Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review
Abstract
1. Introduction
2. Bioavailability
3. Neuroprotective Potential of Hesperidin and Hesperetin
Importance of Hesperidin and Hesperetin Modes of Action in Neuroprotective Activity
4. Antidiabetic Activity of Hesperidin and Hesperetin
The Importance of Hesperidin and Hesperetin Modes of Action in DM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Cardiovascular effects of hesperidin: A flavanone glycoside. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 989–992. [Google Scholar]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Jokić, S.; Šafranko, S.; Jakovljević, M.; Cikoš, A.-M.; Kajić, N.; Kolarević, F.; Babić, J.; Molnar, M. Sustainable green procedure for extraction of hesperidin from selected croatian mandarin peels. Processes 2019, 7, 469. [Google Scholar] [CrossRef]
- De la Rosa, J.D.P.; Ruiz-Palomino, P.; Arriola-Guevara, E.; García-Fajardo, J.; Sandoval, G.; Guatemala-Morales, G.M. A green process for the extraction and purification of hesperidin from mexican lime peel (Citrus aurantifolia Swingle) that is extendible to the citrus genus. Processes 2018, 6, 266. [Google Scholar] [CrossRef]
- Ruviaro, A.R.; Barbosa, P.D.P.M.; Macedo, G.A. Enzyme-assisted biotransformation increases hesperetin content in citrus juice by-products. Food Res. Int. 2019, 124, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential anti-inflammatory effects of hesperidin from the genus citrus. Curr. Med. Chem. 2018, 25, 4929–4945. [Google Scholar] [CrossRef]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phyther. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef]
- Meyer, O.C. Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology 1994, 45, 579–584. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R. Pharmaceutical technologies for enhancing oral bioavailability of poorly soluble drugs. J. Bioequiv. Availab. 2010, 2, 28–36. [Google Scholar] [CrossRef]
- Kiptoo, P.; Calcagno, A.M.; Siahaan, T.J. Physiological, biochemical, and chemical barriers to oral drug delivery. In Drug Delivery: Principles and Applications; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2016; pp. 19–34. [Google Scholar]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Coltescu, A.-R.; Butnariu, M.; Sarac, I. The importance of solubility for new drug Molecules. Biomed. Pharmacol. J. 2020, 13, 577–583. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Srirangam, R. Solubility, Stability, Physicochemical Characteristics and In Vitro Ocular Tissue Permeability of Hesperidin: A Natural Bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef]
- Lund, M.; Petersen, T.S.; Dalhoff, K.P. Clinical implications of P-glycoprotein modulation in drug–drug interactions. Drugs 2017, 77, 859–883. [Google Scholar] [CrossRef]
- Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D.D. Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: Review of clinical drug–drug interaction studies. Clin. Pharmacokinet. 2020, 59, 699–714. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or evade multidrug resistance P-glycoprotein in cancer treatment: Miniperspective. J. Med. Chem. 2017, 61, 5108–5121. [Google Scholar] [CrossRef]
- Kong, W.; Ling, X.; Chen, Y.; Wu, X.; Zhao, Z.; Wang, W.; Wang, S.; Lai, G.; Yu, Z. Hesperetin reverses P-glycoprotein-mediated cisplatin resistance in DDP-resistant human lung cancer cells via modulation of the nuclear factor-κB signaling pathway. Int. J. Mol. Med. 2020, 45, 1213–1224. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Hamdan, D.; Farrag, N.; El-Shazly, A.; Wink, M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur. J. Pharmacol. 2010, 626, 139–145. [Google Scholar] [CrossRef]
- Susidarti, R.A.; Nugroho, A.E.; Meiyanto, E. Increasing sensitivity of MCF-7/DOX cells towards doxorubicin by hesperetin through suppression of P-glycoprotein expression. Indones. J. Pharm. 2014, 25, 84. [Google Scholar]
- Serra, H.; Mendes, T.; Bronze, M.R.; Simplício, A.L. Prediction of intestinal absorption and metabolism of pharmacologically active flavones and flavanones. Bioorg. Med. Chem. 2008, 16, 4009–4018. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Makarova, N.M. Bioavailability and metabolism of flavonoids. Eksp. Klin. Farmakol. 2011, 74, 33–40. [Google Scholar] [PubMed]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Massot-Cladera, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients 2019, 11, 324. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Lei, C.; Song, W.; Fang, R.; Chen, H.; Li, C.; Li, X.; Liang, X.; Huang, Q.; et al. Novel role of hesperidin improve obesity in HFD mice by modulating the composition of the gut microbiota. Res. Sq. 2020. Preprint. [Google Scholar] [CrossRef]
- Stevens, Y.; Van Rymenant, E.; Grootaert, C.; Van Camp, J.; Possemiers, S.; Masclee, A.; Jonkers, D. The intestinal fate of citrus flavanones and their effects on gastrointestinal health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef]
- Van Rymenant, E.; Salden, B.; Voorspoels, S.; Jacobs, G.; Noten, B.; Pitart, J.; Possemiers, S.; Smagghe, G.; Grootaert, C.; Van Camp, J. A critical evaluation of in vitro hesperidin 2S bioavailability in a model combining luminal (microbial) digestion and Caco-2 cell absorption in comparison to a randomized controlled human trial. Mol. Nutr. Food Res. 2018, 62, 1700881. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Trombetta, D. Antioxidant and anti-inflammatory activity of citrus flavanones mix and its stability after in vitro simulated digestion. Antioxidants 2021, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.; Ahmad, I.; Kawish, S.M.; Khan, U.A.; Ahmad, F.J.; Ali, A.; Jain, G.K. Improved cardioprotective effects of hesperidin solid lipid nanoparticles prepared by supercritical antisolvent technology. Colloids Surf. B Biointerfaces 2020, 187, 110628. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Keck, C.M.; Müller, R.H. Oral hesperidin—Amorphization and improved dissolution properties by controlled loading onto porous silica. Int. J. Pharm. 2017, 518, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; Sulaiman, G.M.; Al-Halbosiy, M.M.F.; Jabir, M.S.; Hameed, A.H. Fabrication of hesperidin nanoparticles loaded by poly lactic co-Glycolic acid for improved therapeutic efficiency and cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Corciova, A.; Ciobanu, C.; Poiata, A.; Mircea, C.; Nicolescu, A.; Drobota, M.; Varganici, C.-D.; Pinteala, T.; Marangoci, N. Antibacterial and antioxidant properties of hesperidin:β-cyclodextrin complexes obtained by different techniques. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 71–84. [Google Scholar] [CrossRef]
