Efficacy and Safety of Oral Green Tea Preparations in Skin Ailments: A Systematic Review of Clinical Studies
Abstract
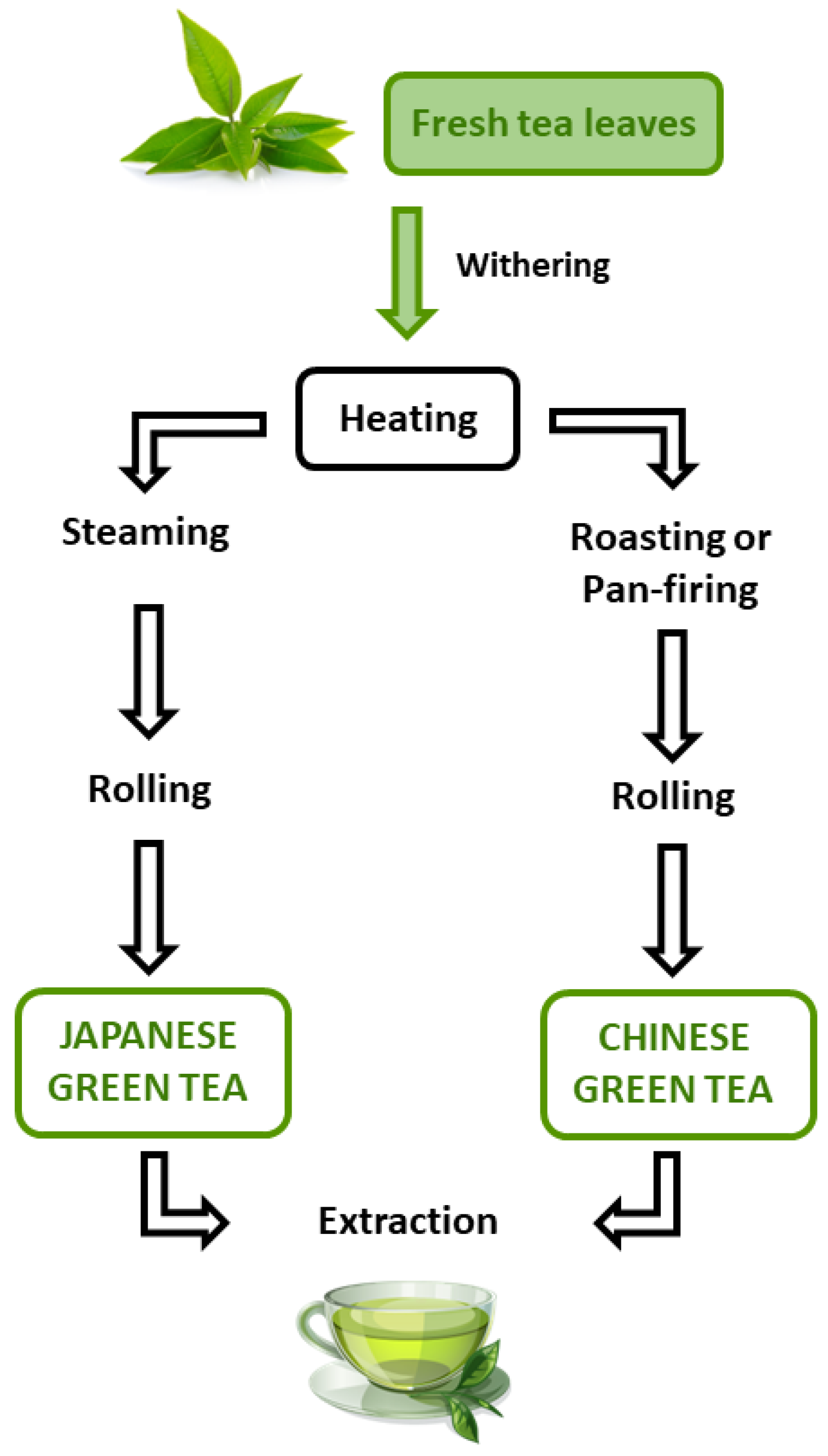
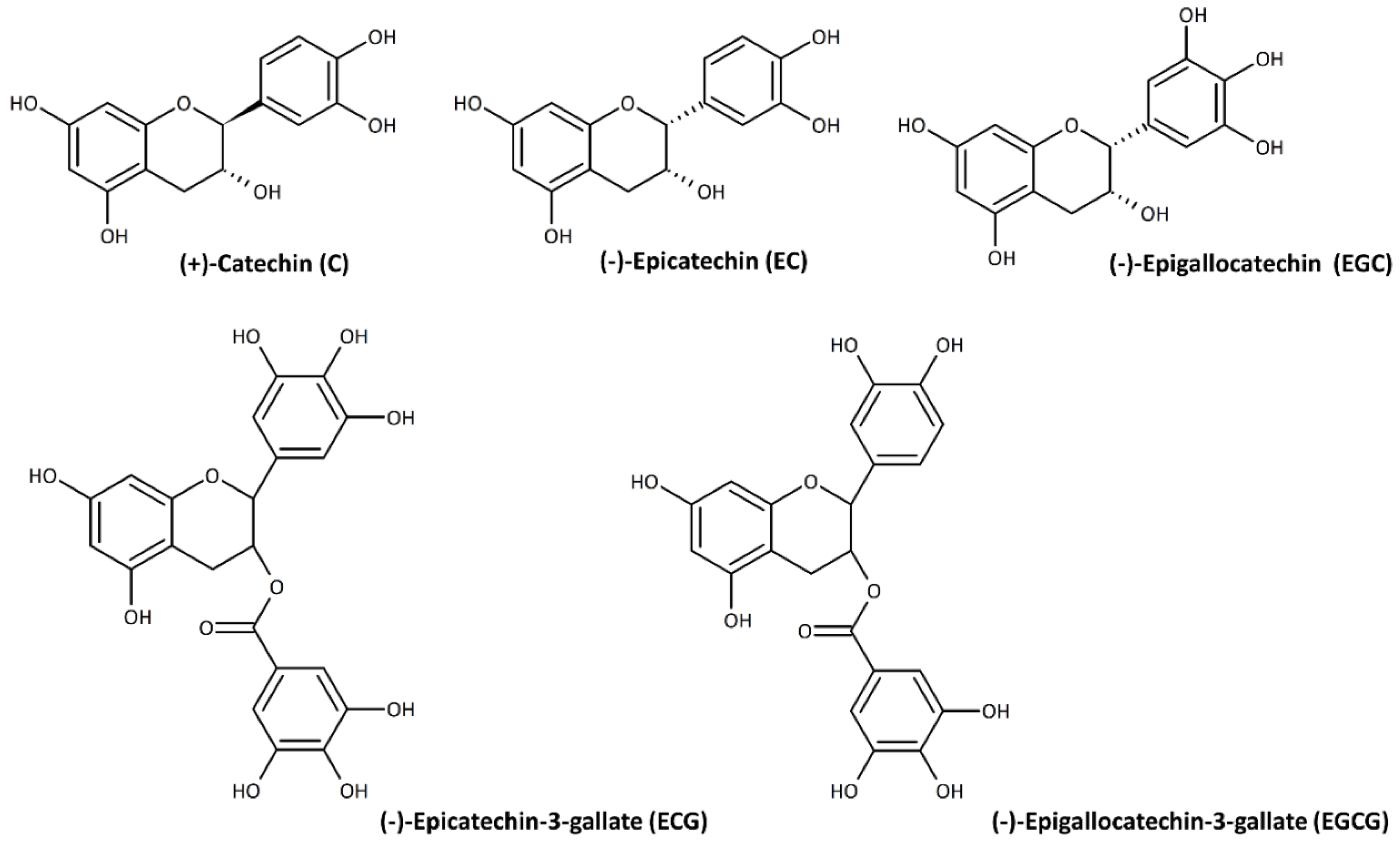
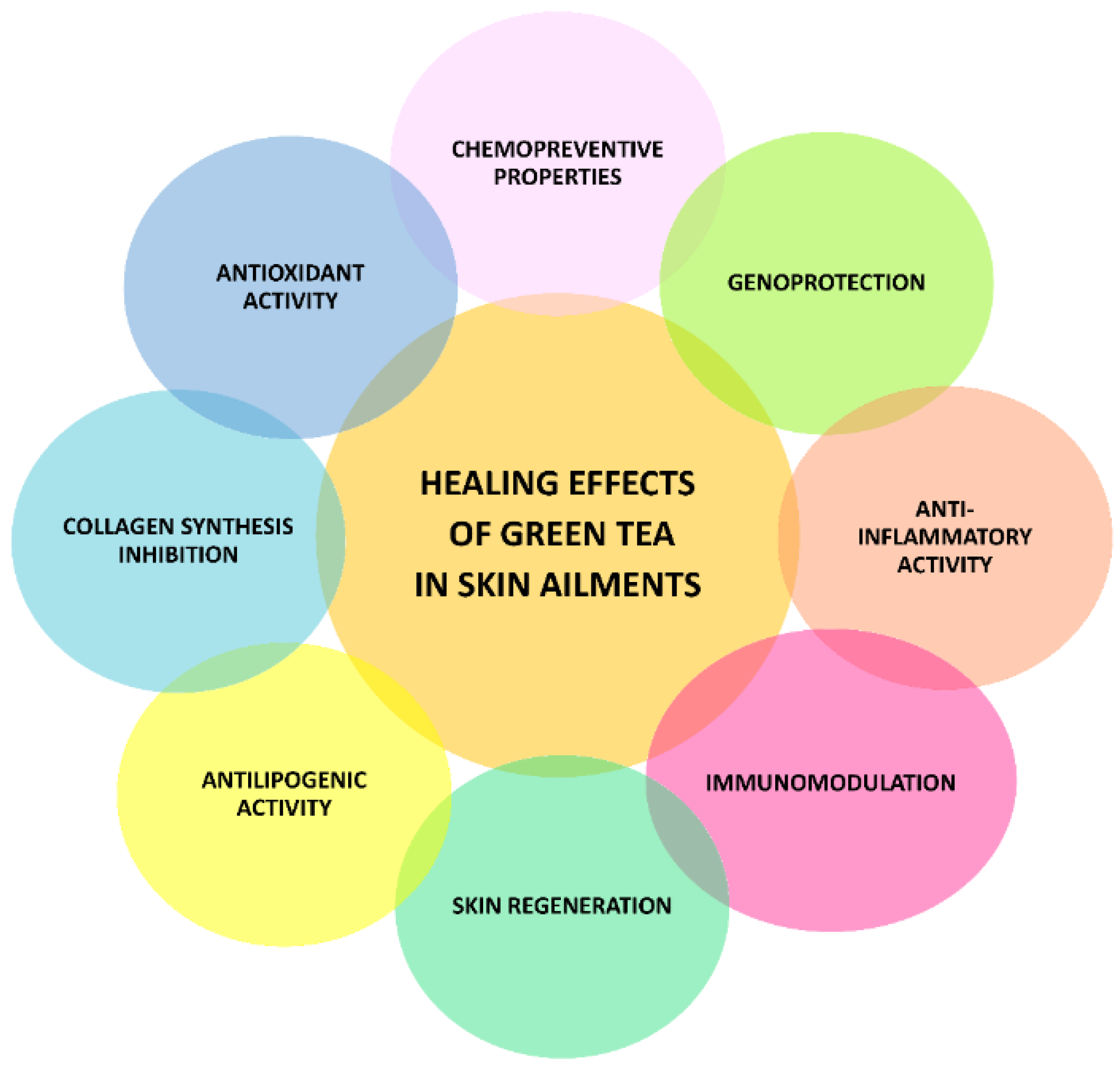
1. Introduction
2. Methodology
3. Results
3.1. Photoaging
3.2. UV-Induced Erythema
3.3. Acne Treatment
3.4. Genodermatosis
3.5. Skin Integrity and Antioxidant Defenses
3.6. Safety Evaluation
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, M.F.; Jiang, C.L.; Kong, Y.S.; Luo, J.L.; Yin, P.; Guo, G.Y. Recent advances in analytical methods for determination of polyphenols in tea: A comprehensive review. Foods 2022, 11, 1425. [Google Scholar] [CrossRef] [PubMed]
- Oyetakin White, P.; Tribout, H.; Baron, E. Protective mechanisms of green tea polyphenols in skin. Oxid. Med. Cell Longev. 2012, 2012, 560682. [Google Scholar]
- Wong, M.; Sirisena, S.; Ng, K. Phytochemical profile of differently processed tea: A review. J. Food Sci. 2022, 87, 1925–1942. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [PubMed]
- Senanayake, S.N. Green tea extract: Chemistry, antioxidant properties and food applications: A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Cao, S.Y.; Zhao, C.N.; Gan, R.Y.; Xu, X.Y.; Wei, X.L.; Corke, H.; Atanasov, A.G.; Li, H.B. Effects and mechanisms of tea and its bioactive compounds for the prevention and treatment of cardiovascular diseases: An updated review. Antioxidants 2019, 8, 166. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Li, F.; Qasim, S.; Li, D.; Dou, Q.P. Updated review on green tea polyphenol epigallocatechin-3-gallate as a cancer epigenetic regulator. Semin. Cancer Biol. 2022, 83, 335–352. [Google Scholar] [CrossRef]
- Martinez Pomier, K.; Ahmed, R.; Melacini, G. Catechins as tools to understand the molecular basis of neurodegeneration. Molecules 2020, 25, 3571. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, T.; Ho, C.T. Redox and other biological activities of tea catechins that may affect health: Mechanisms and unresolved issues. J. Agric. Food Chem. 2022, 70, 7887–7889. [Google Scholar] [CrossRef] [PubMed]
- Zink, A.; Traidl-Hoffmann, C. Green tea in dermatology-myths and facts. J. Dtsch. Dermatol. 2015, 13, 768–775. [Google Scholar] [CrossRef]
- Frasheri, L.; Schielein, M.C.; Tizek, L.; Mikschl, P.; Biedermann, T.; Zink, A. Great green tea ingredient? A narrative literature review on epigallocatechin gallate and its biophysical properties for topical use in dermatology. Phytother. Res. 2020, 34, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Saric, S.; Notay, M.; Sivamani, R.K. Green tea and other tea polyphenols: Effects on sebum production and acne vulgaris. Antioxidants 2016, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial effects of epigallocatechin-3-O-gallate, chlorogenic acid, resveratrol, and curcumin on neurodegenerative diseases. Molecules 2021, 26, 415. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Shida, Y.; Hakariya, T.; Sakai, H. Anti-cancer effects of green tea polyphenols against prostate cancer. Molecules 2019, 24, 193. [Google Scholar] [CrossRef]
