The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome
Abstract
:1. Introduction
2. Obesity
2.1. Definition
2.2. Epidemiology of Overweight and Obesity
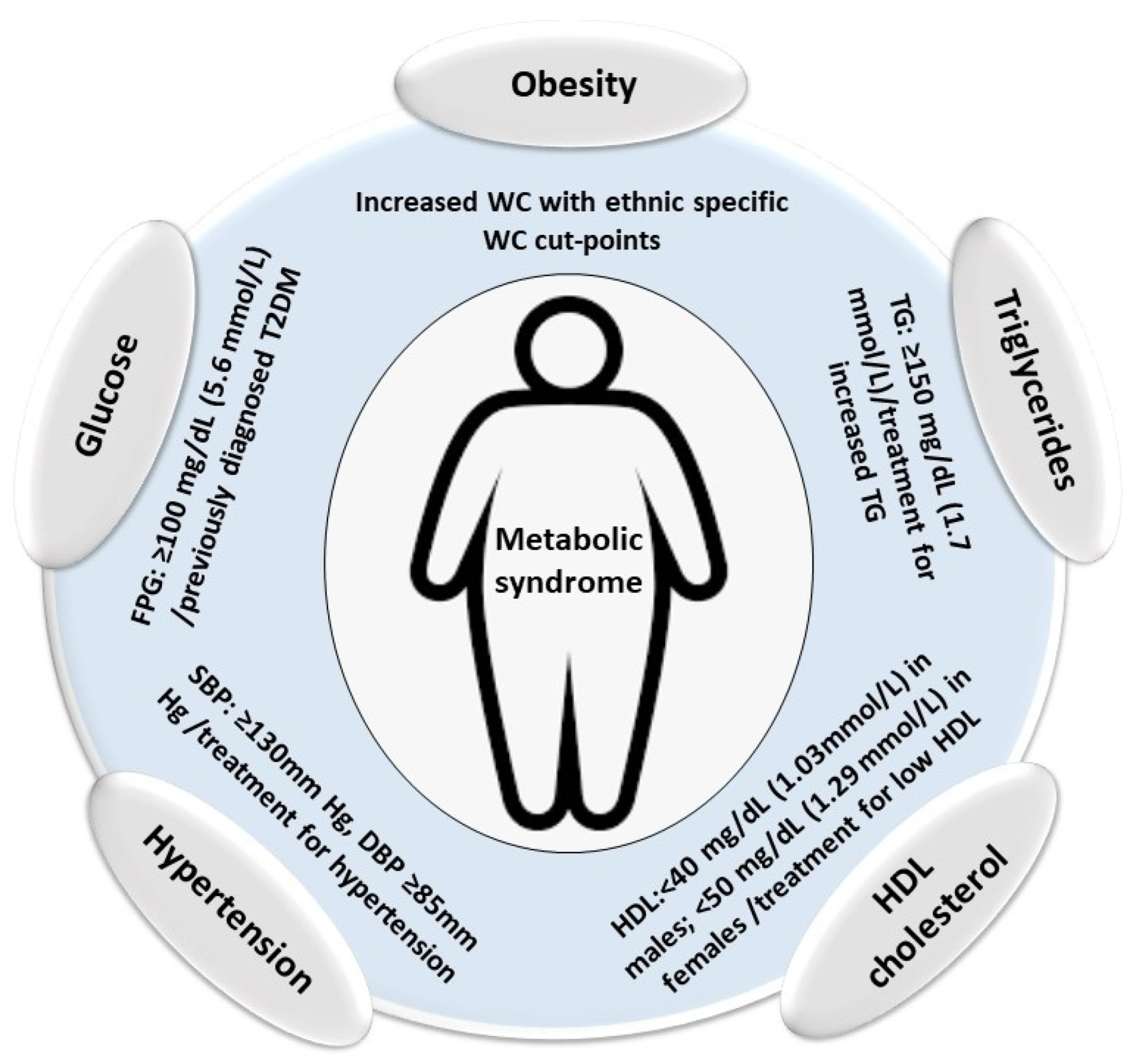
2.3. Metabolic Syndrome
| National Cholesterol Education Program ATP III [77] | International Diabetes Federation (IDF) [78] | |
|---|---|---|
| Any three of the following five abnormalities: | Central obesity plus any two of the following four factors: | |
| Obesity | Abdominal obesity is defined as a waist circumference ≥102 cm in men and ≥88 cm in females | Increased waist circumference, with ethnic-specific waist-circumference cut-off points * |
| Triglycerides | Serum triglycerides ≥ 1.7 mmol/L or drug treatment for elevated triglycerides | Triglycerides ≥ 1.7 mmol/L or drug treatment for elevated triglycerides |
| HDL cholesterol | Serum high-density lipoprotein (HDL) cholesterol <1 mmol/L in males and <1.3 mmol/L in females or drug treatment for low HDL cholesterol | HDL cholesterol < 1.03 mmol/L in men or <1.29 mmol/L in females or drug treatment for low HDL cholesterol |
| Hypertension | Systolic blood pressure ≥ 130mm Hg, diastolic blood pressure ≥ 85 mm Hg or drug treatment for elevated blood pressure | Systolic blood pressure ≥ 130 mm Hg, diastolic blood pressure ≥ 85 mm Hg, or treatment for hypertension |
| Glucose | Fasting plasma glucose (FPG) ≥ 100 mg/dL (5.6 mmol/L) or drug treatment for elevated blood glucose | FPG ≥ 100 mg/dL (5.6 mmol/L) or previously diagnosed type 2 diabetes; an oral glucose tolerance test is recommended for patients with an elevated FPG, but it is not required |
3. Mitochondria, Bioenergetics, Obesity, and MetS
3.1. Mitochondria and Bioenergetics
3.2. Mitochondria and Reactive Oxygen Species (ROS)
3.3. Mitochondrial Biogenesis
3.4. Mitochondrial Dysfunction in Obesity
3.5. Mitochondrial Dysfunction in MetS
4. Diet, Features, and Effects
Mediterranean Diet and Beneficial Effects
5. MD and Mitochondrial Activity
5.1. Preclinical Studies
5.2. Clinical Studies
6. Summary
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| ATP III | Adult Treatment Panel III |
| AMPK | AMP-activated protein kinase |
| APG | Apigenin |
| ApoJ | Apolipoprotein J |
| BMI | Body mass index |
| Bcl2 | B-cell lymphoma 2 |
| Bax | BCl2-associated X |
| CV | Cardiovascular |
| CoQ | Coenzyme Q |
| CoA | Coenzyme A |
| CPT-1 | Carnitine palmitoyltransferase-1 |
| CYP2E1 | Mitochondrial Cytochrome P450 2E1 |
| CVD | Cardiovascular disease |
| CRP | C-reactive protein |
| CGA | Chlorogenic acid |
| Cox | Cytochrome C oxidase |
| Cycs | Cytochrome C |
| CO2 | Carbon dioxide |
| CPT1b | Carnitine palmitoyltransferase 1B |
| DNA | Deoxyribonucleic acid |
| DHAs | Docosahexaenoic acids |
| Drp1 | Dynamin-related protein 1 |
| DHA | Doxosahexaenoic acid |
| ETC | Electron transport chain |
| ERR | Estrogen-related receptors |
| EPAs | Eicosapentaenoic acids |
| EPC | Endothelial progenitor cells |
| EVOO | Extra virgin olive oil |
| ER stress | Endoplasmic reticulum stress |
| EPA | Eicosapenteanoic acid |
| ECM | Extracellular matrix |
| EGCG | Epigallocatechin-3-gallate |
| ENDOG | Endonuclease G |
| FAD | Flavin adenine dinucleotide |
| FFA | Free fatty cid |
| FOXO3a | Forkhead box O3 (transcription factors) |
| FBA | N-(1-carbamoyl-2-phenyl-ethyl) butyramide |
| Fis1 | Mitochondrial fission protein1 |
| FA | Ferulic acid |
| FABPL | Fatty acid binding protein—liver type |
| FASTKD2 | FAST kinase domain-containing protein 2 |
| H2O2 | Hydrogen peroxide |
| HFD | High-fat diet |
| HUVECs | Human endothelial cells |
| HT | Hydroxytyrosol |
| HepG2 | Human liver cancer cell line |
| HDL | High-density lipoprotein |
| IDF | International Diabetes Federation |
| IMM | Inner mitochondrial membrane |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| LYC | Lycopene |
| LFD | Low-fat diet |
| MetS | Metabolic Syndrome |
| MD | Mediterranean diet |
| MHO | Metabolically healthy obesity |
| MAO | Metabolically altered obesity |