- Corciova, A.; Ciobanu, C.; Poiata, A.; Nicolescu, A.; Drobota, M.; Varganici, C.D.; Pinteala, T.; Fifere, A.; Marangoci, N.; Mircea, C. Inclusion complexes of hesperidin with hydroxypropyl-β-cyclodextrin. Physico-chemical characterization and biological assessment. Dig. J. Nanomater. Biostruct. 2014, 9, 1623–1637. [Google Scholar]
- Chalikwar, S.S.; Surana, S.J.; Goyal, S.N.; Chaturvedi, K.K.; Dangre, P.V. Solid self-microemulsifying nutraceutical delivery system for hesperidin using quality by design: Assessment of biopharmaceutical attributes and shelf-life. J. Microencapsul. 2021, 38, 61–79. [Google Scholar] [CrossRef]
- Wei, Q.; Keck, C.M.; Müller, R.H. Solidification of hesperidin nanosuspension by spray drying optimized by design of experiment (DoE). Drug Dev. Ind. Pharm. 2018, 44, 1–12. [Google Scholar] [CrossRef]
- Guo, J.; Lu, S.; Liu, Z.; Tang, W.; Tu, K. Solubilization of hesperidin with octenyl succinic anhydride modified sweet potato starch. Food Chem. 2019, 285, 180–185. [Google Scholar] [CrossRef]
- Varghese, J.J.; Mallya, R. Formulation development and evaluation of antioxidant potential of hesperidin nanocrystals. World J. Pharm. Res. 2015, 4, 1149–1170. [Google Scholar]
- Gao, H.; Chen, Y.; Ma, H.; Zeng, J.; Li, G. Preparation and characterization of hesperidin-PEG 6000 complex. J. Chem. Soc. Pak 2014, 36, 848. [Google Scholar]
- Chadha, K.; Karan, M.; Bhalla, Y.; Chadha, R.; Khullar, S.; Mandal, S.; Vasisht, K. Cocrystals of hesperetin: Structural, pharmacokinetic, and pharmacodynamic evaluation. Cryst. Growth Des. 2017, 17, 2386–2405. [Google Scholar] [CrossRef]
- Chadha, K.; Karan, M.; Chadha, R.; Bhalla, Y.; Vasisht, K. Is failure of cocrystallization actually a failure? Eutectic formation in cocrystal screening of hesperetin. J. Pharm. Sci. 2017, 106, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Abellán, C.; Pérez-Abril, M.; Castillo, J.; Serrano, A.; Mercader, M.T.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Effect of temperature, pH, β- and HP-β-cds on the solubility and stability of flavanones: Naringenin and hesperetin. LWT 2019, 108, 233–239. [Google Scholar] [CrossRef]
- Stahr, P.-L.; Grewal, R.; Eckert, G.P.; Keck, C.M. Investigating hesperetin nanocrystals with tailor-made sizes for the prevention and treatment of Alzheimer’s disease. Drug Deliv. Transl. Res. 2021, 11, 659–674. [Google Scholar] [CrossRef]
- Trendafilova, I.; Mihály, J.; Momekova, D.; Chimshirova, R.; Lazarova, H.; Momekov, G.; Popova, M. Antioxidant activity and modified release profiles of morin and hesperetin flavonoids loaded in Mg-or Ag-modified SBA-16 carriers. Mater. Today Commun. 2020, 24, 101198. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, D.; Tian, Y.; Wang, M.; Liu, R.; Xia, Z.; Huang, Y. Nanoemulsion for Improving the Oral Bioavailability of Hesperetin: Formulation Optimization and Absorption Mechanism. J. Pharm. Sci. 2021, 110, 2555–2561. [Google Scholar] [CrossRef]
- Gurushankar, K.; Gohulkumar, M.; Prasad, N.R.; Krishnakumar, N. Synthesis, characterization and in vitro anti-cancer evaluation of hesperetin-loaded nanoparticles in human oral carcinoma (KB) cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 5, 15006. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Chen, Z.; Qi, X.; Wu, X.; Di, G.; Fan, J.; Guo, C. Improved bioavailability and anticancer efficacy of Hesperetin on breast cancer via a self-assembled rebaudioside A nanomicelles system. Toxicol. Appl. Pharmacol. 2021, 419, 115511. [Google Scholar] [CrossRef]
- Gu, S.-F.; Wang, L.-Y.; Tian, Y.-J.; Zhou, Z.-X.; Tang, J.-B.; Liu, X.-R.; Jiang, H.-P.; Shen, Y.-Q. Enhanced water solubility, antioxidant activity, and oral absorption of hesperetin by D-α-tocopheryl polyethylene glycol 1000 succinate and phosphatidylcholine. J. Zhejiang Univ. B 2019, 20, 273–281. [Google Scholar] [CrossRef]
- Sangpheak, W.; Kicuntod, J.; Schuster, R.; Rungrotmongkol, T.; Wolschann, P.; Kungwan, N.; Viernstein, H.; Mueller, M.; Pongsawasdi, P. Physical properties and biological activities of hesperetin and naringenin in complex with methylated β-cyclodextrin. Beilstein J. Org. Chem. 2015, 11, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Ersoz, M.; Erdemir, A.; Duranoglu, D.; Uzunoglu, D.; Arasoglu, T.; Derman, S.; Mansuroglu, B. Comparative evaluation of hesperetin loaded nanoparticles for anticancer activity against C6 glioma cancer cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Gokuladhas, K.; Jayakumar, S.; Madankumar, A.; Rajan, B.; Elamaran, R.; Pramila, S.; Devaki, T. Synthesis and characterization of biocompatible gold nanoparticles stabilized with hydrophilic polymer coated hesperetin drug for sustained drug delivery to treat hepatocellular carcinoma-derived cancer cells. Int. J. Pharm. Res. 2014, 8, 98–105. [Google Scholar]
- Lazer, L.M.; Sadhasivam, B.; Palaniyandi, K.; Muthuswamy, T.; Ramachandran, I.; Balakrishnan, A.; Pathak, S.; Narayan, S.; Ramalingam, S. Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. Int. J. Biol. Macromol. 2018, 107, 1988–1998. [Google Scholar] [CrossRef]
