Curr. Protoc. Toxicol. 2001, 5, 9–6. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Carlsson, N.-G.; Bergman, E.-L.; Skoglund, E.; Hasselblad, K.; Sandberg, A.-S. Rapid analyzes of inositol phosphates. J. Agric. Food Chem. 2001, 49, 1695–1701. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lee, C.M.; Trevino, B.; Chaiyawat, M. A simple and rapid solvent extraction method for determining total lipids in fish tissue. J. AOAC Int. 1996, 79, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Ax, E.; Lemming, E.W.; Becker, W.; Andersson, A.; Lindroos, A.K.; Cederholm, T.; Sjögren, P.; Fung, T.T. Dietary patterns in Swedish adults; Results from a national dietary survey. Br. J. Nutr. 2016, 115, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Nordic Nutrition Recommendations. Integratin nutrition and physical activity (NNR 2012). In Nordic Nutrition Recommendations Report No: 5; Denmark Nordic Council of Ministers: Copenhagen, Denmark, 2014; pp. 543–571. [Google Scholar]
- Monsen, E.R.; Hallberg, L.; Layrisse, M.; Hegsted, D.M.; Cook, J.; Mertz, W.; Finch, C.A. Estimation of available dietary iron. Am. J. Clin. Nutr. 1978, 31, 134–141. [Google Scholar] [CrossRef]
- Agency, T.S.F. The Swedish Food Agency Food Database, Version 2022-05-24. Available online: https://www7.slv.se/SokNaringsinnehall/ (accessed on 10 June 2022).
- Pretorius, B.; Schönfeldt, H.C.; Hall, N. Total and haem iron content lean meat cuts and the contribution to the diet. Food Chem. 2016, 193, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Hallberg, L.; Hulthén, L. Prediction of dietary iron absorption: An algorithm for calculating absorption and bioavailability of dietary iron. Am. J. Clin. Nutr. 2000, 71, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, K.; Breda, J.; Berdzuli, N.; Rippin, H.; Farrand, C.; Halloran, A. The shift to plant-based diets: Are we missing the point? Glob. Food Secur. 2021, 29, 100530. [Google Scholar] [CrossRef]
- Martínez-Torres, C.; Layrisse, M. Effect of Amino Acids on Iron Absorption from a Staple Vegetable Food. Blood 1970, 35, 669–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Torres, C.; Layrisse, M. Iron absorption from veal muscle. Am. J. Clin. Nutr. 1971, 24, 531–540. [Google Scholar] [CrossRef]
- Layrisse, M.; Martínez-Torres, C.; Roche, M. Effect of Interaction of Various Foods on Iron Absorption. Am. J. Clin. Nutr. 1968, 21, 1175–1183. [Google Scholar] [CrossRef]
- Björn-Rasmussen, E.; Hallberg, L. Effect of Animal Proteins on the Absorption of Food Iron in Man. Ann. Nutr. Metab. 1979, 23, 192–202. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef] [Green Version]
- Petry, N.; Egli, I.; Gahutu, J.B.; Tugirimana, P.L.; Boy, E.; Hurrell, R. Phytic Acid Concentration Influences Iron Bioavailability from Biofortified Beans in Rwandese Women with Low Iron Status. J. Nutr. 2014, 144, 1681–1687. [Google Scholar] [CrossRef] [Green Version]
- Brune, M.; Rossander-Hultén, L.; Hallberg, L.; Gleerup, A.; Sandberg, A.S. Iron absorption from bread in humans: Inhibiting effects of cereal fiber, phytate and inositol phosphates with different numbers of phosphate groups. J. Nutr. 1992, 122, 442–449. [Google Scholar] [CrossRef]
- Davidsson, L.; Dimitriou, T.; Walczyk, T.; Hurrell, R.F. Iron absorption from experimental infant formulas based on pea (Pisum sativum)-protein isolate: The effect of phytic acid and ascorbic acid. Br. J. Nutr. 2001, 85, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Davey, G.K.; Spencer, E.A.; Appleby, P.N.; Allen, N.E.; Knox, K.H.; Key, T.J. EPIC–Oxford: Lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–268. [Google Scholar] [CrossRef]
