Abstract
Meat analogs based on plant protein extracts are rising in popularity as meat consumption declines. A dietary shift away from meat, which has a high iron bioavailability, may have a negative effect on the amount of iron absorbed from the diet. Iron absorption from legumes cultivated in regions not suitable for soy production, such as fava bean, has not yet been explored. The aim of this study was to evaluate non-heme iron absorption from a meal with texturized fava bean protein compared to beef and cod protein meals. The study included two single-blinded iron isotope trials in healthy Swedish women of the ages 18–45 years, each of whom served as their own control. The participants were served matched test meals containing beef and fava bean protein (Study 1) or cod and fava bean protein (Study 2) with radiolabeled non-heme iron 55Fe and 59Fe. The absorption of non-heme iron from test meals was measured by whole-body counting and erythrocyte incorporation. The absorption of non-heme iron, measured as erythrocyte incorporation ratio, from beef protein meal was 4.2 times higher compared to texturized fava bean meal, and absorption from cod protein meal was 2.7 times higher compared to the fava bean meal. The adjusted non-heme iron absorption, normalized to a 40% reference dose uptake, was 9.2% for cod protein meal, 21.7% for beef protein meal, and 4.2% for texturized fava bean meal. A fava bean protein meal has markedly lower iron bioavailability in healthy females compared with a meal of beef or cod protein. Therefore, a dietary shift from meat and fish protein to fava bean protein may increase the risk of iron deficiency.
1. Introduction
Due to concerns regarding the climate impact from food consumption, a shift toward a plant-based diet has been pushed as a means to reduce the climate footprint as well as land and water use [1,2,3,4]. Another strong argument commonly used for such a shift is health since a mainly plant-based diet is related to a decreased risk of metabolic diseases if the diet is composed of high-quality foods [5,6]. Yet, a high-quality plant-based diet has also been linked to an increased risk of micronutrient deficiencies, largely due to limited intake of vitamins D and B12, selenium, iodine, and the low bioavailability of minerals [7].
Rather than increasing the intake of health-promoting, high-quality foods, the use of refined products and meat analogs is becoming increasingly common [8]. A common method to achieve a meat-like structure in the production of meat analogs is extrusion cooking, i.e., the texturization of plant protein extracts. The extraction of plant proteins has been shown to accumulate phytate, a potent inhibitor of iron absorption, as well as other antinutritional components, in the protein-rich fraction later used for extrusion cooking [9,10,11,12]. During extrusion cooking, high pressure and high temperature are used to reshape the protein structures. This processing technique has been reported to affect the composition and nutritional quality of plant protein, the magnitude and effect depending on the specific settings during processing [13,14,15]. Extracted and extruded plant protein differs significantly in terms of composition compared to the raw material, and the health implications of such products are largely unknown.
Meat analogs based on extracted plant protein often have a high content of iron [16,17]. However, the uptake of iron from such products is questionable due to the high content of phytate [18,19]. The inhibitory effect of phytates on mineral absorption is explained by the formation of insoluble and indigestible phytate-mineral compounds in the gut [20]. Excessive levels of phytates in the diet can lead to iron deficiency, highlighting the relevance of investigating iron bioavailability rather than iron content [21,22]. Vegetarians have been shown to have lower iron stores and an increased prevalence of iron deficiency compared with non-vegetarians, mainly explained by the lower bioavailability of iron in plant foods [23,24] as plant foods contain no heme iron, no muscle protein stimulating absorption, and in general have a high phytate content. The current prevalence of iron deficiency in women of fertile ages living in Western countries has been estimated to be 10–30% [25,26,27,28]. Women across Western societies are twice as likely as men to follow a plant-based diet [29], which augments the vulnerability of developing low iron status and iron deficiency.
The use of soy for the production of meat analogs is widespread even in countries with no domestic production of soy. Using domestic crops that are possible to cultivate in regions with short growing seasons, such as fava bean [30], is important in regions currently dependent upon the importation of soybean. Fava bean has been highlighted as a crop with upscaling potential in Sweden [31] and has been suggested to be suitable for the substitution of meat [32]. Moreover, soy protein per se has been shown to inhibit non-heme iron absorption [33,34,35]. The inhibitory effect is attributed to the protein fraction conglycinin [36], which is not present in fava bean [37]. Another advantage is that white-flowered cultivars of fava bean contain comparatively low levels of iron-binding polyphenols [38]. Chemical analyses of the composition of whole fava bean have revealed nutritional differences between cultivars and a generally high content of phytate. The Phytate:Fe molar ratios suggest that the bioavailability of iron in whole fava bean seeds is low [39], with the exception of the Sunrise cultivar, which was analyzed in a previous paper by our group [38].
To the best of our knowledge, iron absorption from a meal of extracted and texturized fava bean protein, industrially produced as meat analogs, has not before been measured in human subjects.
The primary aim was to investigate the absorption of non-heme iron from a meal with texturized fava bean protein compared to matched meals with cod and beef protein.
It was hypothesized that non-heme iron absorption from the plant protein meal would be lower than from the animal protein meals.
2. Materials and Methods
2.1. Subjects
The participants in the study were apparently healthy women aged 18–45. Women of reproductive age were chosen as subjects since this is one of the main risk populations in terms of developing iron deficiency. No iron-containing supplements were allowed two weeks prior to the study, and the donation of blood was not allowed six months prior to the study. Pregnant or lactating women were excluded from the study. Each participant was asked about their current health status and any indications of infection during the weeks prior to the study. Questions regarding infection were repeated at each visit to avoid errors due to infection, as an activated acute-phase reaction influences iron homeostasis and iron absorption [40]. Recruitment was undertaken using flyers set up on university campuses in Gothenburg, Facebook advertisements, and the digital recruitment platform Accindi. Initially, 201 individuals were assessed for eligibility. The sample size (10–15 participants) was determined from the previous literature on similar trials [35,41]. A total of 23 and 22 subjects, respectively, were enrolled in Study Group 1, beef protein vs. fava bean protein meals (beef–fava), and Study Group 2, cod protein vs. fava bean protein meals (cod–fava), respectively (Table 1).