- Fu, H.; Hardy, J.; Duff, K.E. Selective vulnerability in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1350–1358. [Google Scholar] [CrossRef]
- Tamilselvam, K.; Braidy, N.; Manivasagam, T.; Essa, M.M.; Prasad, N.R.; Karthikeyan, S.; Thenmozhi, A.J.; Selvaraju, S.; Guillemin, G.J. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 102741. [Google Scholar] [CrossRef]
- Huang, S.; Tsai, S.; Lin, J.; Wu, C.; Yen, G. Cytoprotective effects of hesperetin and hesperidin against amyloid β-induced impairment of glucose transport through downregulation of neuronal autophagy. Mol. Nutr. Food Res. 2012, 56, 601–609. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Lim, G.T.; Lee, J.; Jun, M. In silico docking and in vitro approaches towards BACE1 and cholinesterases inhibitory effect of citrus flavanones. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef]
- Antunes, M.S.; Goes, A.T.R.; Boeira, S.P.; Prigol, M.; Jesse, C.R. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition 2014, 30, 1415–1422. [Google Scholar] [CrossRef]
- Thenmozhi, A.J.; Raja, T.R.W.; Janakiraman, U.; Manivasagam, T. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem. Res. 2015, 40, 767–776. [Google Scholar] [CrossRef]
- Hong, Y.; An, Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch. Pharm. Res. 2018, 41, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Bandyopadhyay, J.; Chakraborty, S.; Basu, S. Multi-target screening mines hesperidin as a multi-potent inhibitor: Implication in Alzheimer’s disease therapeutics. Eur. J. Med. Chem. 2016, 121, 810–822. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Zhu, X.; Wu, W.; Wang, Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cell. Mol. Neurobiol. 2014, 34, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvam, K.; Nataraj, J.; Janakiraman, U.; Manivasagam, T.; Essa, M.M. Antioxidant and anti-inflammatory potential of hesperidin against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced experimental Parkinson’s disease in mice. Int. J. Nutr. Pharmacol. Neurol. Dis. 2013, 3, 294. [Google Scholar]
- Justin Thenmozhi, A.; William Raja, T.R.; Manivasagam, T.; Janakiraman, U.; Essa, M.M. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr. Neurosci. 2017, 20, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zug, C.; Qu, H.; Schluesener, H.; Zhang, Z. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav. Brain Res. 2015, 281, 32–42. [Google Scholar] [CrossRef]
- Javed, H.; Vaibhav, K.; Ahmed, M.E.; Khan, A.; Tabassum, R.; Islam, F.; Safhi, M.M.; Islam, F. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J. Neurol. Sci. 2015, 348, 51–59. [Google Scholar] [CrossRef]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin confers neuroprotection by regulating Nrf2/TLR4/NF-κB signaling in an Aβ mouse model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Lin, J.-A.; Shih, P.-H.; Yeh, C.-T.; Yen, G.-C. Pro-cellular survival and neuroprotection of citrus flavonoid: The actions of hesperetin in PC12 cells. Food Funct. 2012, 3, 1082–1090. [Google Scholar] [CrossRef]
- Moghaddam, A.H.; Zare, M. Neuroprotective effect of hesperetin and nano-hesperetin on recognition memory impairment and the elevated oxygen stress in rat model of Alzheimer’s disease. Biomed. Pharmacother. 2018, 97, 1096–1101. [Google Scholar]
- Hwang, S.-L.; Yen, G.-C. Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J. Agric. Food Chem. 2008, 56, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Ahn, W.S. Neuroprotective effects of chronic hesperetin administration in mice. Arch. Pharm. Res. 2008, 31, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Shagirtha, K.; Bashir, N.; MiltonPrabu, S. Neuroprotective efficacy of hesperetin against cadmium induced oxidative stress in the brain of rats. Toxicol. Ind. Health 2017, 33, 454–468. [Google Scholar] [CrossRef]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef]
- Kiasalari, Z.; Khalili, M.; Baluchnejadmojarad, T.; Roghani, M. Protective effect of oral hesperetin against unilateral striatal 6-hydroxydopamine damage in the rat. Neurochem. Res. 2016, 41, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Cho, J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch. Pharm. Res. 2006, 29, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Kim, M.E.; Cho, J.H.; Lee, Y.; Lee, J.; Park, Y.-D.; Lee, J.S. Hesperetin inhibits neuroinflammation on microglia by suppressing inflammatory cytokines and MAPK pathways. Arch. Pharm. Res. 2019, 42, 695–703. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, L.; Gu, Z.; Huan, Z.; Fu, H.; Liu, Q. Hesperetin protects SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity via activation of NRF2/ARE signaling pathways. Trop. J. Pharm. Res. 2020, 19, 1197–1201. [Google Scholar] [CrossRef]
- Ishola, I.O.; Jacinta, A.A.; Adeyemi, O.O. Cortico-hippocampal memory enhancing activity of hesperetin on scopolamine-induced amnesia in mice: Role of antioxidant defense system, cholinergic neurotransmission and expression of BDNF. Metab. Brain Dis. 2019, 34, 979–989. [Google Scholar] [CrossRef]
- Ren, H.; Hao, J.; Liu, T.; Zhang, D.; Lv, H.; Song, E.; Zhu, C. Hesperetin suppresses inflammatory responses in lipopolysaccharide-induced RAW 264.7 cells via the inhibition of NF-κB and activation of Nrf2/HO-1 pathways. Inflammation 2016, 39, 964–973. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Li, F.; Maiese, K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005, 75, 207–246. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Smith, M.A.; Zhu, X.; Nunomura, A.; Castellani, R.J.; Perry, G. Oxidative stress and neurodegeneration. Ann. N. Y. Acad. Sci. 2005, 1043, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, M.; Liu, X.; Su Kang, S.; Duong, D.M.; Seyfried, N.T.; Cao, X.; Cheng, L.; Sun, Y.E.; Ping Yu, S. Delta-secretase cleaves amyloid precursor protein and regulates the pathogenesis in Alzheimer’s disease. Nat. Commun. 2015, 6, 8762. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural antioxidant anthocyanins—A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Onyango, I.G.; Khan, S.M. Oxidative stress, mitochondrial dysfunction, and stress signaling in Alzheimer’s disease. Curr. Alzheimer Res. 2006, 3, 339–349. [Google Scholar] [CrossRef]