| MAFLD | Metabolic dysfunction associated fatty liver disease |
| mtROS | Mitochondrial reactive oxygen species |
| mtDNA | Mitochondrial Deoxyribonucleic Acid |
| Mfn1 | Mitofusin-1 |
| Mfn2 | Mitofusin-2 |
| MWCNTs | Multi-walled carbon nanotubes |
| NAFLD | Non-alcoholic fatty liver disease |
| NAD | Nicotinamide adenine dinucleotide |
| NRF | Nuclear respiratory factors |
| NRF-1 | Nuclear respiratory factor 1 |
| NASH | Non-alcoholic steatohepatitis |
| NADPH | Nicotinamide adenine dinucleotide phosphate oxidase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO2-OA | Nitro-fatty acids |
| OMM | Outer mitochondrial membrane |
| OXPHOS | Oxidative phosphorylation |
| ox-LDL | Oxidized low-density lipoprotein |
| PGC-1α | Peroxisome proliferator–activated receptor gamma coactivator-1 α |
| PUFA | Polyunsaturated fatty acid |
| PPAR-α | Peroxisome proliferator–activated receptor-α |
| PolG | DNA polymerase subunit gamma |
| PC-12 | Pheochromocytoma |
| PBMC | Peripheral blood mononuclear cell |
| PRDX6 | Peroxiredoxin 6 |
| PGRMC1 | Progesterone receptor membrane component 1 protein |
| PEBP1 | Phosphatidylethanolamine-binding protein 1 |
| ROS | Reactive oxidative atress |
| RBEE | Rice bran enzymatic extract |
| SO | Sarcopenic obesity |
| SIRT1 | Sirtuin 1 |
| SH-SY5Y | Human neuroblastoma cells |
| SDH | Succinate dehydrogenase |
| SCFAs | Short-chain fatty acids |
| T2DM | Type 2 diabetes mellitus |
| TOFI | Thin-outside-fat-inside |
| t-RNA | Transfer ribonucleic acid |
| TFAM | Mitochondrial transcription factor A |
| TFB2M | Mitochondrial transcription factor B2 |
| TNF-α | Tumor necrosis factor |
| Tfb2m | Transcription factor B2, mitochondria |
| UCP3 | Uncoupling protein 3 |
| UQCRC1 | Ubiquinol cytochrome c reductase |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Di Ciaula, A.; Krawczyk, M.; Filipiak, K.J.; Geier, A.; Bonfrate, L.; Portincasa, P. Noncommunicable diseases, climate change and iniquities: What COVID-19 has taught us about syndemic. Eur. J. Clin. Investig. 2021, 51, e13682. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Thompson, M.A.; Pabelick, C.; Agrawal, A.; Prakash, Y.S. Obesity, mitochondrial dysfunction, and obstructive lung disease. In Mechanisms and Manifestations of Obesity in Lung Disease; Johnston, R.A., Suratt, B.T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 143–167. [Google Scholar]
- Mitchell, T.; Darley-Usmar, V. Metabolic syndrome and mitochondrial dysfunction: Insights from preclinical studies with a mitochondrially targeted antioxidant. Free Radic. Biol. Med. 2012, 52, 838–840. [Google Scholar] [CrossRef] [Green Version]
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsen, B.; Lahti, K.; Nissen, M.; Taskinen, M.R.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Rogge, M.M. The role of impaired mitochondrial lipid oxidation in obesity. Biol. Res. Nurs. 2009, 10, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Kusminski, C.M.; Scherer, P.E. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. TEM 2012, 23, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Bournat, J.C.; Brown, C.W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [Green Version]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef] [Green Version]
- Zorzano, A.; Liesa, M.; Palacin, M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int. J. Biochem. Cell Biol. 2009, 41, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J. Clin. Endocrinol. Metab. 2014, 99, E209–E216. [Google Scholar] [CrossRef] [Green Version]
- Putti, R.; Sica, R.; Migliaccio, V.; Lionetti, L. Diet impact on mitochondrial bioenergetics and dynamics. Front. Physiol. 2015, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 313–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef]
