High-Protein, Low-Glycaemic Meal Replacement Improves Physical Health-Related Quality of Life in High-Risk Persons for Metabolic Syndrome—A Subanalysis of the Randomised-Controlled ACOORH Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Intervention and Meal Replacement Regimen
2.3. Outcomes and Measurements
2.4. Statistics
3. Results
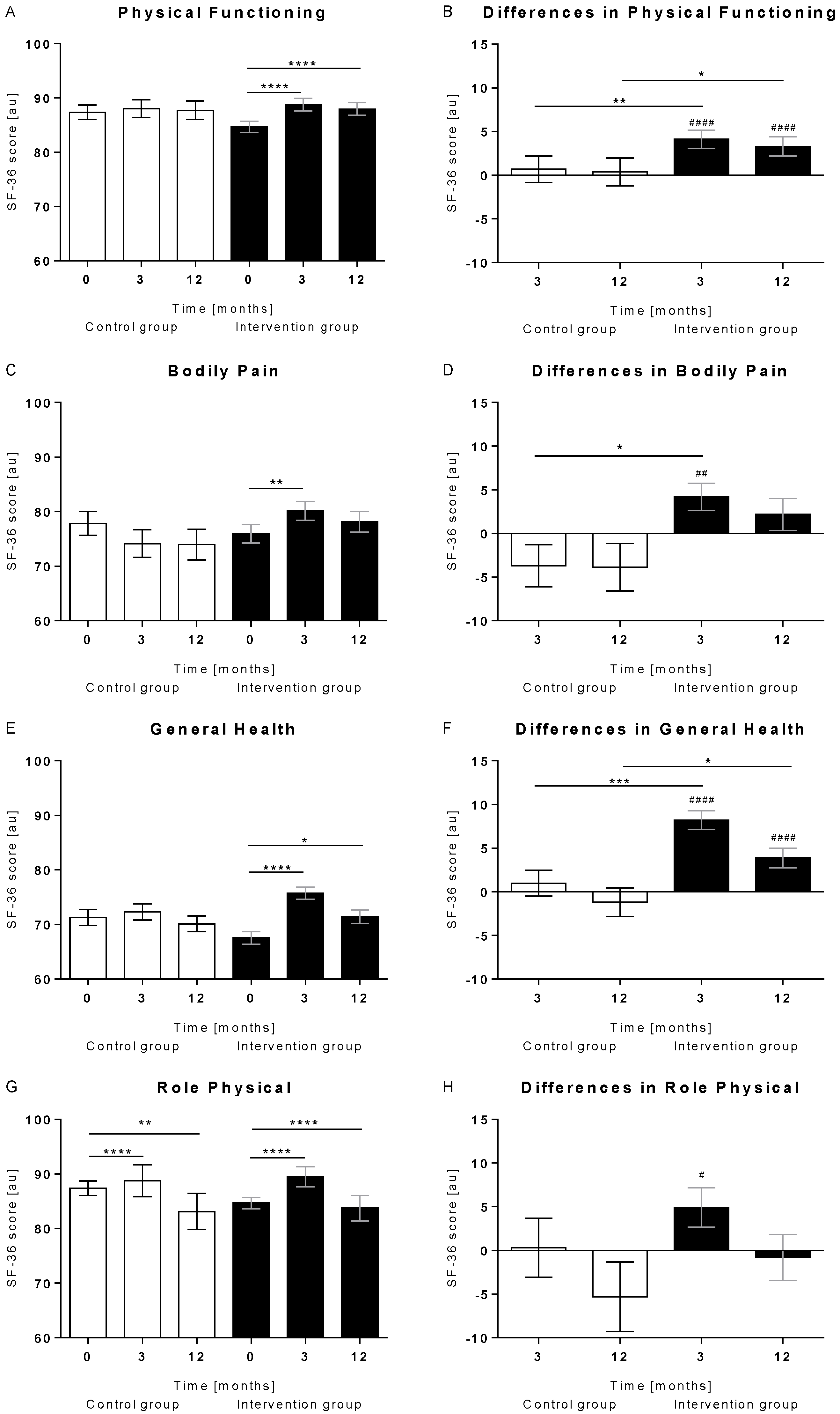
3.1. Overall Increase in PCS Items
3.2. Stronger Improvement of the Physical Component Score in the Intervention Group
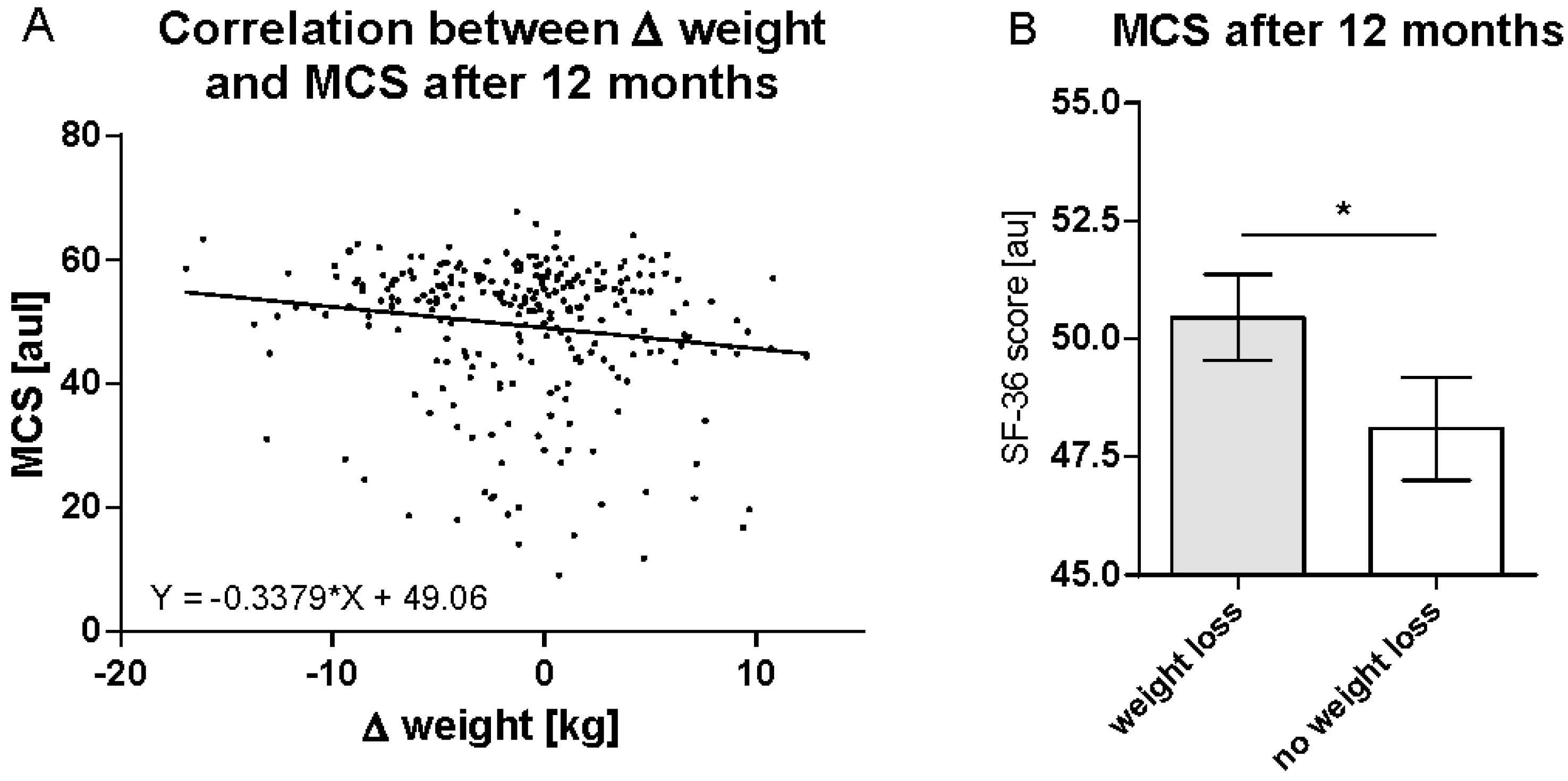
3.3. Weight Reduction Is Predictive for Improvements in MCS after 12 Months
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wadden, T.A.; Phelan, S. Assessment of quality of life in obese individuals. Obes. Res. 2002, 10 (Suppl. 1), 50S–57S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doll, H.A.; Petersen, S.E.; Stewart-Brown, S.L. Obesity and physical and emotional well-being: Associations between body mass index, chronic illness, and the physical and mental components of the SF-36 questionnaire. Obes. Res. 2000, 8, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Saboya, P.P.; Bodanese, L.C.; Zimmermann, P.R.; Gustavo, A.D.; Assumpcao, C.M.; Londero, F. Metabolic syndrome and quality of life: A systematic review. Rev. Lat. Am. Enfermagem 2016, 24, e2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moebus, S.; Hanisch, J.; Bramlage, P.; Losch, C.; Hauner, H.; Wasem, J.; Jockel, K.H. Regional differences in the prevalence of the metabolic syndrome in primary care practices in Germany. Dtsch. Arztebl. Int. 2008, 105, 207–213. [Google Scholar]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Halle, M.; Röhling, M.; Banzer, W.; Braumann, K.M.; Kempf, K.; McCarthy, D.; Schaller, N.; Predel, H.G.; Scholze, J.; Fuhrer-Sakel, D.; et al. Meal replacement by formula diet reduces weight more than a lifestyle intervention alone in patients with overweight or obesity and accompanied cardiovascular risk factors-the ACOORH trial. Eur. J. Clin. Nutr. 2021, 75, 661–669. [Google Scholar] [CrossRef]