- Reynolds, W.F.; Rhees, J.; Maciejewski, D.; Paladino, T.; Sieburg, H.; Maki, R.A.; Masliah, E. Myeloperoxidase polymorphism is associated with gender specific risk for Alzheimer’s disease. Exp. Neurol. 1999, 155, 31–41. [Google Scholar] [CrossRef]
- Derk, J.; MacLean, M.; Juranek, J.; Schmidt, A.M. The receptor for advanced glycation endproducts (RAGE) and mediation of inflammatory neurodegeneration. J. Alzheimer’s Dis. Park. 2018, 8, 421. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin rescue oxidative stress-mediated neuroinflammation/neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef]
- De Felice, F.G.; Velasco, P.T.; Lambert, M.P.; Viola, K.; Fernandez, S.J.; Ferreira, S.T.; Klein, W.L. Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007, 282, 11590–11601. [Google Scholar] [CrossRef]
- Adam-Vizi, V.; Starkov, A.A. Calcium and mitochondrial reactive oxygen species generation: How to read the facts. J. Alzheimer’s Dis. 2010, 20, S413–S426. [Google Scholar] [CrossRef] [PubMed]
- Sakhaei, F.; Keshvari, M.; Asgary, S.; Salehizadeh, L.; Rastqar, A.; Samsam-Shariat, S.Z. Enzymatic antioxidant system and endothelial function in patients with metabolic syndrome. ARYA Atheroscler. 2020, 16, 94. [Google Scholar] [PubMed]
- Zhang, Z.; Qu, J.; Zheng, C.; Zhang, P.; Zhou, W.; Cui, W.; Mo, X.; Li, L.; Xu, L.; Gao, J. Nrf2 antioxidant pathway suppresses Numb-mediated epithelial–mesenchymal transition during pulmonary fibrosis. Cell Death Dis. 2018, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Kavian, N.; Mehlal, S.; Jeljeli, M.; Saidu, N.E.B.; Nicco, C.; Cerles, O.; Chouzenoux, S.; Cauvet, A.; Camus, C.; Ait-Djoudi, M. The Nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Front. Immunol. 2018, 9, 1896. [Google Scholar] [CrossRef]
- Mhillaj, E.; Catino, S.; Miceli, F.M.; Santangelo, R.; Trabace, L.; Cuomo, V.; Mancuso, C. Ferulic acid improves cognitive skills through the activation of the heme oxygenase system in the rat. Mol. Neurobiol. 2018, 55, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Saraswat, G.; Kabir, S.N. α-Dihydroxychalcone-glycoside (α-DHC) isolated from the heartwood of Pterocarpus marsupium inhibits LPS induced MAPK activation and up regulates HO-1 expression in murine RAW 264.7 macrophage. Toxicol. Appl. Pharmacol. 2014, 277, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Shadboorestan, A. Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent. Nutr. Cancer 2016, 68, 29–39. [Google Scholar] [CrossRef]
- Bekdash, R.A. The cholinergic system, the adrenergic system and the neuropathology of alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 1273. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Kamkwalala, A.R.; Newhouse, P.A. Beyond acetylcholinesterase inhibitors: Novel cholinergic treatments for Alzheimer’s disease. Curr. Alzheimer Res. 2017, 14, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Shimouchi, A.; Yokota, H.; Ono, S.; Matsumoto, C.; Tamai, T.; Takumi, H.; Narayanan, S.P.; Kimura, S.; Kobayashi, H.; Caldwell, R.B. Neuroprotective effect of water-dispersible hesperetin in retinal ischemia reperfusion injury. Jpn. J. Ophthalmol. 2016, 60, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Solanki, I.; Parihar, P.; Parihar, M.S. Neurodegenerative diseases: From available treatments to prospective herbal therapy. Neurochem. Int. 2016, 95, 100–108. [Google Scholar] [CrossRef]
- Mairuae, N.; Cheepsunthorn, P. Valproic acid attenuates nitric oxide and interleukin-1β production in lipopolysaccharide-stimulated iron-rich microglia. Biomed. Rep. 2018, 8, 359–364. [Google Scholar] [CrossRef]
- Xu, H.; Qin, W.; Hu, X.; Mu, S.; Zhu, J.; Lu, W.; Luo, Y. Lentivirus-mediated overexpression of OTULIN ameliorates microglia activation and neuroinflammation by depressing the activation of the NF-κB signaling pathway in cerebral ischemia/reperfusion rats. J. Neuroinflamm. 2018, 15, 83. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Cheng, X.; Shen, Y.; Li, R. Targeting TNF: A therapeutic strategy for Alzheimer’s disease. Drug Discov. Today 2014, 19, 1822–1827. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Yakovleva, T.; Bazov, I.; Watanabe, H.; Hauser, K.F.; Bakalkin, G. Transcriptional control of maladaptive and protective responses in alcoholics: A role of the NF-κB system. Brain. Behav. Immun. 2011, 25, S29–S38. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Pepeu, G.; Giovannini, M.G. The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res. 2017, 1670, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, M.T.; Coulson, E.J. Cholinergic basal forebrain lesion decreases neurotrophin signaling without affecting tau hyperphosphorylation in genetically susceptible mice. J. Alzheimer’s Dis. 2017, 55, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Young-Pearse, T.L.; Chen, A.C.; Chang, R.; Marquez, C.; Selkoe, D.J. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev. 2008, 3, 15. [Google Scholar] [CrossRef]
- Van Bulck, M.; Sierra-Magro, A.; Alarcon-Gil, J.; Perez-Castillo, A.; Morales-Garcia, J.A. Novel approaches for the treatment of Alzheimer’s and Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 719. [Google Scholar] [CrossRef]
- Kozlov, S.; Afonin, A.; Evsyukov, I.; Bondarenko, A. Alzheimer’s disease: As it was in the beginning. Rev. Neurosci. 2017, 28, 825–843. [Google Scholar] [CrossRef]
- Cao, J.; Hou, J.; Ping, J.; Cai, D. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 64. [Google Scholar] [CrossRef]