- Huo, R.; Du, T.; Xu, Y.; Xu, W.; Chen, X.; Sun, K.; Yu, X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: A meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 1200–1208. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, K.; Hartley, L.; Clarke, A.; Thorogood, M.; Stranges, S. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2012, 8, CD009825. [Google Scholar] [CrossRef]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Dontas, A.S.; Zerefos, N.S.; Panagiotakos, D.B.; Vlachou, C.; Valis, D.A. Mediterranean diet and prevention of coronary heart disease in the elderly. Clin. Interv. Aging 2007, 2, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Laubinger, W.; Dimroth, P. Characterization of the ATP synthase of Propionigenium modestum as a primary sodium pump. Biochemistry 1988, 27, 7531–7537. [Google Scholar] [CrossRef]
- Medina-Remon, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martinez-Gonzalez, M.A.; Fito, M.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharm. 2017, 83, 114–128. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Fact Sheet: Obesity and Overweight. Available online: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed on 15 June 2022).
- Schwartz, M.W.; Seeley, R.J.; Zeltser, L.M.; Drewnowski, A.; Ravussin, E.; Redman, L.M.; Leibel, R.L. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 2017, 38, 267–296. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, J.; Farr, O.; Perakakis, N.; Ghaly, W.; Mantzoros, C. Obesity as a Disease. Med. Clin. North Am. 2018, 102, 13–33. [Google Scholar] [CrossRef]
- Nam, S.Y. Obesity-related digestive diseases and their pathophysiology. Gut Liver 2017, 11, 323. [Google Scholar] [CrossRef] [Green Version]
- Catalan, V.; Aviles-Olmos, I.; Rodriguez, A.; Becerril, S.; Fernandez-Formoso, J.A.; Kiortsis, D.; Portincasa, P.; Gomez-Ambrosi, J.; Fruhbeck, G. Time to Consider the “Exposome Hypothesis” in the Development of the Obesity Pandemic. Nutrients 2022, 14, 1597. [Google Scholar] [CrossRef]
- Matsuzawa, Y. Therapy Insight: Adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Despres, J.P.; Lemieux, I.; Bergeron, J.; Pibarot, P.; Mathieu, P.; Larose, E.; Rodes-Cabau, J.; Bertrand, O.F.; Poirier, P. Abdominal obesity and the metabolic syndrome: Contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1039–1049. [Google Scholar] [CrossRef]
- Deschênes, D.; Couture, P.; Dupont, P.; Tchernof, A. Subdivision of the Subcutaneous Adipose Tissue Compartment and Lipid-Lipoprotein Levels in Women. Obesity 2003, 11, 469–476. [Google Scholar] [CrossRef]
- Abate, N.; Garg, A.; Peshock, R.M.; Stray-Gundersen, J.; Grundy, S.M. Relationships of generalized and regional adiposity to insulin sensitivity in men. J. Clin. Investig. 1995, 96, 88. [Google Scholar] [CrossRef] [Green Version]
- Vecchie, A.; Dallegri, F.; Carbone, F.; Bonaventura, A.; Liberale, L.; Portincasa, P.; Fruhbeck, G.; Montecucco, F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef]
- Gonzalez-Muniesa, P.; Martinez-Gonzalez, M.A.; Hu, F.B.; Despres, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Aung, K.; Lorenzo, C.; Hinojosa, M.A.; Haffner, S.M. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J. Clin. Endocrinol. Metab. 2014, 99, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Rector, R.S.; Thyfault, J.P.; Ibdah, J.A. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J. Gastroenterol. WJG 2008, 14, 193–199. [Google Scholar] [CrossRef]
- Sharma, A.M.; Kushner, R.F. A proposed clinical staging system for obesity. Int. J. Obes. 2009, 33, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Blundell, J.E.; Dulloo, A.G.; Salvador, J.; Fruhbeck, G.; BMI, E.S.W.G.o. Beyond BMI—Phenotyping the obesities. Obes. Facts 2014, 7, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G.; Busetto, L.; Dicker, D.; Yumuk, V.; Goossens, G.H.; Hebebrand, J.; Halford, J.G.C.; Farpour-Lambert, N.J.; Blaak, E.E.; Woodward, E.; et al. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes. Facts 2019, 12, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Farkouh, M.E.; Newman, J.D.; Garvey, W.T. Cardiometabolic-Based Chronic Disease, Addressing Knowledge and Clinical Practice Gaps: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 539–555. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bauer, J.M.