- Röhling, M.; Kempf, K.; Banzer, W.; Berg, A.; Braumann, K.M.; Tan, S.; Halle, M.; McCarthy, D.; Pinget, M.; Predel, H.G.; et al. Prediabetes Conversion to Normoglycemia Is Superior Adding a Low-Carbohydrate and Energy Deficit Formula Diet to Lifestyle Intervention-A 12-Month Subanalysis of the ACOORH Trial. Nutrients 2020, 12, 2022. [Google Scholar] [CrossRef]
- Röhling, M.; Stensitzky, A.; Oliveira, C.L.P.; Beck, A.; Braumann, K.M.; Halle, M.; Fuhrer-Sakel, D.; Kempf, K.; McCarthy, D.; Predel, H.G.; et al. Effects of a Protein-Rich, Low-Glycaemic Meal Replacement on Changes in Dietary Intake and Body Weight Following a Weight-Management Intervention-The ACOORH Trial. Nutrients 2021, 13, 376. [Google Scholar] [CrossRef]
- Kempf, K.; Röhling, M.; Banzer, W.; Braumann, K.M.; Halle, M.; McCarthy, D.; Predel, H.G.; Schenkenberger, I.; Tan, S.; Toplak, H.; et al. High-Protein, Low-Glycaemic Meal Replacement Decreases Fasting Insulin and Inflammation Markers-A 12-Month Subanalysis of the ACOORH Trial. Nutrients 2021, 13, 1433. [Google Scholar] [CrossRef]
- Röhling, M.; Kempf, K.; Banzer, W.; Braumann, K.M.; Fuhrer-Sakel, D.; Halle, M.; McCarthy, D.; Martin, S.; Scholze, J.; Toplak, H.; et al. A High-Protein and Low-Glycemic Formula Diet Improves Blood Pressure and Other Hemodynamic Parameters in High-Risk Individuals. Nutrients 2022, 14, 1443. [Google Scholar] [CrossRef]
- Kempf, K.; Röhling, M.; Banzer, W.; Braumann, K.M.; Halle, M.; Schaller, N.; McCarthy, D.; Predel, H.G.; Schenkenberger, I.; Tan, S.; et al. Early and Strong Leptin Reduction Is Predictive for Long-Term Weight Loss during High-Protein, Low-Glycaemic Meal Replacement-A Subanalysis of the Randomised-Controlled ACOORH Trial. Nutrients 2022, 14, 2537. [Google Scholar] [CrossRef]
- Skoglund, G.; Nilsson, B.B.; Olsen, C.F.; Bergland, A.; Hilde, G. Facilitators and barriers for lifestyle change in people with prediabetes: A meta-synthesis of qualitative studies. BMC Public Health 2022, 22, 553. [Google Scholar] [CrossRef]
- Hedges, L.V.; Pustejovsky, J.E.; Shadish, W.R. A standardized mean difference effect size for single case designs. Res. Synth. Methods 2012, 3, 224–239. [Google Scholar] [CrossRef]