- Varadarajan, S.; Yatin, S.; Aksenova, M.; Butterfield, D.A. Alzheimer’s amyloid β-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000, 130, 184–208. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gannon, M.; Chen, Y.; Yan, S.; Zhang, S.; Feng, W.; Tao, J.; Sha, B.; Liu, Z.; Saito, T. β-amyloid redirects norepinephrine signaling to activate the pathogenic GSK3β/tau cascade. Sci. Transl. Med. 2020, 12, eaay6931. [Google Scholar] [CrossRef] [PubMed]
- Toral-Rios, D.; Pichardo-Rojas, P.S.; Alonso-Vanegas, M.; Campos-Peña, V. GSK3β and tau protein in Alzheimer’s Disease and epilepsy. Front. Cell. Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef]
- Parameshwaran, K.; Dhanasekaran, M.; Suppiramaniam, V. Amyloid beta peptides and glutamatergic synaptic dysregulation. Exp. Neurol. 2008, 210, 7–13. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Meusburger, S.; Hawkins, C.J.; Riglar, D.T.; Lee, E.F.; Fairlie, W.D.; Huang, D.C.S.; Adams, J.M. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc. Natl. Acad. Sci. USA 2008, 105, 18081–18087. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, T.; Czabotar, P.E. BAX, BAK, and BOK: A coming of age for the BCL-2 family effector proteins. Cold Spring Harb. Perspect. Biol. 2020, 12, a036319. [Google Scholar] [CrossRef]
- Li, J.; Xu, B.; Chen, Z.; Zhou, C.; Liao, L.; Qin, Y.; Yang, C.; Zhang, X.; Hu, Z.; Sun, L. PI 3K/AKT/JNK/p38 signalling pathway-mediated neural apoptosis in the prefrontal cortex of mice is involved in the antidepressant-like effect of pioglitazone. Clin. Exp. Pharmacol. Physiol. 2018, 45, 525–535. [Google Scholar] [CrossRef]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.-M. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, H.; Suzuki, K.; Ma, S.; Liu, C. Linking what we eat to our mood: A review of diet, dietary antioxidants, and depression. Antioxidants 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Davinelli, S.; Drago, F.; De Lorenzo, A.; Oriani, G. Antioxidants as antidepressants. CNS Drugs 2012, 26, 477–490. [Google Scholar] [CrossRef]
- Souza, L.C.; de Gomes, M.G.; Goes, A.T.R.; Del Fabbro, L.; Carlos Filho, B.; Boeira, S.P.; Jesse, C.R. Evidence for the involvement of the serotonergic 5-HT1A receptors in the antidepressant-like effect caused by hesperidin in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 103–109. [Google Scholar] [CrossRef]
- Donato, F.; de Gomes, M.G.; Goes, A.T.R.; Borges Filho, C.; Del Fabbro, L.; Antunes, M.S.; Souza, L.C.; Boeira, S.P.; Jesse, C.R. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: Possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res. Bull. 2014, 104, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Belete, T.M. A recent achievement in the discovery and development of novel targets for the treatment of type-2 diabetes mellitus. J. Exp. Pharmacol. 2020, 12, 1–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aynalem, S.B.; Zeleke, A.J. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 years and above in Mizan-Aman town, Southwest Ethiopia, 2016: A cross sectional study. Int. J. Endocrinol. 2018, 2018, 9317987. [Google Scholar] [CrossRef]
- Dhanya, R.; Jayamurthy, P. In vitro evaluation of antidiabetic potential of hesperidin and its aglycone hesperetin under oxidative stress in skeletal muscle cell line. Cell Biochem. Funct. 2020, 38, 419–427. [Google Scholar] [CrossRef]
- Caglayan, C.; Demir, Y.; Kucukler, S.; Taslimi, P.; Kandemir, F.M.; Gulçin, İ. The effects of hesperidin on sodium arsenite-induced different organ toxicity in rats on metabolic enzymes as antidiabetic and anticholinergics potentials: A biochemical approach. J. Food Biochem. 2019, 43, e12720. [Google Scholar] [CrossRef]
- Gupta, A.; Jacobson, G.A.; Burgess, J.R.; Jelinek, H.F.; Nichols, D.S.; Narkowicz, C.K.; Al-Aubaidy, H.A. Citrus bioflavonoids dipeptidyl peptidase-4 inhibition compared with gliptin antidiabetic medications. Biochem. Biophys. Res. Commun. 2018, 503, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Gauer, J.S.; Crabbe, S.; Cheah, J.W.; Lau, J.; Walsh, R.; Cancalon, P.F.; Williamson, G. Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. Br. J. Nutr. 2019, 121, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Jin, J.; Zou, G.; Sui, Y.; Han, Y.; Zhao, D.; Liu, L. Hesperidin prevents hyperglycemia in diabetic rats by activating the insulin receptor pathway. Exp. Ther. Med. 2021, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.O.; Sharma, P.K.; Shrivastava, B.; Ojha, S.; Upadhya, H.M.; Arya, D.S.; Goyal, S.N. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS ONE 2014, 9, e111212. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Ashraf, S.; Khan, M.I.; Hafizur, R.M.; Ul-Haq, Z. Protein kinase A-dependent insulinotropic effect of selected flavonoids. Int. J. Biol. Macromol. 2018, 119, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef]
- Dokumacioglu, E.; Iskender, H.; Sen, T.M.; Ince, I.; Dokumacioglu, A.; Kanbay, Y.; Erbas, E.; Saral, S. The effects of hesperidin and quercetin on serum tumor necrosis factor-alpha and interleukin-6 levels in streptozotocin-induced diabetes model. Pharmacogn. Mag. 2018, 14, 167–173. [Google Scholar]
- Liu, W.Y.; Liou, S.-S.; Hong, T.-Y.; Liu, I.-M. Protective effects of hesperidin (citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients 2017, 9, 1312. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ahmed, O.M.; Ashour, M.B.; Abdel-Moneim, A. In vivo and in vitro antidiabetic effects of citrus flavonoids; a study on the mechanism of action. Int. J. Diabetes Dev. Ctries. 2015, 35, 250–263. [Google Scholar] [CrossRef]
- Jayaraman, R.; Subramani, S.; Abdullah, S.H.S.; Udaiyar, M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 97, 98–106. [Google Scholar] [CrossRef]