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cruz-Jentoft, A.J.; Dicker, D.; Fruhbeck, G.; Giustina, A.; et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin. Nutr. 2020, 39, 2368–2388. [Google Scholar] [CrossRef]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuthbertson, D.J.; Wilding, J.P.H. Metabolically healthy obesity: Time for a change of heart? Nat. Rev. Endocrinol. 2021, 17, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, S.; Bernardi, S.; Del Bo, C.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; Gonzalez-Dominguez, R.; Peron, G.; Zamora-Ros, R.; et al. Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: Study protocol of the MaPLE randomised controlled trial. BMC Geriatr. 2020, 20, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primeau, V.; Coderre, L.; Karelis, A.D.; Brochu, M.; Lavoie, M.E.; Messier, V.; Sladek, R.; Rabasa-Lhoret, R. Characterizing the profile of obese patients who are metabolically healthy. Int. J. Obes. 2011, 35, 971–981. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Ryu, S.; Suh, B.S.; Yun, K.E.; Kim, C.W.; Cho, S.I. Impact of BMI on the incidence of metabolic abnormalities in metabolically healthy men. Int. J. Obes. 2012, 36, 1187–1194. [Google Scholar] [CrossRef] [Green Version]
- Fortuno, A.; Rodriguez, A.; Gomez-Ambrosi, J.; Muniz, P.; Salvador, J.; Diez, J.; Fruhbeck, G. Leptin inhibits angiotensin II-induced intracellular calcium increase and vasoconstriction in the rat aorta. Endocrinology 2002, 143, 3555–3560. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Sola, G.; Uriarte, I.; Latasa, M.U.; Urtasun, R.; Barcena-Varela, M.; Elizalde, M.; Jimenez, M.; Rodriguez-Ortigosa, C.M.; Corrales, F.J.; Fernandez-Barrena, M.G.; et al. Fibroblast Growth Factor 15/19 in Hepatocarcinogenesis. Dig. Dis. 2017, 35, 158–165. [Google Scholar] [CrossRef]
- Gomez-Ambrosi, J.; Gallego-Escuredo, J.M.; Catalan, V.; Rodriguez, A.; Domingo, P.; Moncada, R.; Valenti, V.; Salvador, J.; Giralt, M.; Villarroya, F.; et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr. 2017, 36, 861–868. [Google Scholar] [CrossRef]
- Bell, J.A.; Sabia, S.; Singh-Manoux, A.; Hamer, M.; Kivimaki, M. Healthy obesity and risk of accelerated functional decline and disability. Int. J. Obes. 2017, 41, 866–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.P.; Fantin, F.; Caliari, C.; Zoico, E.; Mazzali, G.; Zanardo, M.; Bertassello, P.; Zanandrea, V.; Micciolo, R.; Zamboni, M. Dynapenic abdominal obesity as predictor of mortality and disability worsening in older adults: A 10-year prospective study. Clin. Nutr. 2016, 35, 199–204. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin. Nutr. ESPEN 2022, 41, 990–1000. [Google Scholar] [CrossRef]
- Cauley, J.A. An Overview of Sarcopenic Obesity. J. Clin. Densitom. 2015, 18, 499–505. [Google Scholar] [CrossRef]
- Goisser, S.; Kemmler, W.; Porzel, S.; Volkert, D.; Sieber, C.C.; Bollheimer, L.C.; Freiberger, E. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons—A narrative review. Clin. Interv. Aging 2015, 10, 1267–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.N.; Choi, K.M. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J. Cell. Biochem. 2015, 116, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xu, Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int. 2016, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- De Meyts, P.; Delzenne, N. The Brain—Gut—Microbiome Network in Metabolic Regulation and Dysregulation. Front. Endocrinol. 2021, 12, 760558. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Mannalithara, A.; Myer, P.A.; Singh, G. Obesity, Abdominal Obesity, Physical Activity, and Caloric Intake in US Adults: 1988 to 2010. Am. J. Med. 2014, 127, 717–727.e712. [Google Scholar] [CrossRef] [Green Version]
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- Centers for Disease Control and Prevention. Overweight and obesity: Adult obesity facts. 2021. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 28 August 2021).