Table 1.
Exclusion and final inclusion of participants.
A total of 18 participants were excluded during the study, the main reasons being that they were unable to finish the meal (n = 7) and sickness during the study (n = 7). Each participant in both study groups started with a texturized fava bean meal, which was perceived as very dry and, therefore, too difficult for some participants to eat. All of the dropouts caused by this reason occurred during the intake of the first test meal. The participants that developed a fever or an infection during the study were excluded, as this would affect iron metabolism. The high rate of sickness in the two trials can be explained by the simultaneously ongoing COVID-19 pandemic. For a detailed description, see the participant flowchart (Supplementary S1 participant flowchart).
2.2. Experimental Design: Study 1 (Beef-Fava Bean) and Study 2 (Cod-Fava Bean)
The study included two single-blinded double radio-iron isotope trials in healthy Swedish women aged 18–45 years, where each subject served as her own control. The double radio-iron method enabled the effect of texturized fava bean protein compared to meat or fish protein to be measured separately in the same individual. The two trials were performed from winter to spring 2021–2022.
The subjects were served three potato patties baked with a total of 35 g (dry weight, dw) of protein extract. In Study 1, the subjects were served texturized fava bean protein (B) and beef protein (A). In Study 2, the subjects were served texturized fava bean protein (B) and cod protein (A). The subjects fasted before they were served the meals as breakfast for four consecutive days in the order BBAA. The meals were served with 2 dL of water. No food or drinks were allowed within three hours after the intake of the meal. The subjects were measured by a sensitive whole-body counter (WBC) approximately 2 weeks after the meal intake, according to Skoldborn et al. [42], with modifications described by Fredlund et al. [43]. A fasting blood sample followed by the intake of a reference dose containing 59Fe was taken at the Sahlgrenska hospital, Gothenburg, after a WBC measurement. Approximately 2 weeks after the intake of the reference dose, the subjects were again measured by a WBC in order to adjust for individual differences in absorption. The subjects were randomly allocated by computer randomization performed by a colleague not involved in the study. Randomization was restricted to the two study groups (beef–fava or cod–fava).
2.3. Preparation and Serving of Test Meals
Each meal was based on a dough consisting of boiled and mashed potato (180 g, after boiling), 38 mL of tap water, 15 mL of rapeseed oil, and 8 mL of mushroom broth (containing water, salt, E621, E631, yeast extract, sugar, maltodextrin, potato starch, mushroom, E202, E412, E415, flavors, and ascorbic acid). The added content of ascorbic acid was 0.8 mg per dough portion. Since ascorbic acid is a heat-sensitive vitamin, the content in the ready meal was considered negligible and to have no impact on the iron absorption from the meals.
To each dough, 35 g dw of protein was added. Due to the differences in protein content between the three dried and milled protein sources, the cod meal consisted of 44 g of protein concentrate (with a protein content of 83.4%, dw), the beef meal consisted of 42 g of protein concentrate (82.9% protein content, dw), and the fava bean meal contained 67 g of protein concentrate (56.3% protein content, dw). The dough was divided into three equally-sized burger-shaped pieces, and these were baked in a conventional oven at 200 °C for 28 min. After baking, the meal was allowed to cool before the three pieces were placed into a microwave-safe container and stored at −18 °C. The containers were marked with a color code for each type of meal; the code was not visible to the participants. The afternoon before the meals were served, the meals were placed in a refrigerator and allowed to thaw until the following morning when the meals were microwave-heated, and the iron isotope was added. The meals were served outside the clinical nutrition test kitchen at the Sahlgrenska Academy, Gothenburg.
2.4. Radioiron Labeling of Meals
To determine the amount of iron absorbed from the meals, extrinsic radio-labeled 59Fe (A, beef and cod meals) and 55Fe (B, texturized fava bean meals) were added as FeCl3 in HCl to the surface of the heated meals by pipetting immediately before serving. The isotopes 55Fe and 59Fe have been used in iron-absorption studies for nearly 80 years since their introduction in 1939 by Hahn et al. [44]. Extrinsic radio-iron labeling techniques rely on the basis of the “pool concept”, which is the isotopic exchange through diffusion between extrinsically-added iron and the native iron in the food. It has been shown that from a single food that has been biosynthetically labeled with iron using an isotope of either 55Fe or 59Fe and mixed with extrinsically-added iron in the other isotopic form, the absorption of the two isotopes is identical. The absorption of extrinsic and intrinsic isotopes has been shown to remain identical even in meals with different iron bioavailability [45,46], with the exception of foods with a hard outer layer, which inhibits diffusion [47]. In the test meals used in the present study, we expected no interference with the isotopic exchange between the native and extrinsic iron.
2.5. Iron Absorption Measurements
The primary outcome measure was the iron absorption from the test meals, which was measured using two different radio-iron tracers. The average radioactivity each subject received was a total of 74 kBq from 55Fe and 103.6 kBq from 59Fe (34 kBq from the reference dose and 70.0 kBq from the meal).
The iron absorption from the 59Fe-labelled beef and cod protein meals was calculated as the detected whole-body radioactivity content at 11–15 days after the meal intake, corrected for physical decay and background radiation, and compared to the measured 59Fe radioactivity in the served meal. The whole-body content of 59Fe was measured for 10 min per subject by a whole-body counter (WBC) situated in the Sahlgrenska University hospital and consisting of two NaI-detectors (“5 × 4”) located above and below the subject’s thorax region in a stationary lying-on-a-bed geometry [43]. The reference dose, used for the absorption normalization between the individuals, was measured in the WBC, 12–16 days after the intake of the reference dose. For s detailed method description of WBC, see Supplementary Data S2 Detailed method description for whole body counting measurements.