- Cade, J.E.; Burley, V.J.; Greenwood, D.C. The UK Women’s Cohort Study: Comparison of vegetarians, fish-eaters and meat-eaters. Public Health Nutr. 2004, 7, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; de Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Shridhar, K.; Dhillon, P.K.; Bowen, L.; Kinra, S.; Bharathi, A.V.; Prabhakaran, D.; Reddy, K.S.; Ebrahim, S. Nutritional profile of Indian vegetarian diets—The Indian Migration Study (IMS). Nutr. J. 2014, 13, 55. [Google Scholar] [CrossRef]
- Sherf-Dagan, S.; Hod, K.; Buch, A.; Mardy-Tilbor, L.; Regev, Z.; Ben-Porat, T.; Sakran, N.; Goitein, D.; Raziel, A. Health and Nutritional Status of Vegetarian Candidates for Bariatric Surgery and Practical Recommendations. Obes. Surg. 2018, 28, 152–160. [Google Scholar] [CrossRef]
- Cepeda-Lopez, A.C.; Melse-Boonstra, A.; Zimmermann, M.B.; Herter-Aeberli, I. In overweight and obese women, dietary iron absorption is reduced and the enhancement of iron absorption by ascorbic acid is one-half that in normal-weight women. Am. J. Clin. Nutr. 2015, 102, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B.; Zeder, C.; Muthayya, S.; Winichagoon, P.; Chaouki, N.; Aeberli, I.; Hurrell, R.F. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int. J. Obes. 2008, 32, 1098–1104. [Google Scholar] [CrossRef] [Green Version]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.-A.; Cook, J.D. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am. J. Clin. Nutr. 2003, 77, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Donangelo, C.M.; Woodhouse, L.R.; King, S.M.; Toffolo, G.; Shames, D.M.; Viteri, F.E.; Cheng, Z.; Welch, R.M.; King, J.C. Iron and Zinc Absorption from Two Bean (Phaseolus vulgaris L.) Genotypes in Young Women. J. Agric. Food Chem. 2003, 51, 5137–5143. [Google Scholar] [CrossRef]
- Etcheverry, P.; Hawthorne, K.M.; Liang, L.K.; Abrams, S.A.; Griffin, I.J. Effect of beef and soy proteins on the absorption of non-heme iron and inorganic zinc in children. J. Am. Coll. Nutr. 2006, 25, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tetens, I.; Bendtsen, K.M.; Henriksen, M.; Ersbøll, A.K.; Milman, N. The impact of a meat- versus a vegetable-based diet on iron status in women of childbearing age with small iron stores. Eur. J. Nutr. 2007, 46, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Collings, R.; Harvey, L.J.; Hooper, L.; Hurst, R.; Brown, T.J.; Ansett, J.; King, M.; Fairweather-Tait, S.J. The absorption of iron from whole diets: A systematic review. Am. J. Clin. Nutr. 2013, 98, 65–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study Group | ||
|---|---|---|
| Beef-Fava | Cod-Fava | |
| Subjects inititally enrolled *, n | 22 | 23 |
| Subjects excluded, by cause: | ||
| not being able to finish meal, n | 5 | 2 |
| Sensitivity to the fava meal/IBS, n | - | 1 |
| failing to draw blood, n | 1 | - |
| sickness during study, n | 3 | 4 |
| mixed up meals, n | - | 1 |
| broke fasting, n | 1 | - |
| Subjects finishing study and included in analysis, n | 12 | 15 |
| Beef Protein Meal | Cod Protein Meal | Texturized Fava Protein Meal | |
|---|---|---|---|
| Protein (g) | 35 | 35 | 35 |
| Phytic acid (mg) | - | - | 1692 |
| Iron (mg) | 2.7 | 2.3 | 3.7 |
| Zinc (mg) | 0.49 | 0.28 | 3.3 |
| Phy:Fe molar ratio | - | - | 38.7 |
| Phy:Zn molar ratio | - | - | 50.8 |