- Constantin, R.P.; Constantin, R.P.; Bracht, A.; Yamamoto, N.S.; Ishii-Iwamoto, E.L.; Constantin, J. Molecular mechanisms of citrus flavanones on hepatic gluconeogenesis. Fitoterapia 2014, 92, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Revathy, J.; Sheik Abdullah, S. The role of hesperetin in the management of diabetes mellitus and its complications. J. Cancer Treat. Res. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Nerdy, N.; Meliala, L.; Barus, B.R.; Lestari, P.; Ginting, S.; Ariani, P.; Mierza, V.; Bakri, T.K. Effect of hesperetin treatment on blood glucose level, spermatozoa quality, and spermatozoa quantity in alloxan-induced diabetic mice. J. Kedokt. Hewan March 2021, 15, 1–6. [Google Scholar] [CrossRef]
- Samie, A.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci. 2018, 210, 132–139. [Google Scholar] [CrossRef]
- Gong, Y.; Qin, X.-Y.; Zhai, Y.-Y.; Hao, H.; Lee, J.; Park, Y.-D. Inhibitory effect of hesperetin on α-glucosidase: Molecular dynamics simulation integrating inhibition kinetics. Int. J. Biol. Macromol. 2017, 101, 32–39. [Google Scholar] [CrossRef]
- Shokri Afra, H.; Zangooei, M.; Meshkani, R.; Ghahremani, M.H.; Ilbeigi, D.; Khedri, A.; Shahmohamadnejad, S.; Khaghani, S.; Nourbakhsh, M. Hesperetin is a potent bioactivator that activates SIRT1-AMPK signaling pathway in HepG2 cells. J. Physiol. Biochem. 2019, 75, 125–133. [Google Scholar] [CrossRef]
- Teng, J.; Li, J.; Zhao, Y.; Wang, M. Hesperetin, a dietary flavonoid, inhibits AGEs-induced oxidative stress and inflammation in RAW264. 7 cells. J. Funct. Foods 2021, 81, 104480. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Kong, L.; Tang, Z.-Z.; Zhang, Y.-M.; Liu, Y.; Wang, T.-Y.; Liu, Y.-W. Hesperetin ameliorates diabetic nephropathy in rats by activating Nrf2/ARE/glyoxalase 1 pathway. Biomed. Pharmacother. 2019, 111, 1166–1175. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Mechanisms of hyperglycemic damage in diabetes. In Atlas of Diabetes; Springer: Berlin/Heidelberg, Germany, 2012; pp. 217–231. [Google Scholar]
- Rorsman, P.; Braun, M. Regulation of insulin secretion in human pancreatic islets. Annu. Rev. Physiol. 2013, 75, 155–179. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Martin, D.S.; Xu, R.A.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Styskal, J.; Van Remmen, H.; Richardson, A.; Salmon, A.B. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic. Biol. Med. 2012, 52, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Holley, C.T.; Duffy, C.M.; Butterick, T.A.; Long, E.K.; Lindsey, M.E.; Cabrera, J.A.; Ward, H.B.; McFalls, E.O.; Kelly, R.F. Expression of uncoupling protein-2 remains increased within hibernating myocardium despite successful coronary artery bypass grafting at 4 wk post-revascularization. J. Surg. Res. 2015, 193, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Flagg, T.P.; Enkvetchakul, D.; Koster, J.C.; Nichols, C.G. Muscle KATP channels: Recent insights to energy sensing and myoprotection. Physiol. Rev. 2010, 90, 799–829. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, A.E.; Reyes, S.; Yamada, S.; Hodgson-Zingman, D.M.; Sattiraju, S.; Zhu, Z.; Sierra, A.; Gerbin, M.; Coetzee, W.A.; Goldhamer, D.J. Sarcolemmal ATP-sensitive K+ channels control energy expenditure determining body weight. Cell Metab. 2010, 11, 58–69. [Google Scholar] [CrossRef]
- Wang, Z.; Thurmond, D.C. Mechanisms of biphasic insulin-granule exocytosis–roles of the cytoskeleton, small GTPases and SNARE proteins. J. Cell Sci. 2009, 122, 893–903. [Google Scholar] [CrossRef]
- Kim, J.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Withers, D.J.; Gutierrez, J.S.; Towery, H.; Burks, D.J.; Ren, J.-M.; Previs, S.; Zhang, Y.; Bernal, D.; Pons, S.; Shulman, G.I. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998, 391, 900–904. [Google Scholar] [CrossRef]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- Langlais, P.; Yi, Z.; Finlayson, J.; Luo, M.; Mapes, R.; De Filippis, E.; Meyer, C.; Plummer, E.; Tongchinsub, P.; Mattern, M. Global IRS-1 phosphorylation analysis in insulin resistance. Diabetologia 2011, 54, 2878–2889. [Google Scholar] [CrossRef][Green Version]
- Tirosh, A.; Potashnik, R.; Bashan, N.; Rudich, A. Oxidative stress disrupts insulin-induced cellular redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes: A putative cellular mechanism for impaired protein kinase B activation and GLUT4 translocation. J. Biol. Chem. 1999, 274, 10595–10602. [Google Scholar] [CrossRef] [PubMed]
- Lazo-de-la-Vega-Monroy, M.L.; Fernández-Mejía, C. Oxidative stress in diabetes mellitus and the role of vitamins with antioxidant actions. In Oxidative Stress and Chronic Degenerative Diseases: A Role for Antioxidants; IntechOpen: London, UK, 2013; pp. 209–232. [Google Scholar]
- Kaneto, H.; Matsuoka, T.; Kawashima, S.; Yamamoto, K.; Kato, K.; Miyatsuka, T.; Katakami, N.; Matsuhisa, M. Role of MafA in pancreatic beta-cells. Adv. Drug Deliv. Rev. 2009, 61, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diab. Rep. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, L.-J.; Yu, J.; Wang, H.-J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef]
- Parwani, K.; Mandal, P. Role of advanced glycation end products and insulin resistance in diabetic nephropathy. Arch. Physiol. Biochem. 2020, 1–13. [Google Scholar] [CrossRef]
- Yang, P.; Feng, J.; Peng, Q.; Liu, X.; Fan, Z. Advanced glycation end products: Potential mechanism and therapeutic target in cardiovascular complications under diabetes. Oxid. Med. Cell. Longev. 2019, 2019, 9570616. [Google Scholar] [CrossRef]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011, 286, 1374–1380. [Google Scholar] [CrossRef]
- Unnikrishnan, M.K.; Veerapur, V.; Nayak, Y.; Mudgal, P.P.; Mathew, G. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoids. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 143–161. [Google Scholar]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 241. [Google Scholar]
- Bermudez, V.; Finol, F.; Parra, N.; Parra, M.; Pérez, A.; Penaranda, L.; Vílchez, D.; Rojas, J.; Arráiz, N.; Velasco, M. PPAR-γ agonists and their role in type 2 diabetes mellitus management. Am. J. Ther. 2010, 17, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. New insights for oxidative stress and diabetes mellitus. Oxid. Med. Cell. Longev. 2015, 2015, 875961. [Google Scholar] [CrossRef] [PubMed]