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grattagliano, I.; Di Ciaula, A.; Baj, J.; Molina-Molina, E.; Shanmugam, H.; Garruti, G.; Wang, D.Q.; Portincasa, P. Protocols for Mitochondria as the Target of Pharmacological Therapy in the Context of Nonalcoholic Fatty Liver Disease (NAFLD). Methods Mol. Biol. 2021, 2310, 201–246. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Calamita, G.; Shanmugam, H.; Khalil, M.; Bonfrate, L.; Wang, D.Q.; Baffy, G.; Portincasa, P. Mitochondria Matter: Systemic Aspects of Nonalcoholic Fatty Liver Disease (NAFLD) and Diagnostic Assessment of Liver Function by Stable Isotope Dynamic Breath Tests. Int. J. Mol. Sci. 2021, 22, 7702. [Google Scholar] [CrossRef]
- Grattagliano, I.; Montezinho, L.P.; Oliveira, P.J.; Fruhbeck, G.; Gomez-Ambrosi, J.; Montecucco, F.; Carbone, F.; Wieckowski, M.R.; Wang, D.Q.; Portincasa, P. Targeting mitochondria to oppose the progression of nonalcoholic fatty liver disease. Biochem. Pharmacol. 2019, 160, 34–45. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jedrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014, 383, 970–983. [Google Scholar] [CrossRef] [Green Version]
- Faienza, M.F.; Chiarito, M.; Molina-Molina, E.; Shanmugam, H.; Lammert, F.; Krawczyk, M.; D’Amato, G.; Portincasa, P. Childhood obesity, cardiovascular and liver health: A growing epidemic with age. World J. Pediatrics WJP 2020, 16, 438–445. [Google Scholar] [CrossRef]
- Faienza, M.F.; Wang, D.Q.H.; Frühbeck, G.; Garruti, G.; Portincasa, P. The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Intern. Emerg. Med. 2016, 11, 175–182. [Google Scholar] [CrossRef]
- Akil, L.; Ahmad, H.A. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. J. Health Care Poor Underserved 2011, 22, 61. [Google Scholar] [CrossRef] [Green Version]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5.24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef] [Green Version]
- American Heart, A.; National Heart, L.; Blood, I.; Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol. Rev. 2005, 13, 322–327. [Google Scholar]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html (accessed on 23 June 2022).
- Ford, E.S. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care 2005, 28, 2745–2749. [Google Scholar] [CrossRef] [Green Version]
- Beltran-Sanchez, H.; Harhay, M.O.; Harhay, M.M.; McElligott, S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J. Am. Coll. Cardiol. 2013, 62, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Ravikiran, M.; Bhansali, A.; Ravikumar, P.; Bhansali, S.; Dutta, P.; Thakur, J.S.; Sachdeva, N.; Bhadada, S.; Walia, R. Prevalence and risk factors of metabolic syndrome among Asian Indians: A community survey. Diabetes Res. Clin. Pract. 2010, 89, 181–188. [Google Scholar] [CrossRef]
- Zuo, H.; Shi, Z.; Hu, X.; Wu, M.; Guo, Z.; Hussain, A. Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metab. Clin. Exp. 2009, 58, 1102–1108. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q. Novel Insights into the Pathogenesis and Management of the Metabolic Syndrome. Pediatric Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Gastaldi, G.; Giacobino, J.P.; Ruiz, J. Metabolic syndrome, a mitochondrial disease? Rev. Med. Suisse 2008, 4, 1387–1388. [Google Scholar] [PubMed]
- Beuther, D.A. Recent insight into obesity and asthma. Curr. Opin. Pulm. Med. 2010, 16, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; Barbato, A.; Siani, A.; Cappuccio, F.P.; Versiero, M.; Schiattarella, P.; Russo, O.; Avallone, S.; della Valle, E.; Farinaro, E. Diagnostic criteria for metabolic syndrome: A comparative analysis in an unselected sample of adult male population. Metab. Clin. Exp. 2008, 57, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Clementi, E.; Carruba, M.O.; Moncada, S. Defective mitochondrial biogenesis: A hallmark of the high cardiovascular risk in the metabolic syndrome? Circ. Res. 2007, 100, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Krawczyk, M.; Wang, D.Q.; Portincasa, P.; Lammert, F. Dissecting the genetic heterogeneity of gallbladder stone formation. Semin. Liver Dis. 2011, 31, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Cholesterol gallstones: A fellow traveler with metabolic syndrome? Am. J. Clin. Nutr. 2004, 80, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Portincasa, P.; Moschetta, A.; Palasciano, G. Cholesterol gallstone disease. Lancet 2006, 368, 230–239. [Google Scholar] [CrossRef]