- Li, M.J.; Yin, Y.C.; Wang, J.; Jiang, Y.F. Green tea compounds in breast cancer prevention and treatment. World J. Clin. Oncol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Roh, E.; Kim, J.E.; Kwon, J.Y.; Park, J.S.; Bode, A.M.; Dong, Z.; Lee, K.W. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit. Rev. Food Sci. Nutr. 2017, 57, 1631–1637. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Conney, A.H.; Wang, Z.Y.; Huang, M.T.; Ho, C.T.; Yang, C.S. Inhibitory effect of green tea on tumorigenesis by chemicals and ultraviolet light. Prev. Med. 1992, 21, 361–369. [Google Scholar] [CrossRef]
- Xu, F.W.; Lv, Y.L.; Zhong, Y.F.; Xue, Y.N.; Wang, Y.; Zhang, L.Y.; Hu, X.; Tan, W.Q. Beneficial effects of green tea EGCG on skin wound healing: A comprehensive review. Molecules 2021, 26, 6123. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P Acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Feily, A.; Saki, J.; Maraghi, S.; Moosavi, Z.; Khademvatan, S.; Siahpoosh, A. In vitro activity of green tea extract against Leishmania major promastigotes. Int. J. Clin. Pharmacol. Ther. 2012, 50, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Di Sotto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Oketch-Rabah, H.A.; Roe, A.L.; Rider, C.V.; Bonkovsky, H.L.; Giancaspro, G.I.; Navarro, V.; Paine, M.F.; Betz, J.M.; Marles, R.J.; Casper, S.; et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol. Rep. 2020, 7, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Welch, V.; Petticrew, M.; Petkovic, J.; Moher, D.; Waters, E.; White, H.; Tugwell, P. Extending the PRISMA statement to equity- 664 focused systematic reviews (PRISMA-E 2012): Explanation and elaboration. Int. J. Equity Health 2015, 14, 92. [Google Scholar] [CrossRef]
- Chiu, A.E.; Chan, J.L.; Kern, D.G.; Kohler, S.; Rehmus, W.E.; Kimball, A.B. Double-blinded, placebo-controlled trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol. Surg. 2005, 31, 855–860. [Google Scholar] [CrossRef]
- Janjua, R.; Munoz, C.; Gorell, E.; Rehmus, W.; Egbert, B.; Kern, D.; Chang, A.L.S. A two-year, double-blind, randomized placebo-controlled trial of oral green tea polyphenols on the long-term clinical and histologic appearance of photoaging skin. Dermatol. Surg. 2009, 35, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.; Aladren, S.; Delgado, J.; Garre, A.; Trullas, C.; Gilaberte, Y. Prospective evaluation of the efficacy of a food supplement in increasing photoprotection and improving selective markers related to skin photo-ageing. Dermatol. Ther. 2020, 10, 163–178. [Google Scholar] [CrossRef]
- Heinrich, U.; Moore, C.E.; De Spirt, S.; Tronnier, H.; Stahl, W. Green tea polyphenols provide photoprotection, increase microcirculation, and modulate skin properties of women. J. Nutr. 2011, 141, 1202–1208. [Google Scholar] [CrossRef]