- Kaku, K.; Kadowaki, T.; Terauchi, Y.; Okamoto, T.; Sato, A.; Okuyama, K.; Arjona Ferreira, J.C.; Goldstein, B.J. Sitagliptin improves glycaemic excursion after a meal or after an oral glucose load in J apanese subjects with impaired glucose tolerance. Diabetes Obes. Metab. 2015, 17, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. 2019, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar]
- Maedler, K.; Dharmadhikari, G.; Schumann, D.M.; Størling, J. Interleukin-1 beta targeted therapy for type 2 diabetes. Expert Opin. Biol. Ther. 2009, 9, 1177–1188. [Google Scholar] [CrossRef]
- Senn, J.J.; Klover, P.J.; Nowak, I.A.; Mooney, R.A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002, 51, 3391–3399. [Google Scholar] [CrossRef]
- Kim, H.-E.; Choi, S.-E.; Lee, S.-J.; Lee, J.-H.; Lee, Y.-J.; Kang, S.S.; Chun, J.; Kang, Y. Tumour necrosis factor-a-induced glucose-stimulated insulin secretion inhibition in INS-1 cells is ascribed to a reduction of the glucose-stimulated Ca2+ influx. J. Endocrinol. 2008, 198, 549–560. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Lee, S.C.; Pervaiz, S. Apoptosis in the pathophysiology of diabetes mellitus. Int. J. Biochem. Cell Biol. 2007, 39, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kass, G.E.N.; Szegezdi, E.; Joseph, B. The mitochondrial death pathway: A promising therapeutic target in diseases. J. Cell. Mol. Med. 2009, 13, 1004–1033. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, S.; Chung, H.-T.; Pae, H.-O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar] [PubMed]
- Serras, F. The benefits of oxidative stress for tissue repair and regeneration. Fly 2016, 10, 128–133. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Reversal of Insulin Resistance in Overweight and Obese Subjects by trans-Resveratrol and Hesperetin Combination—Link to Dysglycemia, Blood Pressure, Dyslipidemia, and Low-Grade Inflammation. Nutrients 2021, 13, 2374. [Google Scholar] [CrossRef]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.-B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G. Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes 2016, 65, 2282–2294. [Google Scholar] [CrossRef]
- Shams-Rad, S.; Mohammadi, M.; Ramezani-Jolfaie, N.; Zarei, S.; Mohsenpour, M.; Salehi-Abargouei, A. Hesperidin supplementation has no effect on blood glucose control: A systematic review and meta-analysis of randomized controlled clinical trials. Br. J. Clin. Pharmacol. 2020, 86, 13–22. [Google Scholar] [CrossRef]
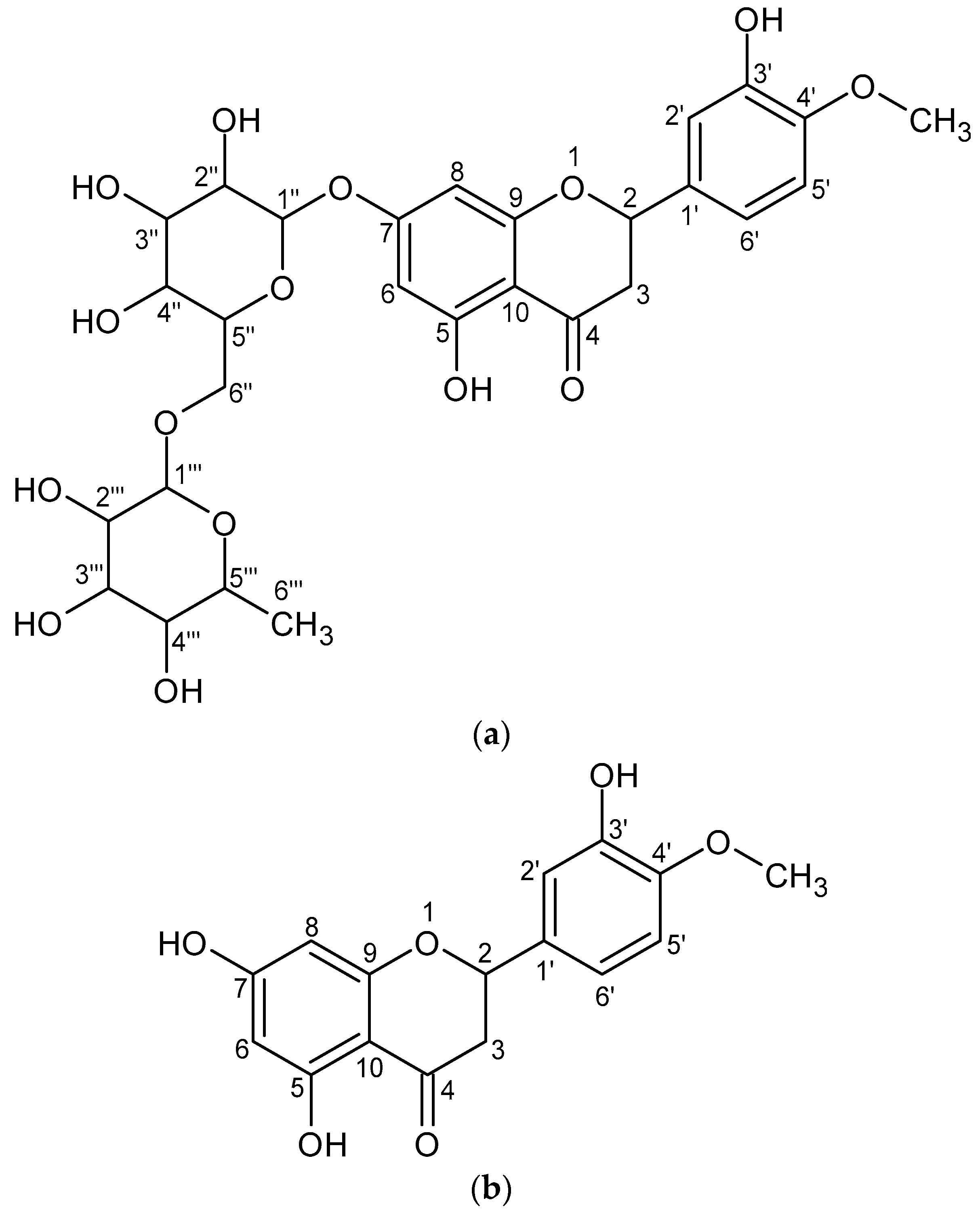
| Hesperidin | ||
|---|---|---|
| Technique | Observations | Reference |
| Hesperidin–chitosan complexes | The enhancement of solubility by 1.6-, 2.7-, and 3.8-fold and visible correlation between improved solubility and antioxidant activity. The greater the solubility improvement was, the better antioxidant activity reported | [16] |
| Inclusion complex of hesperidin with HP-β-CD | Obtaining the complex translated into increased solubility by 95-fold with respect to unmodified compound | [17] |
| Solid lipid nanoparticles loaded with Hesperidin | The increase of solubility by 20-fold. Impact on apparent permeability, leading to enhancement nearly by 5-fold. After oral administration, the overall bioavailability increased by 4.5-times in the study performed in a rat model. The obtained system affected biological activity as well, providing attenuation of Doxorubicin-induced cardiotoxicity and oxidative stress | [34] |
| Amorphous systems of Hesperidin with mesoporous material | Significant improvement in solubility by 51-fold for the best system and an impact on dissolution rate, better dissolution behavior in terms of apparent solubility | [35] |
| Nanoparticles of Hesperidin loaded by PLGA-Poloxamer 407 | In in vitro release profiles, sustained and slow release, and higher apparent solubility were observed. This modification provided stronger inhibitory activity on the breast cancer cells | [36] |
| Hesperidin-β-CD inclusion complexes | The systems showed better behavior in dissolution studies and also demonstrated an enhancement of antibacterial and antioxidant activity compared with unmodified hesperidin | [37] |
| Inclusion complexes of Hesperidin with HP-β-CD | The obtained complexes showed an improvement in dissolution rate, and antioxidant as well as antimicrobial activity | [38] |
| A Solid self-microemulsifying system with Hesperidin composing of Maisine CC, Tween 80 and PEG 400 | Significantly better dissolution rate profiles than that of free hesperidin, which enabled the release of almost all polyphenol from the system (>98%) after 60 min. Moreover, formulation showed better therapeutic activity for the management of diabetes mellitus in vivo | [39] |
| Solid nanocrystals | In the solubility studies, the system provided enhancement in solubility by 4.8-fold with respect to pure compound, faster dissolution, and higher apparent solubility | [40] |
| Inclusion system of Hesperidin with octenyl succinic anhydride modified sweet potato starch | The increase in solubility by 6.52-fold in the optimal conditions | [41] |
| Nanocrystals by combining Hesperidin with HPMC E5 and Poloxamer 188 | The systems enhanced the solubility by 5-times as well as the drug dissolution rate. The systems were characterized by comparable antioxidant activity with regard to pure compound. | [42] |
| Hesperidin-PEG 6000 complex | Enhancement of solubility by 21-fold. | [43] |
| Hesperetin | ||
| Cocrystals with different excipients such as caffeine, nicotinamide and picolinic acid | It translated into about 5-times better solubility as compared with pure substance. The parachute effect was observed in dissolution rate studies. Moreover, significant improvements in biological activity and pharmacokinetic profile were noticed. | [44] |