- Wang, D.Q.H.; Portincasa, P.; Wang, H.H. Bile Formation and Pathophysiology of Gallstones. In Encyclopedia of Gastroenterology, 2nd ed.; Kujpers, E.J., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Oxford, UK, 2020; pp. 287–306. [Google Scholar]
- Portincasa, P.; Molina-Molina, E.; Garruti, G.; Wang, D.Q. Critical Care Aspects of Gallstone Disease. J. Crit. Care Med. 2019, 5, 6–18. [Google Scholar] [CrossRef] [Green Version]
- Portincasa, P.; van Erpecum, K.J.; Di Ciaula, A.; Wang, D.Q. The physical presence of gallstone modulates ex vivo cholesterol crystallization pathways of human bile. Gastroenterol. Rep. 2019, 7, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Wang, D.Q.; Portincasa, P. Cholesterol cholelithiasis: Part of a systemic metabolic disease, prone to primary prevention. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 157–171. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Bugianesi, E.; Gish, R.G.; Lammert, F.; Tilg, H.; Nguyen, M.H.; Sarin, S.K.; Fabrellas, N.; Zelber-Sagi, S.; Fan, J.G.; et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet. Gastroenterol. Hepatol. 2022, 7, 388–390. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Bonfrate, L.; Portincasa, P. The role of microbiota in nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2022, 52, e13768. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Passarella, S.; Shanmugam, H.; Noviello, M.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Nonalcoholic Fatty Liver Disease (NAFLD). Mitochondria as Players and Targets of Therapies? Int. J. Mol. Sci. 2021, 22, 5375. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef]
- Molina-Molina, E.; Shanmugam, H.; Di Palo, D.; Grattagliano, I.; Portincasa, P. Exploring Liver Mitochondrial Function by (13)C-Stable Isotope Breath Tests: Implications in Clinical Biochemistry. Methods Mol. Biol. 2021, 2310, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Baldini, F.; Fabbri, R.; Eberhagen, C.; Voci, A.; Portincasa, P.; Zischka, H.; Vergani, L. Adipocyte hypertrophy parallels alterations of mitochondrial status in a cell model for adipose tissue dysfunction in obesity. Life Sci. 2021, 265, 118812. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin. Sci. 2008, 114, 195–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Schurr, A.; Portincasa, P. Mitochondrial Transport in Glycolysis and Gluconeogenesis: Achievements and Perspectives. Int. J. Mol. Sci. 2021, 22, 2620. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Tu, B.P.; Tang, Y. Eight Kinetically Stable but Thermodynamically Activated Molecules that Power Cell Metabolism. Chem. Rev. 2018, 118, 1460–1494. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhou, Q.; Pang, X.; Xu, Y.; Rao, Z. Revealing various coupling of electron transfer and proton pumping in mitochondrial respiratory chain. Curr. Opin. Struct. Biol. 2013, 23, 526–538. [Google Scholar] [CrossRef]
- Sazanov, L.A. A giant molecular proton pump: Structure and mechanism of respiratory complex I. Nat. Rev. Mol. Cell Biol. 2015, 16, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fabra, M.; Navas, P.; Brea-Calvo, G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta 2016, 1857, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, I.N.; Montgomery, M.G.; Runswick, M.J.; Leslie, A.G.; Walker, J.E. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc. Natl. Acad. Sci. USA 2010, 107, 16823–16827. [Google Scholar] [CrossRef] [Green Version]
- Schatz, G. Mitochondrial oxidative phosphorylation. Angew Chem. Int. Ed. Engl. 1967, 6, 1035–1046. [Google Scholar] [CrossRef]
- Brookes, P.S. Mitochondrial H(+) leak and ROS generation: An odd couple. Free Radic. Biol. Med. 2005, 38, 12–23. [Google Scholar] [CrossRef]
- Venditti, P.; Di Stefano, L.; Di Meo, S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013, 13, 71–82. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Weinstein, H.; Jamison, E.; Brenowitz, M. A detailed interpretation of OH radical footprints in a TBP-DNA complex reveals the role of dynamics in the mechanism of sequence-specific binding. J. Mol. Biol. 2000, 304, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Zweier, J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014, 114, 524–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef] [Green Version]
- Karnati, S.; Lüers, G.; Pfreimer, S.; Baumgart-Vogt, E. Mammalian SOD2 is exclusively located in mitochondria and not present in peroxisomes. Histochem. Cell Biol. 2013, 140, 105–117. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Van Veldhoven, P.P.; Brees, C.; Rubio, N.; Nordgren, M.; Apanasets, O.; Kunze, M.; Baes, M.; Agostinis, P.; Fransen, M. Mitochondria are targets for peroxisome-derived oxidative stress in cultured mammalian cells. Free Radic. Biol. Med. 2013, 65, 882–894. [Google Scholar] [CrossRef] [Green Version]