- Rhodes, L.E.; Darby, G.; Massey, K.A.; Clarke, K.A.; Dew, T.P.; Farrar, M.D.; Bennet, S.; Watson, R.E.B.; Williamson, G.; Nicolaou, A. Oral green tea catechin metabolites are incorporated into human skin and protect against UV radiation-induced cutaneous inflammation in association with reduced production of pro-inflammatory eicosanoid 12-hydroxyeicosatetraenoic acid. Br. J. Nutr. 2013, 110, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.D.; Nicolaou, A.; Clarke, K.A.; Mason, S.; Massey, K.A.; Dew, T.P.; Watson, R.E.B.; Williamson, G.; Rhodes, L.E. A randomized controlled trial of green tea catechins in protection against ultraviolet radiation–induced cutaneous inflammation, 2. Am. J. Clin. Nutr. 2015, 102, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.D.; Huq, R.; Mason, S.; Nicolaou, A.; Clarke, K.A.; Dew, T.P.; Williamson, G.; Watson, R.E.B.; Rhodes, L.E. Oral green tea catechins do not provide photoprotection from direct DNA damage induced by higher dose solar simulated radiation: A randomized controlled trial. J. Am. Acad. Dermatol. 2018, 78, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Charoenchon, N.; Rhodes, L.E.; Nicolaou, A.; Williamson, G.; Watson, R.E.B.; Farrar, M.D. Ultraviolet radiation-induced degradation of dermal extracellular matrix and protection by green tea catechins: A randomized controlled trial. Clin. Exp. Dermatol. 2022, 47, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.H.; Hsu, C.H. Does supplementation with green tea extract improve acne in post-adolescent women? A randomized, double-blind, and placebo-controlled clinical trial. Complement Ther. Med. 2016, 25, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Chiaverini, C.; Roger, C.; Fontas, E.; Bourrat, E.; Bourdon-Lanoy, E.; Labrèze, C.; Mazereeuw, J.; Vabres, P.; Bodemer, C.; Lacour, J.P. Oral epigallocatechin-3-gallate for treatment of dystrophic epidermolysis bullosa: A multicentre, randomized, crossover, double-blind, placebo-controlled clinical trial. Orphanet. J. Rare Dis. 2016, 11, 31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiu, H.F.; Lin, T.Y.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Improvement of green tea polyphenol with milk on skin with respect to antioxidation in healthy adults: A double-blind placebo-controlled randomized crossover clinical trial. Food Funct. 2016, 7, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Megow, I.; Darvin, M.E.; Meinke, M.C.; Lademann, J. A randomized controlled trial of green tea beverages on the in vivo radical scavenging activity in human skin. Skin Pharmacol. Physiol. 2017, 30, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Chien, A.L. Photoaging: A review of current literature. Curr. Derm. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and photoaging: A review of current literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the contribution of oxidative stress in skin aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and skin aging-from the perspective of food nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Jiang, S.J.; Chu, A.W.; Lu, Z.F.; Pan, M.H.; Che, D.F.; Zhou, X.J. Ultraviolet B-induced alterations of the skin barrier and epidermal calcium gradient. Exp. Dermatol. 2007, 16, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Emri, G.; Paragh, G.; Tósaki, Á.; Janka, E.; Kollár, S.; Hegedűs, C.; Gellén, E.; Horkay, I.; Koncz, G.; Remenyik, É. Ultraviolet radiation-mediated development of cutaneous melanoma: An update. J. Photochem. Photobiol. B 2018, 185, 169–175. [Google Scholar] [CrossRef]