| Heme iron | - | ||
| Total Phenolic Content (mg GAE/g) | - | - | 0.39 |
| Total fat (g/100 g) | 8.3 | 8.4 | 6.4 |
| 1. Beef-Fava | 2. Cod-Fava | ||||
|---|---|---|---|---|---|
| Median (25th–75th) | Median (25th–75th) | p-Value 1 | |||
| Included subjects (n) | 12 | 15 | - | ||
| Age (yrs) | 30.5 | (25.3–37.3) | 27 | (23–36) | NS |
| Height (cm) | 168 | (162–172) | 168 | (163–172) | NS |
| Weight (kg) | 71 | (65–80) | 63 | (59–72) | 0.036 |
| BMI (kg/m2) | 26 | (24–28) | 23 | (21–24) | 0.002 |
| S-Iron (umol/L) | 18 | (15–24) | 19 | (13–25) | NS |
| TIBC (umol/L) | 69 | (62–75) | 70 | (65–79) | NS |
| TSAT (%) | 27.5 | (22–32) | 26 | (21–34) | NS |
| Serum ferritin (ug/L) | 45 | (24.8–71.8) | 57 | (24–81) | NS |
| Hemoglobin (g/L) | 133 | (126–135) | 131 | (127–135) | NS |
| CRP (mg/L) | 0.84 | (0.6–1.9) | 0.66 | (0.51–2.9) | NS |
| Absorbance quota between animal protein/fava protein meals 2 | 4.2 p = 0.0012 | (3.2–6.7) | 2.7 p = 0.001 | (1.8–3.4) | 0.013 3 |
| Reference dose absorption (%) | 28 | (21–33) | 37 | (27–45) | NS (0.079) |
| Absorption of 55Fe from fava meals (unadjusted/adjusted), (%) | 3.8/5.7 | 3.0/3.2 | NS/NS | ||
| Reference Diet | Scenario 1 | Scenario 2 | ||
|---|---|---|---|---|
| Swedish National Diet | 100% Exchange | 50% Exchange | ||
| Total Meat (88 g) | Texturized Fava Bean (88 g) | Meat (44 g) | Texturized Fava Bean (44 g) | |
| Protein (g) | 19.7 | 16.6 | 9.8 | 8.3 |
| Zinc (mg) | 2.8 | 1.6 | 1.4 | 0.8 |
| Total iron (mg) | 1.7 | 1.8 | 0.8 | 0.9 |
| Non-heme iron (mg) | 1.0 | 1.8 | 0.5 | 0.9 |
| Heme iron 1 (mg) | 0.67 | 0 | 0.3 | 0 |
| Non-heme iron absorption 2 | 21.7% | 4.2% | 21.7% | 4.2% |
| Assumed heme iron absorption 3 | 25% | - | 25% | - |
| Calculated iron absorbed (mg) | 0.39 | 0.07 | 0.19 | 0.04 |
| 0.23 | ||||
| Difference in iron absorbed 4 | −82% | −41% | ||
| Percentage of daily iron need (2.22 mg) | 18% | 3% | 11% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer Labba, I.-C.; Hoppe, M.; Gramatkovski, E.; Hjellström, M.; Abdollahi, M.; Undeland, I.; Hulthén, L.; Sandberg, A.-S. Lower Non-Heme Iron Absorption in Healthy Females from Single Meals with Texturized Fava Bean Protein Compared to Beef and Cod Protein Meals: Two Single-Blinded Randomized Trials. Nutrients 2022, 14, 3162. https://doi.org/10.3390/nu14153162
Mayer Labba I-C, Hoppe M, Gramatkovski E, Hjellström M, Abdollahi M, Undeland I, Hulthén L, Sandberg A-S. Lower Non-Heme Iron Absorption in Healthy Females from Single Meals with Texturized Fava Bean Protein Compared to Beef and Cod Protein Meals: Two Single-Blinded Randomized Trials. Nutrients. 2022; 14(15):3162. https://doi.org/10.3390/nu14153162
Chicago/Turabian StyleMayer Labba, Inger-Cecilia, Michael Hoppe, Elisabeth Gramatkovski, Martin Hjellström, Mehdi Abdollahi, Ingrid Undeland, Lena Hulthén, and Ann-Sofie Sandberg. 2022. "Lower Non-Heme Iron Absorption in Healthy Females from Single Meals with Texturized Fava Bean Protein Compared to Beef and Cod Protein Meals: Two Single-Blinded Randomized Trials" Nutrients 14, no. 15: 3162. https://doi.org/10.3390/nu14153162
APA StyleMayer Labba, I.-C., Hoppe, M., Gramatkovski, E., Hjellström, M., Abdollahi, M., Undeland, I., Hulthén, L., & Sandberg, A.-S. (2022). Lower Non-Heme Iron Absorption in Healthy Females from Single Meals with Texturized Fava Bean Protein Compared to Beef and Cod Protein Meals: Two Single-Blinded Randomized Trials. Nutrients, 14(15), 3162. https://doi.org/10.3390/nu14153162