Since the activity of 55Fe cannot be detected by a WBC due to a lack of appropriate gamma-ray emissions, the blood sample that was drawn immediately after the first WBC (11–15 days after meal intake) was used to determine the ratio between the two isotopes using a liquid scintillator. The isotope ratio was then used to calculate the 55Fe absorption based on the measured 59Fe activity from the WBC measurements. The analysis was performed according to the method described by Eakins et al. [48], with slight modifications by Hoppe et al. [49]. Duplicates of whole blood corresponding to 10 mg of Fe were pre-treated and analyzed in a liquid scintillator (Tri-Carb, Packard Instruments, Dallas, TX, USA) to determine the radiation from 55Fe and 59Fe.
2.6. Oral Reference Dose
By relating the absorption from the test meals to the absorption of a reference dose, the variation depending on individual differences in iron absorption capacity, primarily dependent on iron status, can be corrected [50]. Therefore, a reference dose of ferrous ascorbate with added 59Fe was administered to the participants. Iron absorption was normalized to a 40% absorption from the reference dose, corresponding to borderline iron-deficient individuals who have not developed anemia, as this is a standard procedure and allows for comparisons between the two trials as well as other iron absorption studies [50]. The reference doses are usually administered to participants two days in a row in order to limit day-to-day variations within the same individual [51,52], but due to the global shortage of 59Fe as a consequence of the ongoing COVID-19 pandemic, it was only possible to use one reference dose.
2.7. Statistical Analyses
The data analysis was performed with an IBM SPSS version 28 (IBM, New York, NY, USA). To investigate the differences between the groups and to account for the small sample size and skew, a Mann–Whitney U test was used, and p-values below 0.05 were considered significant. The absorption quota is presented as the ratio between 59Fe/55Fe in erythrocytes. The percentage of absorption is presented as the median uptake using combined WBC/erythrocyte data, and for fava bean meal absorption, this was calculated using the combined data from Study 1 and Study 2 as no significant difference in 55Fe absorption was found between the two study groups.
2.8. Hematological Analysis
On the first day of the study, each participant gave a fasting blood sample at the Sahlgrenska hospital, Gothenburg, by venipuncture prior to the intake of the first meal. The blood was analyzed at an accredited reference laboratory (Clinical Chemistry Laboratory, Sahlgrenska University Hospital, Gothenburg, Sweden) for C-reactive protein (CRP) and iron status: serum iron concentration, total iron binding capacity (TIBC), transferrin saturation (TSAT), serum ferritin, soluble transferrin receptor (sTfR), and hemoglobin. CRP was analyzed to avoid any errors caused by ongoing infection.
2.9. Extraction of Beef- and Cod Protein
Fresh, ice-covered Atlantic cod (Gadus morhua) filleting co-products, including the head and tail, were provided by Fisk Idag AB and Sjöboden AB (Gothenburg, Sweden). Fresh boneless beef loins were also bought from a local store. Right after arrival, the co-products and beef samples were separately ground using a table-top meat grinder (C/E22 N, Minerva Omega Group, Italy) equipped with a plate with 4.5 mm holes. Then, the mince produced from the co-products and beef samples were packed in polyethylene Ziplock bags and frozen and stored at −80 °C until further usage.
To extract protein from the minced samples, they were subjected to alkaline solubilization and isoelectric precipitation following the main steps reported by Abdollahi and Undeland (2019) [53] with minor modifications. For a detailed description of the method, see Supplementary Data S3 Extraction of beef and cod protein.
2.10. Analysis of Food Composition
The three meals were analyzed for their content of Fe and Zn, protein, total fat, and the presence of total phenolics. The meat and cod meals were analyzed for their content of heme iron, and the fava bean meal was analyzed for its content of phytate.
The total iron and zinc contents in the protein extracts were determined in duplicate by atomic absorption spectrometry (200 Series AA System; Agilent, Santa Clara, CA, USA). The digestion of the samples prior to analysis was performed using the microwave technique, as previously described by Fredriksson et al. [54]. The heme-iron content in the cod and meat protein isolates was determined in triplicate by HPLC, according to the protocol developed by Ryter and Tyrell [55]. The protein content was determined by the Dumas principle using a LECO Trumac nitrogen analyzer (LECO Corporation, St. Joseph, MI, USA). The total protein content of the meat was calculated using a nitrogen-to-protein conversion factor of 5.42, fish was 5.58, and fava bean was 5.4, according to Mariotti et al. [56]. The total phenolic content (TPC) was determined in duplicate by the Folin–Ciocalteu method, based on the technique of Howard et al. [57]. Phytate was analyzed as inositol hexaphosphate (InsP6) by high-performance ion chromatography (HPIC), according to Carlsson et al. [58]. The lipids were analyzed and extracted with chloroform and methanol (2:1), according to Bligh and Dyer [59], as modified by Lee et al. [60]. The molar ratios Phy:Fe and Phy:Zn in the fava bean protein were calculated to estimate the relative bioavailabilities of iron and zinc, respectively, and to give an indication of the inhibitory effects of phytate on these minerals. A molecular mass for phytate of 660.3 g/mol was used for the calculations.
2.11. Ethics
The study protocol was approved by the Ethical Review Board in Gothenburg, Registration Diary number 573-18. The subjects were given comprehensive written and oral information about the aims and procedure prior to study inclusion. A written consent form was signed by the subjects before the study started. The trial was registered at www.clinicaltrials.gov as NCT05392816.
3. Results
3.1. Chemical Composition of Meals
The protein content was matched between the three test meals (Table 2), while the content of iron and zinc was higher in the fava bean meal compared to the cod and beef meals. The fava bean meal contained a high level of phytate, with a phytate-to-iron molar ratio of 39, and a phytate-to-zinc molar ratio of 51.