- Mullenders, L.H. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Brem, R.; Guven, M.; Karran, P. Oxidatively-generated damage to DNA and proteins mediated by photosensitized UVA. Free Radicals Biol. Med. 2017, 107, 101–109. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Panich, U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front. Pharmacol. 2022, 13, 823881. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Heinrich, U.; Neukam, K.; Tronnier, H.; Sies, H.; Stahl, W. Long-term ingestion of high flavanol cocoa provides photoprotection against UV induced erythema and improves skin condition in women. J. Nutr. 2006, 136, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, Q.Y.; Wong, Y.F.; Zhang, Z.; Chung, H.Y. Stabilizing effect of ascorbic acid on green tea catechins. J. Agric. Food Chem. 1998, 46, 2512–2516. [Google Scholar] [CrossRef]
- McArdle, F.; Rhodes, L.E.; Parslew, R.; Jack, C.I.; Friedmann, P.S.; Jackson, M.J. UVR-induced oxidative stress in human skin in vivo: Effects of oral vitamin C supplementation. Free Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [CrossRef]
- Türkoğlu, M.; Uğurlu, T.; Gedik, G.; Yılmaz, A.M.; Yalçin, A.S. In vivo evaluation of black and green tea dermal products against UV radiation. Drug Discov. Ther. 2010, 4, 362–367. [Google Scholar]
- Kapoor, M.P.; Sugita, M.; Fukuzawa, Y.; Timm, D.; Ozeki, M.; Okubo, T. Green tea catechin association with ultraviolet radiation-induced erythema: A systematic review and meta-analysis. Molecules 2021, 26, 3702. [Google Scholar] [CrossRef]
- Huang, A.; Honda, Y.; Li, P.; Tanaka, T.; Baba, S. Integration of epigallocatechin gallate in gelatin sponges attenuates matrix metalloproteinase-dependent degradation and increases bone formation. Int. J. Mol. Sci. 2019, 20, 6042. [Google Scholar] [CrossRef]
- DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.P.; Moatshe, G.; LaPrade, R.F. Efficacy of vitamin C supplementation on collagen synthesis and oxidative stress after musculoskeletal injuries: A systematic review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef]
- Melnik, B.C.; John, S.M.; Schmitz, G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: Lessons learnt from laron syndrome. Nutr. Metab. 2011, 8, 41. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Eady, A.; Philpott, M.; Goldsmith, L.A.; Orfanos, C.; Cunliffe, W.C.; Rosenfield, R. What is the pathogenesis of acne? Exp. Dermatol. 2005, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973. [Google Scholar] [CrossRef] [PubMed]
- Elsaie, M.L.; Abdelhamid, M.F.; Elsaaiee, L.T.; Emam, H.M. The efficacy of topical 2% green tea lotion in mild-to-moderate acne vulgaris. J. Drugs Dermatol. 2009, 8, 358–364. [Google Scholar]
- Sharquie, K.E.; Al-Turfi, I.A.; Al-Shimary, W.M. Treatment of acne vulgaris with 2% topical tea lotion. Saudi Med. J. 2006, 27, 83–85. [Google Scholar] [PubMed]
- Sharquie, K.E.; Noaimi, A.A.; Al-Salih, M.M. Topical therapy of acne vulgaris using 2% tea lotion in comparison with 5% zinc sulphate solution. Saudi Med. J. 2008, 29, 1757–1761. [Google Scholar]