- Lopez-Gomez, J.J.; Izaola-Jauregui, O.; Torres-Torres, B.; Gomez-Hoyos, E.; Castro Lozano, M.A.; Ortola-Buigues, A.; Martin Ferrero, M.A.; De Luis-Roman, D.A. Influence of a meal-replacement diet on quality of life in women with obesity and knee osteoarthritis before orthopedic surgery. Nutr. Hosp. 2018, 35, 71–77. [Google Scholar] [CrossRef] [Green Version]
- De Luis, D.A.; Izaola, O.; Garcia, A.M.; Aller, R.; Cabezas, G.; de la Fuente, B. Effect of a hypocaloric diet with a commercial formula in weight loss and quality of life in obese patients with chronic osteoarthritis. Nutr. Hosp. 2012, 27, 1648–1654. [Google Scholar]
- Marcos-Delgado, A.; Hernandez-Segura, N.; Fernandez-Villa, T.; Molina, A.J.; Martin, V. The Effect of Lifestyle Intervention on Health-Related Quality of Life in Adults with Metabolic Syndrome: A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 887. [Google Scholar] [CrossRef]
- Koohkan, S.; Schaffner, D.; Milliron, B.J.; Frey, I.; Konig, D.; Deibert, P.; Vitolins, M.; Berg, A. The impact of a weight reduction program with and without meal-replacement on health related quality of life in middle-aged obese females. BMC Womens Health 2014, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Kempf, K.; Altpeter, B.; Berger, J.; Reuss, O.; Fuchs, M.; Schneider, M.; Gartner, B.; Niedermeier, K.; Martin, S. Efficacy of the Telemedical Lifestyle intervention Program TeLiPro in Advanced Stages of Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2017, 40, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.H.; Sun, L.H.; Yang, W.; Li, B.J.; Cui, R.J. Potential role of insulin on the pathogenesis of depression. Cell Prolif. 2020, 53, e12806. [Google Scholar] [CrossRef] [Green Version]
- Perry, B.I.; Khandaker, G.M.; Marwaha, S.; Thompson, A.; Zammit, S.; Singh, S.P.; Upthegrove, R. Insulin resistance and obesity, and their association with depression in relatively young people: Findings from a large UK birth cohort. Psychol. Med. 2020, 50, 556–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.T.; Huang, W.Y.; Kor, C.T.; Liu, K.H.; Chen, T.Y.; Lin, P.T.; Wu, H.M. Relationships between depression and anxiety symptoms and adipocyte-derived proteins in postmenopausal women. PLoS ONE 2021, 16, e0248314. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho-Ferreira, J.P.; Masquio, D.C.; da Silveira Campos, R.M.; Dal Molin, N.B.; Corgosinho, F.C.; Sanches, P.L.; Tock, L.; Tufik, S.; de Mello, M.T.; Finlayson, G.; et al. Is there a role for leptin in the reduction of depression symptoms during weight loss therapy in obese adolescent girls and boys? Peptides 2015, 65, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parameshwar, A.; Maiya, G.A.; Kamath, S.U.; Shastry, B.A.; Ravishankar. Lifestyle Modification with Physical Activity Promotion on Leptin Resistance and Quality of Life in Metabolic Syndrome—A Systematic Review with Meta-Analysis. Curr. Diabetes Rev. 2021, 17, 345–355. [Google Scholar] [CrossRef]


| Parameters | Control Group (n = 80) | Intervention Group (n = 183) |
|---|---|---|
| Sex [%] female/male | 57.5/42.5 | 65.0/35.0 |
| Age [years] | 50.1 ± 9.8 | 51.5 ± 9.0 |
| Body mass index [kg/m2] | 30.8 ± 2.3 | 29.9 ± 2.3 * |
| Weight [kg] | 92.2 ± 12.5 | 87.2 ± 12.7 ** |
| PCS [au] | 50.6 ± 5.9 | 49.4 ± 6.4 |
| MCS [au] | 50.7 ± 12.2 | 49.5 ± 10.1 |
| Fasting insulin [µU/mL] | 14.1 ± 7.2 | 13.4 ± 8.6 |
| Leptin [µg/L] | 13.5 ± 9.5 | 14.6 ± 10.3 |
| PCS after 12 Months | Δ PCS after 12 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | r | p | ß | p # | r | p | ß | p # |
| weight (baseline) | 0.012 | 0.847 | −0.065 | 0.464 | −0.056 | 0.362 | −0.68 | 0.465 |
| weight (3 months) | −0.023 | 0.711 | −0.117 | 0.186 | −0.074 | 0.230 | −0.121 | 0.186 |
| weight (12 months) | −0.040 | 0.519 | −0.141 | 0.085 | −0.088 | 0.155 | −0.145 | 0.085 |
| Δ weight (3 months) | −0.178 | 0.004 | −0.102 | 0.065 | −0.090 | 0.145 | −0.105 | 0.065 |
| Δ weight (12 months) | −0.096 | 0.120 | −0.113 | 0.040 | −0.067 | 0.277 | −0.117 | 0.040 |
| insulin (baseline) | −0.107 | 0.084 | −0.140 | 0.018 | −0.052 | 0.402 | −0.145 | 0.018 |
| insulin (3 months) | −0.058 | 0.346 | −0.72 | 0.195 | −0.036 | 0.557 | −0.075 | 0.196 |
| insulin (12 months) | −0.055 | 0.372 | −0.53 | 0.353 | −0.020 | 0.749 | −0.055 | 0.352 |
| Δ insulin (3 months) | 0.036 | 0.566 | 0.041 | 0.453 | 0.027 | 0.664 | 0.043 | 0.453 |
| Δ insulin (12 months) | 0.011 | 0.858 | 0.059 | 0.295 | 0.001 | 0.987 | 0.060 | 0.296 |
| leptin (baseline) | −0.038 | 0.536 | 0.081 | 0.234 | 0.099 | 0.111 | 0.084 | 0.234 |
| leptin (3 months) | −0.181 | 0.003 | −0.110 | 0.097 | 0.000 | 0.995 | −0.114 | 0.097 |