Table 2.
Composition of test meals.
3.2. Iron Absorption from Meals
The hematological data and age did not significantly differ between the two study groups (Table 3). However, the BMI was significantly higher in the beef–fava study. The average BMI in the beef–fava group was 25.5, which corresponds to overweight. The average BMI in the cod–fava group was 22.6, which corresponds to a normal weight. Large individual differences were found as expected within the two study groups with respect to iron status. The CRP level of the participants was low on the first day of the trial; no exclusions were made based on the blood analysis.

Table 3.
Subject data for Study Group 1 (beef–fava) and Study Group 2 (cod–fava).
The absorbance quota, analyzed by the erythrocyte-incorporated iron using liquid scintillation, was 2.7 times greater for the cod-protein meal compared to the texturized fava bean meal and 4.2 times greater for the beef-protein meal compared to the texturized fava bean meal.
The absolute measurements of 59Fe absorbance from WBC were combined with the relative 59Fe/55Fe absorbance quota from the erythrocyte incorporation analysis by liquid scintillation in order to calculate the absorbance of 55Fe. The beef- and cod-protein meals were labeled with 59Fe, while the texturized fava bean protein meals were labeled with 55Fe.
As seen in Figure 1, the adjusted median fractional absorption of non-heme iron from the texturized fava bean protein meal was 4.2% (3.2% unadjusted), 9.2% for the cod-protein meal (9.7% unadjusted), and 21.7% for the beef-protein meal (13.9% unadjusted). The beef–fava group had an insignificant trend of lower absorption from the reference dose (Table 3). As we adjusted the absorption based on the reference dose uptake, the correction for this group was higher, which explains the larger difference between the adjusted and unadjusted absorption from the beef-protein meal compared to the cod-protein meal. There was no significant difference in the fava bean meal iron absorption between the two study groups.
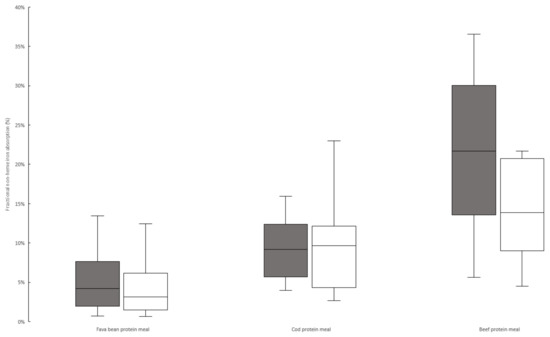
Figure 1.
Box plot of adjusted and unadjusted non-heme iron absorption from texturized fava bean meal, cod-protein meal, and beef-protein meal. Absorption of texturized fava protein is based on combined data from Study 1 and Study 2. Grey boxes represent data adjusted to a 40% reference dose absorption; white boxes represent unadjusted data. Adjusted and unadjusted data based on combined data from absorption quota and WBC was significantly (p < 0.05) different between each of the three meals, analyzed by Mann–Whitney U test.
According to the Swedish national dietary survey, Riksmaten, Swedish women of fertile ages have a total meat intake of 88 g per day on average [61]. Total meat includes red meat, poultry, offal, blood products, and sausages. According to the Nordic Nutrition Recommendations, the daily need for iron is 2.22 mg per day for women of fertile ages [62]. The contribution to the daily iron need from current meat intake was calculated to be 18% (Table 4). The calculations were based on a non-heme iron absorption of 21.7% from meat, as found in this study, and an assumption of 25% absorption from heme iron, as previously reported by Monsen et al. [63].

Table 4.
Scenario calculations on the effects of absorbed iron when 100%, and 50% of the total meat intake is exchanged to texturized fava bean meals.
4. Discussion
Non-heme iron absorption, measured as 59Fe/55Fe ratio in erythrocytes, from meals containing beef or cod protein was 4.2 and 2.7 times higher, respectively, compared to a meal with texturized fava bean protein. Combined data from WBC and erythrocyte analysis showed that the absorption of non-heme iron was 9.2% from the cod protein meal (9.7% unadjusted), 21.7% from the beef protein meal (13.9% unadjusted), and 4.2% from the texturized fava bean meal (combined data from Study 1 and Study 2. Unadjusted 3.2%). Apart from the non-heme iron, a large contribution to total iron absorbed can be expected from heme iron in the beef and cod proteins. Approximately 40% of the total iron in these animal protein sources was found to be heme iron, from which up to 25% is considered to be absorbed [66]. Based on this assumption, the total amount of iron absorbed was estimated to be 0.62 mg from the beef protein meal (out of 2.7 mg total iron), 0.36 mg from the cod protein meal (out of 2.3 mg total iron), and 0.16 mg from the fava bean meal (out of 3.7 mg total iron). Despite having the highest amount of total iron, the actual iron absorbed from the fava bean meal was significantly lower compared to the beef and cod protein meals. Compared to the daily iron need of women of fertile ages, which is estimated to be 2.22 mg/day (90th percentile) according to the Nordic Nutrition Recommendations [62], the contribution from the fava bean meal was 7.0%. The contribution to the daily need for iron from the beef protein meal was 28.0% and 16.3% from the cod protein meal. A total exchange from current meat intake among Swedish women into texturized fava bean protein was calculated to result in a decrease of 82% of absorbed iron from what was expected from the meat fraction, as the texturized fava bean was calculated to contribute with 3% of the daily iron need (Table 4). Exchanging half of the current total meat intake was calculated to result in a 41% decrease of absorbed iron from what was expected from the meat fraction and a total contribution of 11% to the daily iron need.