- Mahmood, T.; Akhtar, N.; Khan, B.A. Outcomes of 3% green tea emulsion on skinsebum production in male volunteers. Bosn. J. Basic Med. Sci. 2010, 10, 260–264. [Google Scholar] [CrossRef]
- Jung, M.K.; Ha, S.; Son, J.A.; Song, J.H.; Houh, Y.; Cho, E.; Chun, J.H.; Yoon, S.R.; Yang, Y.; Bang, S.I.; et al. Polyphenon-60 displays a therapeutic effect on acne by suppression of TLR2 and IL-8 expression via down-regulating the ERK1/2 pathway. Arch. Dermatol. Res. 2012, 304, 655–663. [Google Scholar] [CrossRef]
- Waranuch, N.; Phimnuan, P.; Yakaew, S.; Nakyai, W.; Grandmottet, F.; Onlom, C.; Srivilai, J.; Viyoch, J. Antiacne and antiblotch activities of a formulated combination of Aloe barbadensis leaf powder, Garcinia mangostana peel extract, and Camellia sinensis leaf extract. Clin. Cosmet. Investig. Dermatol. 2019, 12, 383–391. [Google Scholar] [CrossRef]
- Bruckner-Tuderman, L. Dystrophic epidermolysis bullosa: Pathogenesis and clinical features. Dermatol. Clin. 2010, 28, 107–114. [Google Scholar] [CrossRef]
- Chow, H.H.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001, 10, 53–58. [Google Scholar]
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Consumption of black tea elicits an increase in plasma antioxidant potential in humans. Int. J. Food Sci. Nutr. 2000, 5, 309–315. [Google Scholar]
- Dubeau, S.; Samson, G.; Tajmir-Riahi, H. Dual effect of milk on the antioxidant capacity of green, Darjeeling, and English breakfast teas. Food Chem. 2010, 122, 539–545. [Google Scholar] [CrossRef]
- Egert, S.; Tereszczuk, J.; Wein, S.; Müller, M.J.; Frank, J.; Rimbach, G.; Wolffram, S. Simultaneous ingestion of dietary proteins reduces the bioavailability of galloylated catechins from green tea in humans. Eur. J. Nutr. 2013, 52, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Jochmann, N.; von Krosigk, A.; Martus, P.; Baumann, G.; Stangl, K.; Stangl, V. Addition of milk prevents vascular protective effects of tea. Eur. Heart J. 2007, 28, 219–223. [Google Scholar] [CrossRef]
- Zhang, B.; Rusciano, D.; Osborne, N.N. Orally administered epigallocatechin gallate attenuates retinal neuronal death in vivo and light-induced apoptosis in vitro. Brain Res. 2008, 1198, 141–152. [Google Scholar] [CrossRef]
- Percevault, S.; Charpiat, B.; Lebossé, F.; Mabrut, J.Y.; Vial, T.; Colom, M. Green tea and hepatoxicity: Two case reports. Therapie 2021, S0040-5957(21)00209-2. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic effects of EGCG: A patent review. Expert Opin. Ther. Pat. 2016, 26, 907–916. [Google Scholar]
- Tzellos, T.; Sardeli, C.; Lallas, A.; Papazisis, G.; Chourdakis, M.; Kouvelas, D. Efficacy, safety and tolerability of green tea catechins in the treatment of external anogenital warts: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Chang, H.K.; Baek, S.Y.; Chung, J.O.; Rha, C.S.; Kim, S.Y.; Kim, B.J.; Kim, M.N. Treatment of Atopic Dermatitis Associated with Malassezia sympodialis by Green Tea Extracts Bath Therapy: A Pilot Study. Mycobiology 2012, 40, 124–128. [Google Scholar] [CrossRef]