- Stowe, D.F.; Bienengraeber, M.; Camara, A.K.S. Mitochondrial Approaches to Protect Against Cardiac Ischemia and Reperfusion Injury. Front. Physiol. 2011, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Stowe, D.F.; Camara, A.K.S. Mitochondrial Reactive Oxygen Species Production in Excitable Cells: Modulators of Mitochondrial and Cell Function. Antioxid. Redox Signal. 2009, 11, 1373–1414. [Google Scholar] [CrossRef] [Green Version]
- Camara, A.K.S.; Lesnefsky, E.J.; Stowe, D.F. Potential Therapeutic Benefits of Strategies Directed to Mitochondria. Antioxid. Redox Signal. 2010, 13, 279–347. [Google Scholar] [CrossRef] [Green Version]
- Storz, G.; Imlay, J.A. Oxidative stress. Curr. Opin. Microbiol. 1999, 2, 188–194. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Collins, Y.; Logan, A.; Murphy, M.P. Mitochondrial oxidative stress and the metabolic syndrome. Trends Endocrinol. Metab. 2012, 23, 429–434. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial genetic medicine. Nat. Genet. 2018, 50, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
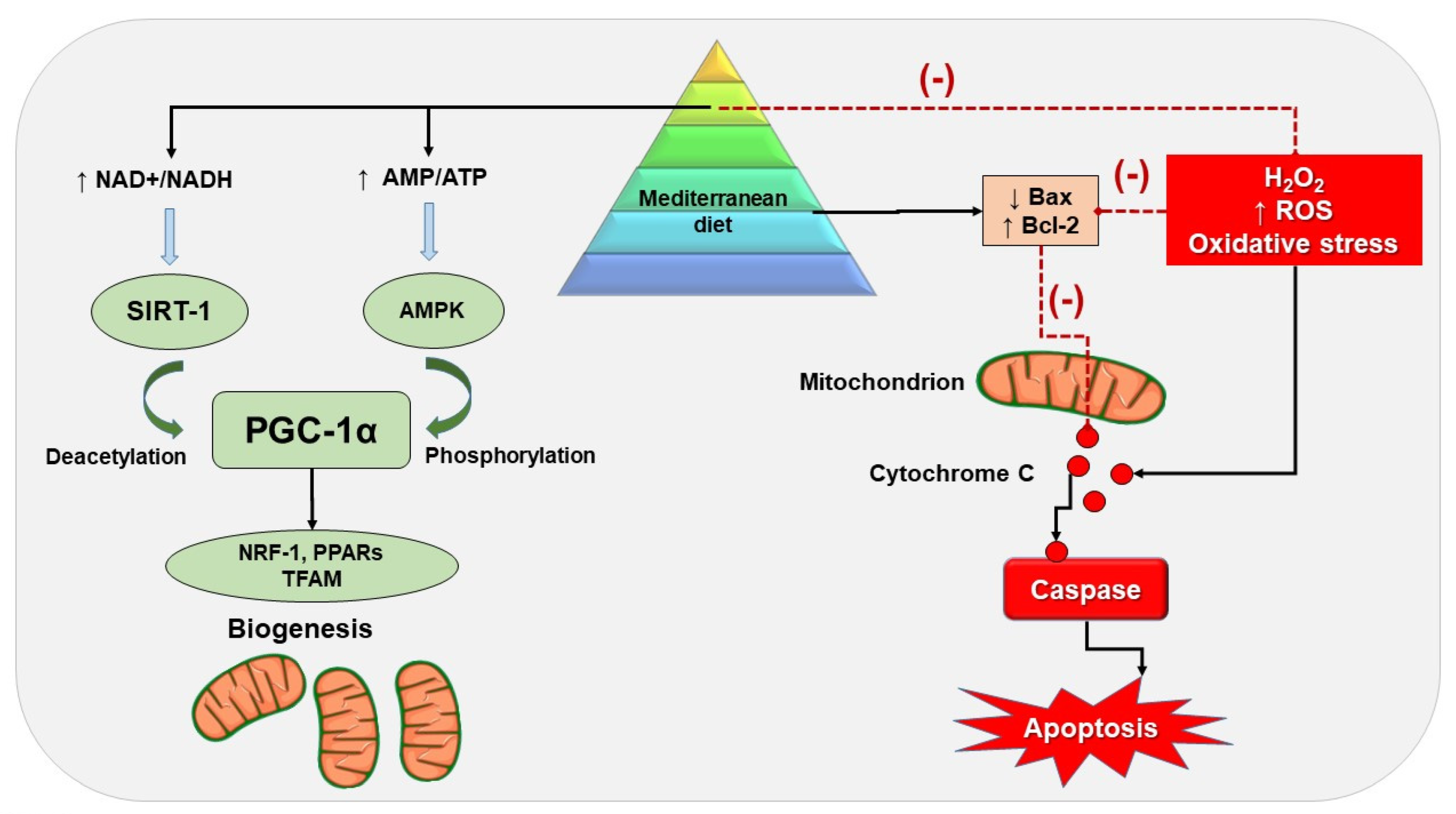
- Austin, S.; St-Pierre, J. PGC1alpha and mitochondrial metabolism—Emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef] [Green Version]
- Di, W.; Lv, J.; Jiang, S.; Lu, C.; Yang, Z.; Ma, Z.; Hu, W.; Yang, Y.; Xu, B. PGC-1: The Energetic Regulator in Cardiac Metabolism. Curr. Issues Mol. Biol. 2018, 28, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Islam, H.; Edgett, B.A.; Gurd, B.J. Coordination of mitochondrial biogenesis by PGC-1alpha in human skeletal muscle: A re-evaluation. Metab. Clin. Exp. 2018, 79, 42–51. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Barshad, G.; Marom, S.; Cohen, T.; Mishmar, D. Mitochondrial DNA Transcription and Its Regulation: An Evolutionary Perspective. Trends Genet. 2018, 34, 682–692. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardie, D.G. Minireview: The AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology 2003, 144, 5179–5183. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Dominy, J.E., Jr.; Lee, Y.; Gerhart-Hines, Z.; Puigserver, P. Nutrient-dependent regulation of PGC-1alpha’s acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochim. Biophys. Acta 2010, 1804, 1676–1683. [Google Scholar] [CrossRef] [Green Version]
- Benton, C.R.; Wright, D.C.; Bonen, A. PGC-1alpha-mediated regulation of gene expression and metabolism: Implications for nutrition and exercise prescriptions. Appl. Physiol. Nutr. Metab. 2008, 33, 843–862. [Google Scholar] [CrossRef]
- Bonen, A. PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity. Appl. Physiol. Nutr. Metab. 2009, 34, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Li Ji, L. Role of PGC-1alpha signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef]
- Wu, Z.; Boss, O. Targeting PGC-1 alpha to control energy homeostasis. Expert Opin. Targets 2007, 11, 1329–1338. [Google Scholar] [CrossRef]
- Handschin, C.; Spiegelman, B.M. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 2006, 27, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Komen, J.C.; Thorburn, D.R. Turn up the power—Pharmacological activation of mitochondrial biogenesis in mouse models. Br. J. Pharmacol. 2014, 171, 1818–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero, T. Mitochondrial biogenesis: Pharmacological approaches. Curr. Pharm. Des. 2014, 20, 5507–5509. [Google Scholar] [CrossRef]
- Radika, M.K.; Anuradha, C.V. Activation of insulin signaling and energy sensing network by AICAR, an AMPK activator in insulin resistant rat tissues. J. Basic Clin. Physiol. Pharm. 2015, 26, 563–574. [Google Scholar] [CrossRef]
- Yarnoz-Esquiroz, P.; Olazaran, L.; Aguas-Ayesa, M.; Perdomo, C.M.; Garcia-Goni, M.; Silva, C.; Fernandez-Formoso, J.A.; Escalada, J.; Montecucco, F.; Portincasa, P.; et al. ‘Obesities’: Position statement on a complex disease entity with multifaceted drivers. Eur. J. Clin. Investig. 2022, 52, e13811. [Google Scholar] [CrossRef] [PubMed]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.X.; Qiu, Y.; Hansen, M.K.; Zhu, L.; Zhang, V.; Xie, M.; Okamoto, Y.; Mattie, M.D.; Higashiyama, H.; Asano, S.; et al. Adipose Mitochondrial Biogenesis Is Suppressed in db/db and High-Fat Diet–Fed Mice and Improved by Rosiglitazone. Diabetes 2007, 56, 1751–1760. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Lee, T.Y.; Kwok, C.F.; Hsu, Y.P.; Shih, K.C.; Lin, Y.J.; Ho, L.T. Cannabinoid receptor type 1 mediates high-fat diet-induced insulin resistance by increasing forkhead box O1 activity in a mouse model of obesity. Int. J. Mol. Med. 2016, 37, 743–754. [Google Scholar] [CrossRef]