Our results highlight the difficulties of covering daily iron needs with plant-protein-extract products as a substitute for beef or cod meals, especially for women of fertile ages as well as growing individuals with high iron needs. This is an important finding as the majority of meat substitutes currently on the market are based on legume protein extracts, with extrusion as the principal technique for building structure [8]. The effects on health and nutrition of a diet composed of such products are currently unknown [67].
Apart from containing heme iron, meat and fish have been demonstrated many times to enhance non-heme iron absorption ascribed to the meat factor, which is thought to be related to the protein, or peptide digestion products, of muscle tissue [68,69,70,71]. Hurrell et al. showed that extracted heme-free beef protein enhances non-heme iron absorption to the same extent as the native muscle tissue [33].
There was a higher mineral content in the fava bean meal compared to the beef and cod protein meals. Since we wanted the meals to reflect actual protein meals, adjustment of iron content was deemed undesirable. The fava bean meal contained a high content of phytate, which reflects an actual meal based on plant protein extract. The meal was not designed to be high in phytate; rather, the extract naturally had a very high content since phytate is accumulated in the protein fraction of legumes [18]. The molar ratio of phytate to iron was 39, which can be considered to reflect a very low theoretical bioavailability of non-heme iron. A maximum ratio of 1, or ideally 0.4, has been recommended [72]. The molar ratio of phytate-to-zinc was 51, which similarly reflects a very low bioavailability of zinc [73]. In the present study, the low theoretical value of non-heme iron absorption was confirmed by the clinical trial results. Reducing phytic acid is an essential means of achieving adequate non-heme iron absorption from plant-based meals [74,75,76]. The total iron content has been reported to be higher among plant-based dietary patterns compared with omnivore dietary patterns [77,78,79,80], while individuals following plant-based diets have been reported to have an increased prevalence of low iron stores and iron deficiency [23,24,81]. Our findings suggest that the content of iron in plant protein should be evaluated in a different way, taking into account the bioavailability, rather than solely total content.
As mentioned, only one reference dose of 59Fe was administered to the participants. Day-to-day variations of iron absorption are known to be significant. Therefore, it is most likely that among the participants in the study, day-to-day variations have had an impact on the amount of iron absorbed from the reference dose. Despite this limitation, one reference dose was still used as an attempt to adjust for individual differences and comparisons between the two study groups as well as other trials. The absorption of the reference doses was 39% in the cod–fava study group and 30% in the beef–fava study group. The mean reference dose absorption did not differ significantly between the two study groups (p ≥ 0.05), but the adjustment of 59Fe in the beef-fava study group was higher due to a trend of lower reference dose absorption in the beef group, which might be explained by day-to-day variations. Since overweight and obesity are related to an impairment of iron absorption [82,83], the beef–fava group may potentially have absorbed less iron from the meals and reference dose compared to the cod–fava group, but no difference could be found in the absorption of 55Fe (fava bean meals) between the two groups.
Our results are in line with previous studies on plant foods in single meals [35,74,84,85,86] as well as reports on iron status in long-term interventions [87]. Even though single meal absorption studies tend to exaggerate the inhibitory effects compared to long-term studies [88], our results highlight a need to update recommendations with regard to iron and low-bioavailability meals. Today, most recommendations on iron intake are calculated on a relatively high bioavailability of iron in the diet. For example, Nordic Nutrition Recommendations (NNR) calculate recommended daily intake of iron based on a bioavailability of 15%, which corresponds to a diet containing meat [62]. There are no specific recommendations for low-bioavailability diets, such as vegetarian or vegan diets. As mentioned, the prevalence of iron deficiency among women of fertile ages across western societies today is approximately 25% [25]. This proportion is at risk of increasing as a result of the protein shift, especially for individuals in vulnerable groups such as women of fertile ages, children, and adolescents, due to their high iron requirements. It is of critical importance to include nutrition and bioavailability in policy-making, calculations, and processes concerning the protein shift in order to counteract potential negative health effects of a dietary shift.
5. Conclusions
Clearly, the substitution of beef and cod protein with texturized fava bean protein has a serious negative effect on the amount of iron absorbed from a meal; here, for the first time, it was shown when serving subjects these protein sources in the form of protein extracts. There is a need to develop plant-protein products with high iron bioavailability, especially meat substitutes and meals aimed at young women and children. The results also point out a need to update guidelines on dietary iron intake from plant-based food patterns with low iron bioavailability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14153162/s1, Supplementary S1: Participant flow chart; Supplementary S2: Whole-body counting method description; Supplementary S3: Detailed method description of beef and cod protein extraction.
Author Contributions
Conceptualization, A.-S.S. and L.H.; methodology, L.H., M.H. (Michael Hoppe), M.H. (Martin Hjellström) and A.-S.S.; formal analysis, M.H. (Martin Hjellström), M.H. (Michael Hoppe) and I.-C.M.L.; investigation, E.G., M.H. (Martin Hjellström), M.H. (Michael Hoppe) and I.-C.M.L.; resources, I.-C.M.L., M.A., I.U. and A.-S.S.; writing—original draft preparation, I.-C.M.L.; writing—review and editing, A.-S.S.; visualization, I.-C.M.L.; supervision, A.-S.S.; project administration, A.-S.S. and I.-C.M.L.; funding acquisition, A.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish Research Council FORMAS, grant number 2021-01123, to Ann-Sofie Sandberg and by Västra Götaland Region, RUN 202-00041 to Ann-Sofie Sandberg.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Gothenburg (Diary number 573-18).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval.