- Clouth, A.; Schöfer, H. Treatment of recalcitrant facial verrucae vulgares with sinecatechins (greentea catechins) ointment. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Ahmad, R.S.; Sultan, M.T.; Qayyum, M.M.; Naz, A. Green Tea and Anticancer Perspectives: Updates from Last Decade. Crit. Rev. Food Sci. Nutr. 2015, 55, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Mohan, R.R.; Agarwal, R.; Mukhtar, H. Protection against induction of mouse skin papillomas with low and high risk of conversion to malignancy by green tea polyphenols. Carcinogenesis 1997, 18, 497–502. [Google Scholar] [CrossRef]
- Camouse, M.M.; Domingo, D.S.; Swain, F.R.; Conrad, E.P.; Matsui, M.S.; Maes, D.; Declercq, L.; Cooper, K.D.; Stevens, S.R.; Baron, E.D. Topical application of green and white tea extracts provides protection from solar-simulated ultraviolet light in human skin. Exp. Dermatol. 2009, 18, 522–526. [Google Scholar] [CrossRef]
- Mittal, A.; Piyathilake, C.; Hara, Y.; Katiyar, S.K. Exceptionally high protection of photocarcinogenesis by topical application of (–)-epigallocatechin-3-gallate in hydrophilic cream in SKH-1 hairless mouse model: Relationship to inhibition of UVB-induced global DNA hypomethylation. Neoplasia 2003, 5, 555–565. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Abdulla, M.A.; Sanusi, J. The Effect of Camellia sinensis on Wound Healing Potential in an Animal Model. Evid Based Complement Alternat Med. 2013, 2013, 386734. [Google Scholar] [CrossRef] [PubMed]
- Kessels, J.; Voeten, L.; Nelemans, P.; Cleutjens, J.; Hillen, L.M.; Mosterd, K.; Kelleners-Smeets, N.W.J. Topical Sinecatechins, 10%, Ointment for Superficial Basal Cell Carcinoma: A Randomized Clinical Trial. JAMA Dermatol. 2017, 153, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Akhtar, N. Combined topical application of lotus and green tea improves facial skin surface parameters. Rejuvenation Res. 2013, 16, 91–97. [Google Scholar] [CrossRef]
- Dal Belo, S.E.; Gaspar, L.R.; Maia Campos, P.M. Photoprotective effects of topical formulations containing a combination of Ginkgo biloba and green tea extracts. Phytother. Res. 2011, 25, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Morré, D.J.; Geilen, C.C.; Welch, A.M.; Morré, D.M. Response of carcinoma in situ (actinic keratosis) to green tea concentrate plus capsicum. J. Diet. Suppl. 2009, 6, 385–389. [Google Scholar] [CrossRef] [PubMed]

| Authors, Year [Ref.] | Study Design | Participants/N. (Years) | Treatment (Dosage) Duration | Product/Composition | Endpoints | Outcomes | Adverse Events |
|---|---|---|---|---|---|---|---|
| Photoaging | |||||||
| Chiu et al., 2005 [27] | R, DB, PC | Moderate photoaging/ 40 healthy women | Green tea supplement (300 mg) plus 10% green tea cream twice daily 8 weeks | Undefined decaffeinated green tea extract/38% EGCG, 14% ECG, 7% EC, 6% EGC, 4% GCG, 1% C, <0.5% caffeine | Evaluation of clinical and histological parameters of skin photoaging | Improved skin elasticity | Local irritation due to 10% green tea cream |
| Janjua et al., 2009 [28] | R, DB, PC | Moderate to advanced photoaging/56 (25–75) | Green tea supplement (250 mg) daily2 years | Undefined decaffeinated green tea extract/70% catechins (38% EGCG, 14% ECG, 7% EC, 6% EGC, 4% GCG, 1% C) and <0.5% caffeine | Clinical and histological parameters of skin photoaging | Lacking effects | Like placebo nonserious events. 2 cases of nonrelated serious events (i.e., appendicitis and retinal detachment) |