| leptin (12 months) | −0.131 | 0.033 | −0.014 | 0.828 | 0.046 | 0.462 | −0.014 | 0.828 |
| Δ leptin (3 months) | −0.207 | 0.001 | −0.136 | 0.015 | −0.191 | 0.002 | −0.141 | 0.015 |
| Δ leptin (12 months) | −0.127 | 0.041 | −0.060 | 0.281 | −0.033 | 0.591 | −0.062 | 0.281 |
| MCS after 12 months | Δ MCS after 12 months | |||||||
| Parameters | r | p | ß | p # | r | p | ß | p # |
| weight (baseline) | 0.093 | 0.132 | 0.118 | 0.176 | −0.079 | 0.203 | 0.119 | 0.176 |
| weight (3 months) | 0.079 | 0.203 | 0.068 | 0.427 | −0.115 | 0.064 | 0.069 | 0.427 |
| weight (12 months) | 0.017 | 0.778 | −0.037 | 0.642 | −0.141 | 0.022 | −0.038 | 0.642 |
| Δ weight (3 months) | −0.065 | 0.296 | −0.085 | 0.117 | −0.202 | 0.001 | −0.086 | 0.117 |
| Δ weight (12 months) | −0.148 | 0.016 | −0.161 | 0.003 | −0.198 | 0.001 | −0.163 | 0.003 |
| insulin (baseline) | −0.104 | 0.093 | −0.068 | 0.239 | −0.144 | 0.020 | −0.069 | 0.239 |
| insulin (3 months) | −0.132 | 0.033 | −0.142 | 0.009 | −0.238 | <0.001 | −0.144 | 0.009 |
| insulin (12 months) | −0.062 | 0.314 | −0.098 | 0.082 | −0.161 | 0.009 | −0.099 | 0.082 |
| Δ insulin (3 months) | −0.036 | 0.557 | −0.087 | 0.107 | −0.135 | 0.028 | −0.088 | 0.108 |
| Δ insulin (12 months) | 0.066 | 0.287 | −0.036 | 0.515 | −0.010 | 0.878 | −0.036 | 0.515 |
| leptin (baseline) | −0.088 | 0.157 | 0.074 | 0.271 | 0.060 | 0.334 | 0.074 | 0.271 |
| leptin (3 months) | −0.122 | 0.049 | 0.000 | 0.998 | −0.045 | 0.472 | 0.000 | 0.998 |
| leptin (12 months) | −0.194 | 0.002 | −0.108 | 0.079 | −0.117 | 0.058 | −0.110 | 0.079 |
| Δ leptin (3 months) | −0.007 | 0.913 | −0.056 | 0.310 | −0.101 | 0.101 | −0.056 | 0.310 |
| Δ leptin (12 months) | −0.117 | 0.059 | −0.137 | 0.012 | −0.144 | 0.020 | −0.138 | 0.012 |
| PCS after 12 Months | MCS after 12 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | r | p | ß | p # | r | p | ß | p # |
| Group | 0.094 | 0.130 | 0.084 | 0.136 | −0.026 | 0.679 | 0.045 | 0.402 |
| Sex | −0.133 | 0.031 | −0.060 | 0.298 | −0.126 | 0.042 | −0.043 | 0.431 |
| Age | −0.194 | 0.002 | −0.095 | 0.095 | 0.139 | 0.024 | 0.035 | 0.521 |
| BMI (at baseline) | −0.123 | 0.046 | 0.016 | 0.791 | −0.080 | 0.198 | −0.023 | 0.675 |