Acknowledgments
Authors thank Hannah Steinhausen and Linnéa Almius for their help with serving test meals.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; de Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO) of the United Nations. The State of Food Insecurity in the World, Addressing Food Insecurity in Protracted Crises; FAO, Ed.; FAO: Rome, Italy, 2010. [Google Scholar]
- Micha, R.; Shulkin, M.L.; Peñalvo, J.L.; Khatibzadeh, S.; Singh, G.M.; Rao, M.; Fahimi, S.; Powles, J.; Mozaffarian, D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS ONE 2017, 12, e0175149. [Google Scholar]
- Hemler, C.E.; Hu, F.B. Plant-Based Diets for Cardiovascular Disease Prevention: All Plant Foods Are Not Created Equal. Curr. Atheroscler. Rep. 2019, 21, 18. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2022, 14, 29. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Van Der Poel, T.F.B.; Aarts, H.L.M.; Kik, M.J.L. Air classification of bean flour—Effects on protein, antinutritional factors and the effect of a fines fraction on cultured explants of small intestinal mucosa. J. Sci. Food Agric. 1990, 53, 143–157. [Google Scholar] [CrossRef]
- Gamel, T.H.; Linssen, J.P.; Mesallam, A.S.; Damir, A.A.; Shekib, L.A. Seed treatments affect functional and antinutritional properties of amaranth flours. J. Sci. Food Agric. 2006, 86, 1095–1102. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2014, 193, 34–42. [Google Scholar] [CrossRef]
- Sandberg, A.-S. Developing functional ingredient. A case study of pea protein. In Functional Foods. Concept to Product, 2nd ed.; Saarela, M., Ed.; Woodhead Publishing Ltd.: Abington, PA, USA; CRC Press LLC: Boca Raton, FL, USA, 2011; Chapter 15; pp. 358–383. [Google Scholar]
- El-Hady, E.-S.A.A.; Habiba, R.A.A. Effect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. LWT Food Sci. Technol. 2003, 36, 285–293. [Google Scholar] [CrossRef]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Batista, K.A.; Prudêncio, S.H.; Fernandes, K.F. Changes in the functional properties and antinutritional factors of extruded hard-to-cook common beans (Phaseolus vulgaris L.). J. Food Sci. 2010, 75, C286–C290. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Moshtaghian, H.; Bianchi, M.; Hallström, E. Nutritional assessment of plant-based meat analogues on the Swedish market. Int. J. Food Sci. Nutr. 2022, 6, 1–13. [Google Scholar] [CrossRef]
- Fredrikson, M.; Biot, P.; Alminger, M.L.; Carlsson, N.-G.; Sandberg, A.-S. Production Process for High-Quality Pea-Protein Isolate with Low Content of Oligosaccharides and Phytate. J. Agric. Food Chem. 2001, 49, 1208–1212. [Google Scholar] [CrossRef]
- Harnack, L.; Mork, S.; Valluri, S.; Weber, C.; Schmitz, K.; Stevenson, J.; Pettit, J. Nutrient Composition of a Selection of Plant-Based Ground Beef Alternative Products Available in the United States. J. Acad. Nutr. Diet. 2021, 121, 2401–2408.e12. [Google Scholar] [CrossRef]
- Sandstrom, B. Bioavailability of Zinc. Eur. J. Clin. Nutr. 1997, 51, 17–19. [Google Scholar]
- Zhou, J.R.; Erdman, J.W. Phytic acid in health and disease. Crit. Rev. Food Sci. Nutr. 1995, 35, 495–508. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chaouki, N.; Hurrell, R.F. Iron deficiency due to consumption of a habitual diet low in bioavailable iron: A longitudinal cohort study in Moroccan children. Am. J. Clin. Nutr. 2005, 81, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Haider, L.M.; Schwingshackl, L.; Hoffmann, G.; Ekmekcioglu, C. The effect of vegetarian diets on iron status in adults: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 1359–1374. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Berger, J.; Hines, I. Iron Status of Vegetarian Adults: A Review of Literature. Am. J. Lifestyle Med. 2016, 12, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Umbreit, J. Iron deficiency: A concise review. Am. J. Hematol. 2005, 78, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Greene-Finestone, L.; Lowell, H.; Levesque, J.; Robinson, S. Iron sufficiency of Canadians. Health Rep. 2012, 23, 41–48. [Google Scholar] [PubMed]
- Hercberg, S.; Preziosi, P.; Galan, P. Iron deficiency in Europe. Public Health Nutr. 2001, 4, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Lahti-Koski, M.; Valsta, L.M.; Alfthan, G.; Tapanainen, H.; Aro, A. Iron status of adults in the capital area of Finland. Eur. J. Nutr. 2003, 42, 287–292. [Google Scholar] [CrossRef]
- Modlinska, K.; Adamczyk, D.; Maison, D.; Pisula, W. Gender Differences in Attitudes to Vegans/Vegetarians and Their Food Preferences, and Their Implications for Promoting Sustainable Dietary Patterns—A Systematic Review. Sustainability 2020, 12, 6292. [Google Scholar] [CrossRef]
- Stoddard, F.; Hämäläinen, K. Towards the World’s earliest maturing faba beans. Grain Legumes 2011, 56, 9–10. [Google Scholar]
- Röös, E.; Carlsson, G.; Ferawati, F.; Hefni, M.; Stephan, A.; Tidåker, P.; Witthöft, C. Less meat, more legumes: Prospects and challenges in the transition toward sustainable diets in Sweden. Renew. Agric. Food Syst. 2020, 35, 192–205. [Google Scholar] [CrossRef] [Green Version]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.; Cook, J.D. Meat Protein Fractions Enhance Nonheme Iron Absorption in Humans. J. Nutr. 2006, 136, 2808–2812. [Google Scholar] [CrossRef]
- Weinborn, V.; Pizarro, F.; Olivares, M.; Brito, A.; Arredondo, M.; Flores, S.; Valenzuela, C. The Effect of Plant Proteins Derived from Cereals and Legumes on Heme Iron Absorption. Nutrients 2015, 7, 8977–8986. [Google Scholar] [CrossRef]