| Granger et al., 2020 [29] | Open prospective and monocentric | Moderate photoaging/30 (40–65) | Multicomponent food supplement/one capsule twice daily 12 weeks | VitAoX ultra® formula/50 mg Camellia sinensis L. Kuntze extract, 480 mg Polypodium leucotomos Poir, 10.1 mg Vitis vinifera L. extract, 40 mg vitamin C, 12 mg vitamin E, 5 µg vitamin D3, 41.5 µg selenium (sodium selenite), 800 µg vitamin A, 8 mg lycopene and 8 mg lutein per capsule | Minimal erythemal dose (MED), antioxidant capacity, skin parameters of aging and treatment tolerability | ↑ MED, improved antioxidant capacity and aging parameters | 2 cases of not clearly related ailments (i.e., slight stomach burns, digestive difficulties) |
| UV-induced erythema | |||||||
| Heinrich et al., 2011 [30] | R, DB, PC | Healthy/ 60 (40–65) | Green tea beverage/1 L daily (1402 mg GTC) 12 weeks | Beverage/1402 mg total catechins (70% EGCG, 17% ECG, 7.1% EC, 3.1% GCG, 1.6% C, 0.6% CG, 0.4% EGC, 0.2% GC) and 119 mg ascorbic acid per L | Photoprotection (reddening), skin function and structure, skin blood flow and catechin serum levels | ↓UV-induced erythema Improved skin texture and structure ↑ Microcirculation and catechin serum levels | Lacking data |
| R, DB, PC | Healthy/ 15 (40–65; 5 people per dose-treatment) | Decaffeinatedgreen tea extract (500 mg capsule)/0.5, 1.0, or 2.0 g daily 12 weeks | Sunphenol 90 decaffeinated (SP 90 DCF-T)/66.5% total catechins (51% EGCG, 8% EC, 4% EGC, 2.3% GC, 1.2% C) | Skin blood flow and catechin serum levels | Transient but not dose-dependent alteration of blood flow and ↑ epicatechin serum levels | Lacking data | |
| Rhodes et al., 2013 [31] | Open intervention study | Healthy/ 16 | Oral green tea supplement plus vitamin C/1350 mg green tea extract (corresponding to 540 mg catechins) plus 50 mg vitamin C daily 12 weeks | Undefined green tea extract (450 mg per capsule)/40% catechins (40% EGCG, 27% EGC, 14% ECG, 6.9% EC, 6.9% GC, 2.5% GCG, 1.2% C, 0.2% GA, 0.2% CG) | UV skin sensitivity | ↓ UV-induced erythema | 4 cases of mild nausea after ingestion |
| Farrar et al., 2015 [32] | R, DB, PC | Healthy male and female/ 50 (18–65) | Oral green tea supplement plus vitamin C/ 2700 mg green tea extract (corre-sponding to 1080 mg catechins) plus 10 mg vita-min C daily 12 weeks | Undefined green tea extract (450 mg per capsule)/40% catechins (16% EGCG, 11% EGC, 14% ECG, 6.9% EC, 6.9% GC, 2.5% GCG, 1.2% C, 0.2% GA, 0.2% CG) | UVR-induced inflammation | Lacking effects | Mild nausea after ingestion |
| Farrar et al., 2018 [33] | R, DB, PC | Healthy male and female/ 50 (18–65) | Oral green tea supplement plus vitamin C/ 2700 mg green tea extract (corre-sponding to 1080 mg catechins) plus 10 mg vita-min C daily 12 weeks | Undefined green tea extract (450 mg per capsule)/as for [32] | UVR-induced inflammation | Lacking effects | Lacking data |