| Eutectic mixtures | In dissolution studies, the increase of apparent solubility was evident and reached about 3-times higher than the pure compound. The biological models revealed a direct impact of solubility on antioxidant and antihemolytic activity | [45] |
| Complexes of Hesperetin with β-CD and HP-β-CD | Higher solubility by 25-fold for β-CD and 467-fold for HP-β-CD complexes. | [46] |
| Nanocrystals | Significant enhancement in dissolution rate and apparent solubility was reported. In dissolution rate studies, authors reported the spring effect, leading to a dramatic increase in solubility in a short time from the beginning. However, the amount of dissolved substance decreased over time, and thus the parachute effect was not observed. | [47] |
| The systems of Hesperetin with Mg- or Ag-modified SBA-16 carriers | In dissolution studies, higher apparent solubility and dissolution velocity were reported. However, the total drug release was unnoticed. | [48] |
| Nanoemulsion | The authors reported 5.67-fold higher oral bioavailability | [49] |
| Nanoparticles composed of Hesperetin and Eudragit E 100 | Systems were characterized by sustained release with a pattern of initial rapid release of about 30% of the drug in the first 8 h, followed by a slow and continuous release of approximately 82% drug release in the next 24 h. | [50] |
| Self-assembling rebaudioside A nanomicelles with hesperetin | A drug release study revealed that prepared systems considerably increased apparent solubility and provided sustained release of the compound, reaching almost 81% at 24 h time point. This approach had a positive impact on the biological activity of hesperidin with respect to anticancer efficacy. | [51] |
| Formulations of hesperetin-D-alpha-tocopheryl polyethylene glycol 1000 succinate micelles and hesperetin-phosphatidylcholine complexes | The micelles formation was connected to an increase of solubility of 21.5-fold, whereas phosphatidylcholine complexes by 20.7-fold. Moreover, the solubility enhancement translated into a 4.2-fold boost in antioxidant activity for micelles and 3.9-fold for complexes. A significant improvement in bioavailability was also reported. The AUC increased by 16.2-fold for micelles formulation, whereas for complexes it was 18.0-fold. | [52] |
| Hesperetin complexes with β-CD and methylated-β-CD | The complexation caused an increase in apparent solubility and improved the dissolution profile. It also helped to increase the anti-inflammatory activity by reducing IL-6 secretion from LPS-stimulated macrophages. | [53] |
| Hesperetin-PLGA nanoparticles | Sustained release from formulation, which enabled a constant, slow-release within 7 days. Enhancement in the cytotoxic activity of prepared delivery system as compared with free compound. | [54] |
| Biocompatible gold nanoparticles of hesperetin | Sustained release of hesperetin from nanoparticles and increased cytotoxicity on cancer cells. | [55] |
| Chitosan-based nanoparticles | Sustained release of hesperetin and enhanced anticancer activity by an increase of inhibitory effect on colon cancer cell growth by 6-fold. | [56] |
| Hesperidin | ||
|---|---|---|
| Model | Observations/proposed mechanism | Reference |
| Human neuroblastoma SK-N-SH cells |
| [58] |
| Neuro-2A cells |
| [59] |
| In silico In vitro |
| [60] |
| female C57 BL/6 mice |
| [61] |
| Male Albino Wistar rats |
| [62] |
| Male APP/PS1 mice |
| [63] |
| In silico In vitro |
| [64] |
| APPswe/PS1dE9 mice |
| [65] |
| Adult male C57BL/6 mice |
| [66] |
| Male Wistar rats |
| [67] |
| male transgenic APP/PS1–21 mice |
| [68] |
| Swiss male albino mice |
| [69] |
| Hesperetin | ||
| adult male mice (C57BL/6N, wild type) HT22 cells |
| [70] |
| PC12 cells |
| [71] |
| Wistar rats |
| [72] |
| PC12 cells |
| [73] |
| Neuro-2A cells |
| [59] |
| In silico In vitro |
| [60] |
| ICR female mice |
| [74] |
| Male albino Wistar rats |
| [75] |
| Male C57BL/6 N mice |
| [76] |
| Male adult Wistar rats PD |
| [77] |
| Cortical cells |
| [78] |
| C57/BL6 male mice BV-2 microglial cells |
| [79] |
| SH-SY5Y cells |
| [80] |
| Male albino mice |
| [81] |
| RAW 264.7 Cells |
| [82] |
| Hesperidin | ||
|---|---|---|
| Model | Observations/proposed mechanism | Reference |
| Rat skeletal muscle cell lines, L6 myoblasts |
| [141] |
| Male Sprague Dawley rats |
| [142] |
| In vitro In silico |
| [143] |
| In vitro—Caco-2/TC7 cells and Xenopus laevis oocytes In vivo—human |
| [144] |
| Male Sprague Dawley rats |
| [145] |
| Male Wistar rats |
| [146] |
| In silico In vitro—pancreas of male BALB/c mice |
| [147] |
| White male albino rats |
| [148] |
| Male Wistar albino rats |
| [149] |
| Retinal ganglion cell 5 (RGC-5) cells |
| [150] |
| Male albino rats |
| [151] |
| Hesperetin | ||
| Rat skeletal muscle cell lines, L6 myoblasts |
| [141] |
| Male albino Wistar rats |
| [152] |
| Wistar rats |
| [153] |
| Adult male Wistar albino rats |
| [154] |
| Male mice |
| [155] |
| Wistar rats |
| [156] |
| In vitro In silico |
| [157] |
| In vitro In silico |
| [143] |
| HepG2 cells |
| [158] |
| RAW264.7 cells |
| [159] |
| Male Sprague Dawley rats |
| [160] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients 2022, 14, 2647. https://doi.org/10.3390/nu14132647
Wdowiak K, Walkowiak J, Pietrzak R, Bazan-Woźniak A, Cielecka-Piontek J. Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients. 2022; 14(13):2647. https://doi.org/10.3390/nu14132647
Chicago/Turabian StyleWdowiak, Kamil, Jarosław Walkowiak, Robert Pietrzak, Aleksandra Bazan-Woźniak, and Judyta Cielecka-Piontek. 2022. "Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review" Nutrients 14, no. 13: 2647. https://doi.org/10.3390/nu14132647
APA StyleWdowiak, K., Walkowiak, J., Pietrzak, R., Bazan-Woźniak, A., & Cielecka-Piontek, J. (2022). Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients, 14(13), 2647. https://doi.org/10.3390/nu14132647