- Hallberg, L.; Rossander, L. Effect of soy protein on nonheme iron absorption in man. Am. J. Clin. Nutr. 1982, 36, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S.R.; Dassenko, S.A.; Cook, J.D.; Juillerat, M.A.; Hurrell, R.F. Inhibitory effect of a soybean-protein--related moiety on iron absorption in humans. Am. J. Clin. Nutr. 1994, 60, 567–572. [Google Scholar] [CrossRef]
- Warsame, A.O.; Michael, N.; O’Sullivan, D.M.; Tosi, P. Identification and Quantification of Major Faba Bean Seed Proteins. J. Agric. Food Chem. 2020, 68, 8535–8544. [Google Scholar] [CrossRef]
- Mayer Labba, I.-C.; Frøkiær, H.; Sandberg, A.-S. Nutritional and antinutritional composition of fava bean (Vicia faba L., var. minor) cultivars. Food Res. Int. 2021, 140, 110038. [Google Scholar] [CrossRef]
- Lazarte, C.E.; Carlsson, N.-G.; Almgren, A.; Sandberg, A.-S.; Granfeldt, Y. Phytate, zinc, iron and calcium content of common Bolivian food, and implications for mineral bioavailability. J. Food Compos. Anal. 2015, 39, 111–119. [Google Scholar] [CrossRef]
- Bothwell, T.H.; Finch, C.A. Iron Metabolism; Little Brown: Boston, MA, USA, 1962. [Google Scholar]
- Reddy, M.B.; Hurrell, R.F.; Juillerat, M.A.; Cook, J.D. The influence of different protein sources on phytate inhibition of nonheme-iron absorption in humans. Am. J. Clin. Nutr. 1996, 63, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Skoldborm, H.; Arvidsson, B.; Andersson, M. A new whole body monitoring laboratory. Acta Radiol. 1972, 313, 233–241. [Google Scholar] [CrossRef]
- Fredlund, K.; Isaksson, M.; Rossander-Hulthén, L.; Almgren, A.; Sandberg, A.-S. Absorption of zinc and retention of calcium: Dose-dependent inhibition by phytate. J. Trace Elem. Med. Biol. 2006, 20, 49–57. [Google Scholar] [CrossRef]
- Hahn, P.F.; Bale, W.F.; Lawrence, E.O.; Whipple, G.H. Radioactive Iron and Its Metabolism in Anemia: Its Absorption, Transportation, and Utilization. J. Exp. Med. 1939, 69, 739–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.D.; Layrisse, M.; Martinez-Torres, C.; Walker, R.; Monsen, E.; Finch, C.A. Food iron absorption measured by an extrinsic tag. J. Clin. Investig. 1972, 51, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Björn-Rasmussen, E. Determination of iron absorption from whole diet. A new two-pool model using two radioiron isotopes given as haem and non-haem iron. Scand. J. Haematol. 1972, 9, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L. Bioavailability of dietary iron in man. Annu. Rev. Nutr. 1981, 1, 123–147. [Google Scholar] [CrossRef]
- Eakins, J.D.; Brown, D.A. An improved method for the simultaneous determination of iron-55 and iron-59 in blood by liquid scintillation counting. Int. J. Appl. Radiat. Isot. 1966, 17, 391–397. [Google Scholar] [CrossRef]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, B.; Björn-Rassmussen, E.; Hallberg, L.; Rossander, L. Iron absorption in relation to iron status. Model proposed to express results to food iron absorption measurements. Scand. J. Haematol. 1981, 27, 201–208. [Google Scholar] [CrossRef]
- Brise, H.; Hallberg, L. A method for comparative studies on iron absorption in man using two radioiron isotopes. Acta Med. Scand. 1962, 376, 7–22. [Google Scholar] [CrossRef]
- Björn-Rasmussen, E.; Halberg, L.; Magnusson, B.; Rossander, L.; Svanberg, B.; Arvidsson, B. Measurement of iron absorption from compositite meals. Am. J. Clin. Nutr. 1976, 29, 772–778. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, M.; Undeland, I. Physicochemical and gel-forming properties of protein isolated from salmon, cod and herring by-products using the pH-shift method. LWT 2019, 101, 678–684. [Google Scholar] [CrossRef]
- Fredriksson, M.; Carlsson, N.-G.; Almgren, A.; Sandberg, A.-S. Simultaneous and sensitive analysis of Cu, Ni, Zn, Co, Mn, and Fe in food and biological samples by ion chromatography. J. Agric. Food Chem. 2002, 50, 59–65. [Google Scholar] [CrossRef]
- Ryter, S.W.; Tyrrell, R.M. An HPLC method to detect heme oxygenase activity. Curr. Protoc. Toxicol. 2001, 5, 9–6. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Carlsson, N.-G.; Bergman, E.-L.; Skoglund, E.; Hasselblad, K.; Sandberg, A.-S. Rapid analyzes of inositol phosphates. J. Agric. Food Chem. 2001, 49, 1695–1701. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lee, C.M.; Trevino, B.; Chaiyawat, M. A simple and rapid solvent extraction method for determining total lipids in fish tissue. J. AOAC Int. 1996, 79, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Ax, E.; Lemming, E.W.; Becker, W.; Andersson, A.; Lindroos, A.K.; Cederholm, T.; Sjögren, P.; Fung, T.T. Dietary patterns in Swedish adults; Results from a national dietary survey. Br. J. Nutr. 2016, 115, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Nordic Nutrition Recommendations. Integratin nutrition and physical activity (NNR 2012). In Nordic Nutrition Recommendations Report No: 5; Denmark Nordic Council of Ministers: Copenhagen, Denmark, 2014; pp. 543–571. [Google Scholar]
- Monsen, E.R.; Hallberg, L.; Layrisse, M.; Hegsted, D.M.; Cook, J.; Mertz, W.; Finch, C.A. Estimation of available dietary iron. Am. J. Clin. Nutr. 1978, 31, 134–141. [Google Scholar] [CrossRef]
- Agency, T.S.F. The Swedish Food Agency Food Database, Version 2022-05-24. Available online: https://www7.slv.se/SokNaringsinnehall/ (accessed on 10 June 2022).