| PCS (at baseline) | 0.464 | <0.001 | 0.444 | <0.001 | 0.056 | 0.368 | 0.155 | 0.006 |
| MCS (at baseline) | −0.094 | 0.127 | 0.043 | 0.433 | 0.486 | <0.001 | 0.491 | <0.001 |
| Δ weight | −0.096 | 0.120 | −0.064 | 0.268 | −0.148 | 0.016 | −0.123 | 0.038 |
| insulin a | −0.107 | 0.084 | −0.116 | 0.052 | −0.132 | 0.033 | −0.145 | 0.007 |
| Δ leptin b | −0.207 | 0.001 | −0.103 | 0.080 | −0.117 | 0.059 | −0.077 | 0.199 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempf, K.; Röhling, M.; Banzer, W.; Braumann, K.M.; Halle, M.; Schaller, N.; McCarthy, D.; Predel, H.G.; Schenkenberger, I.; Tan, S.; et al. High-Protein, Low-Glycaemic Meal Replacement Improves Physical Health-Related Quality of Life in High-Risk Persons for Metabolic Syndrome—A Subanalysis of the Randomised-Controlled ACOORH Trial. Nutrients 2022, 14, 3161. https://doi.org/10.3390/nu14153161
Kempf K, Röhling M, Banzer W, Braumann KM, Halle M, Schaller N, McCarthy D, Predel HG, Schenkenberger I, Tan S, et al. High-Protein, Low-Glycaemic Meal Replacement Improves Physical Health-Related Quality of Life in High-Risk Persons for Metabolic Syndrome—A Subanalysis of the Randomised-Controlled ACOORH Trial. Nutrients. 2022; 14(15):3161. https://doi.org/10.3390/nu14153161
Chicago/Turabian StyleKempf, Kerstin, Martin Röhling, Winfried Banzer, Klaus Michael Braumann, Martin Halle, Nina Schaller, David McCarthy, Hans Georg Predel, Isabelle Schenkenberger, Susanne Tan, and et al. 2022. "High-Protein, Low-Glycaemic Meal Replacement Improves Physical Health-Related Quality of Life in High-Risk Persons for Metabolic Syndrome—A Subanalysis of the Randomised-Controlled ACOORH Trial" Nutrients 14, no. 15: 3161. https://doi.org/10.3390/nu14153161
APA StyleKempf, K., Röhling, M., Banzer, W., Braumann, K. M., Halle, M., Schaller, N., McCarthy, D., Predel, H. G., Schenkenberger, I., Tan, S., Toplak, H., Martin, S., Berg, A., & on behalf of the ACOORH Study Group. (2022). High-Protein, Low-Glycaemic Meal Replacement Improves Physical Health-Related Quality of Life in High-Risk Persons for Metabolic Syndrome—A Subanalysis of the Randomised-Controlled ACOORH Trial. Nutrients, 14(15), 3161. https://doi.org/10.3390/nu14153161