| Charoenchon et al., 2022 [34] | R, DB, PC | Healthy/ 50 (18–65) | Oral green tea supplement plus vitamin C/ 2700 mg green tea extract (corre-sponding to 1080 mg catechins) plus 10 mg vita-min C daily 12 weeks | Undefined green tea extract (450 mg per gelatin capsule)/as for [31] | UVR-induced inflammation | UVR protection to fibulin-5 | Lacking data |
| Acne | |||||||
| Lu et al., 2016 [35] | R, DB, PC | Women carrying moderate to severe acne 80 (25–45) | Decaffeinated green tea extract/1500 mg daily 4 weeks | Undefined green tea extract (500 mg per capsule)/90% catechins (57% EGCG, 16% ECG, 8% EGC, 5% EC, 4% GCG, GC) and <0.07% caffeine | Inflammatory lesion counts | ↓ nose, perioral, and chin lesionsTotal lesion unaffected | One case of mild constipation and two with abdominal discomfort |
| Genodermatosis | |||||||
| Chiaverini et al., 2016 [36] | R, DB, PC, crossover | RDEB patients/ 17 (19.4 ± 16.2 SD) | Green tea extract/400 to 800 mg daily based on body weight 4 months | Polyphenon E® green tea extract (200 mg per capsule)/65% EGCG,9% EC, 6% ECG, 4% EGC, 4% GCG, 0.2% CG, 0.2% GC, 1.1% C, 0.7% caffein | Improvement of RDEB | Lacking effects | Some cases of gastroenteritis, vomiting, odynophagia, esophageal blister and pain, constipation, pruritus, asthenia and bronchitis |
| Skin integrity and antioxidant defenses | |||||||
| Chiu et al., 2016 [37] | R, DB, PC crossover | Healthy subjects/ 44 (25–80) | Green tea supplement plus milk/2 packs daily 6 months treatment + 6 months placebo | GTPM (green tea polyphenol milk)/131.4 ± 9.2 mg TP a and 91.7 b ± 0.5 mg TF per mg dry extract | Skin integrity in relation to oxidative status | ↑ skin integrity and texture ↑ antioxidant index ↓ lipid peroxidation | Lacking data |
| Megow et al., 2017 [38] | R, PC | Healthy male and female/ 37 (20–55) | Freshly prepared green tea beverages/600 mL daily (corresponding to 6 g tea leaves) 2 weeks | Benifuuki and Yabukita teas (lacking chemical characterization) | Skin radical scavenging activity | ↑ Skin radical scavenging activity | Symptoms of illness (usually common cold) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sotto, A.; Gullì, M.; Percaccio, E.; Vitalone, A.; Mazzanti, G.; Di Giacomo, S. Efficacy and Safety of Oral Green Tea Preparations in Skin Ailments: A Systematic Review of Clinical Studies. Nutrients 2022, 14, 3149. https://doi.org/10.3390/nu14153149
Di Sotto A, Gullì M, Percaccio E, Vitalone A, Mazzanti G, Di Giacomo S. Efficacy and Safety of Oral Green Tea Preparations in Skin Ailments: A Systematic Review of Clinical Studies. Nutrients. 2022; 14(15):3149. https://doi.org/10.3390/nu14153149
Chicago/Turabian StyleDi Sotto, Antonella, Marco Gullì, Ester Percaccio, Annabella Vitalone, Gabriela Mazzanti, and Silvia Di Giacomo. 2022. "Efficacy and Safety of Oral Green Tea Preparations in Skin Ailments: A Systematic Review of Clinical Studies" Nutrients 14, no. 15: 3149. https://doi.org/10.3390/nu14153149
APA StyleDi Sotto, A., Gullì, M., Percaccio, E., Vitalone, A., Mazzanti, G., & Di Giacomo, S. (2022). Efficacy and Safety of Oral Green Tea Preparations in Skin Ailments: A Systematic Review of Clinical Studies. Nutrients, 14(15), 3149. https://doi.org/10.3390/nu14153149