- Pretorius, B.; Schönfeldt, H.C.; Hall, N. Total and haem iron content lean meat cuts and the contribution to the diet. Food Chem. 2016, 193, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Hallberg, L.; Hulthén, L. Prediction of dietary iron absorption: An algorithm for calculating absorption and bioavailability of dietary iron. Am. J. Clin. Nutr. 2000, 71, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, K.; Breda, J.; Berdzuli, N.; Rippin, H.; Farrand, C.; Halloran, A. The shift to plant-based diets: Are we missing the point? Glob. Food Secur. 2021, 29, 100530. [Google Scholar] [CrossRef]
- Martínez-Torres, C.; Layrisse, M. Effect of Amino Acids on Iron Absorption from a Staple Vegetable Food. Blood 1970, 35, 669–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Torres, C.; Layrisse, M. Iron absorption from veal muscle. Am. J. Clin. Nutr. 1971, 24, 531–540. [Google Scholar] [CrossRef]
- Layrisse, M.; Martínez-Torres, C.; Roche, M. Effect of Interaction of Various Foods on Iron Absorption. Am. J. Clin. Nutr. 1968, 21, 1175–1183. [Google Scholar] [CrossRef]
- Björn-Rasmussen, E.; Hallberg, L. Effect of Animal Proteins on the Absorption of Food Iron in Man. Ann. Nutr. Metab. 1979, 23, 192–202. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef] [Green Version]
- Petry, N.; Egli, I.; Gahutu, J.B.; Tugirimana, P.L.; Boy, E.; Hurrell, R. Phytic Acid Concentration Influences Iron Bioavailability from Biofortified Beans in Rwandese Women with Low Iron Status. J. Nutr. 2014, 144, 1681–1687. [Google Scholar] [CrossRef] [Green Version]
- Brune, M.; Rossander-Hultén, L.; Hallberg, L.; Gleerup, A.; Sandberg, A.S. Iron absorption from bread in humans: Inhibiting effects of cereal fiber, phytate and inositol phosphates with different numbers of phosphate groups. J. Nutr. 1992, 122, 442–449. [Google Scholar] [CrossRef]
- Davidsson, L.; Dimitriou, T.; Walczyk, T.; Hurrell, R.F. Iron absorption from experimental infant formulas based on pea (Pisum sativum)-protein isolate: The effect of phytic acid and ascorbic acid. Br. J. Nutr. 2001, 85, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Davey, G.K.; Spencer, E.A.; Appleby, P.N.; Allen, N.E.; Knox, K.H.; Key, T.J. EPIC–Oxford: Lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–268. [Google Scholar] [CrossRef]
- Cade, J.E.; Burley, V.J.; Greenwood, D.C. The UK Women’s Cohort Study: Comparison of vegetarians, fish-eaters and meat-eaters. Public Health Nutr. 2004, 7, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; de Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Shridhar, K.; Dhillon, P.K.; Bowen, L.; Kinra, S.; Bharathi, A.V.; Prabhakaran, D.; Reddy, K.S.; Ebrahim, S. Nutritional profile of Indian vegetarian diets—The Indian Migration Study (IMS). Nutr. J. 2014, 13, 55. [Google Scholar] [CrossRef]
- Sherf-Dagan, S.; Hod, K.; Buch, A.; Mardy-Tilbor, L.; Regev, Z.; Ben-Porat, T.; Sakran, N.; Goitein, D.; Raziel, A. Health and Nutritional Status of Vegetarian Candidates for Bariatric Surgery and Practical Recommendations. Obes. Surg. 2018, 28, 152–160. [Google Scholar] [CrossRef]
- Cepeda-Lopez, A.C.; Melse-Boonstra, A.; Zimmermann, M.B.; Herter-Aeberli, I. In overweight and obese women, dietary iron absorption is reduced and the enhancement of iron absorption by ascorbic acid is one-half that in normal-weight women. Am. J. Clin. Nutr. 2015, 102, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B.; Zeder, C.; Muthayya, S.; Winichagoon, P.; Chaouki, N.; Aeberli, I.; Hurrell, R.F. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int. J. Obes. 2008, 32, 1098–1104. [Google Scholar] [CrossRef] [Green Version]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.-A.; Cook, J.D. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am. J. Clin. Nutr. 2003, 77, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Donangelo, C.M.; Woodhouse, L.R.; King, S.M.; Toffolo, G.; Shames, D.M.; Viteri, F.E.; Cheng, Z.; Welch, R.M.; King, J.C. Iron and Zinc Absorption from Two Bean (Phaseolus vulgaris L.) Genotypes in Young Women. J. Agric. Food Chem. 2003, 51, 5137–5143. [Google Scholar] [CrossRef]
- Etcheverry, P.; Hawthorne, K.M.; Liang, L.K.; Abrams, S.A.; Griffin, I.J. Effect of beef and soy proteins on the absorption of non-heme iron and inorganic zinc in children. J. Am. Coll. Nutr. 2006, 25, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tetens, I.; Bendtsen, K.M.; Henriksen, M.; Ersbøll, A.K.; Milman, N. The impact of a meat- versus a vegetable-based diet on iron status in women of childbearing age with small iron stores. Eur. J. Nutr. 2007, 46, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Collings, R.; Harvey, L.J.; Hooper, L.; Hurst, R.; Brown, T.J.; Ansett, J.; King, M.; Fairweather-Tait, S.J. The absorption of iron from whole diets: A systematic review. Am. J. Clin. Nutr. 2013, 98, 65–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).