- Konopka, A.R.; Asante, A.; Lanza, I.R.; Robinson, M.M.; Johnson, M.L.; Dalla Man, C.; Cobelli, C.; Amols, M.H.; Irving, B.A.; Nair, K.S. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes 2015, 64, 2104–2115. [Google Scholar] [CrossRef] [Green Version]
- Semple, R.K.; Crowley, V.C.; Sewter, C.P.; Laudes, M.; Christodoulides, C.; Considine, R.V.; Vidal-Puig, A.; O’Rahilly, S. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2004, 28, 176–179. [Google Scholar] [CrossRef] [Green Version]
- Diogo, C.V.; Grattagliano, I.; Oliveira, P.J.; Bonfrate, L.; Portincasa, P. Re-wiring the circuit: Mitochondria as a pharmacological target in liver disease. Curr. Med. Chem. 2011, 18, 5448–5465. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; Russmann, S.; Diogo, C.; Bonfrate, L.; Oliveira, P.J.; Wang, D.Q.; Portincasa, P. Mitochondria in chronic liver disease. Curr. Drug Targets 2011, 12, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.H.; Swerdlow, R.H.; Khan, E.M.; Iezzoni, J.C.; Hespenheide, E.E.; Parks, J.K.; Parker, W.D., Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J. Hepatol. 1999, 31, 430–434. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef] [Green Version]
- Simoes, I.C.M.; Karkucinska-Wieckowska, A.; Janikiewicz, J.; Szymanska, S.; Pronicki, M.; Dobrzyn, P.; Dabrowski, M.; Dobrzyn, A.; Oliveira, P.J.; Zischka, H.; et al. Western Diet Causes Obesity-Induced Nonalcoholic Fatty Liver Disease Development by Differentially Compromising the Autophagic Response. Antioxidants 2020, 9, 995. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef] [Green Version]
- Longo, M.; Meroni, M.; Paolini, E.; Macchi, C.; Dongiovanni, P. Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): New perspectives for a fairy-tale ending? Metab. Clin. Exp. 2021, 117, 154708. [Google Scholar] [CrossRef]
- Ajaz, S.; McPhail, M.J.; Gnudi, L.; Trovato, F.M.; Mujib, S.; Napoli, S.; Carey, I.; Agarwal, K. Mitochondrial dysfunction as a mechanistic biomarker in patients with non-alcoholic fatty liver disease (NAFLD). Mitochondrion 2021, 57, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E.; Ragavan, M.; Palavicini, J.P.; Fourcaudot, M.; Bakewell, T.M.; Valdez, I.A.; Ayala, I.; Jin, E.S.; Madesh, M.; Han, X.; et al. Insulin resistance is mechanistically linked to hepatic mitochondrial remodeling in non-alcoholic fatty liver disease. Mol. Metab. 2021, 45, 101154. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Yang, M.; Wei, L.; Wu, H.; Wang, Q.; Shi, H. Physiological evidence of mitochondrial permeability transition pore opening caused by lipid deposition leading to hepatic steatosis in db/db mice. Free Radic. Biol. Med. 2021, 162, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, I.; Santoro, N.; Chen, Y.; Hoque, R.; Ouyang, X.; Caprio, S.; Shlomchik, M.J.; Coffman, R.L.; Candia, A.; Mehal, W.Z. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J. Clin. Investig. 2016, 126, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Ou, Z.; Cai, C.; Li, P.; Gong, J.; Ruan, X.Z.; He, K. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell Immunol. 2018, 332, 111–120. [Google Scholar] [CrossRef]
- Pirola, C.J.; Garaycoechea, M.; Flichman, D.; Castano, G.O.; Sookoian, S. Liver mitochondrial DNA damage and genetic variability of Cytochrome b—A key component of the respirasome—Drive the severity of fatty liver disease. J. Intern. Med. 2021, 289, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Czajka, A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 2013, 13, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.A.; Fromenty, B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: Mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637. [Google Scholar] [CrossRef]
- Weltman, M.D.; Farrell, G.C.; Liddle, C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology 1996, 111, 1645–1653. [Google Scholar] [CrossRef]
- Chalasani, N.; Gorski, J.C.; Asghar, M.S.; Asghar, A.; Foresman, B.; Hall, S.D.; Crabb, D.W. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 2003, 37, 544–550. [Google Scholar] [CrossRef]
- Piccinin, E.; Villani, G.; Moschetta, A. Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: The role of PGC1 coactivators. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 160–174. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jezek, P.; Hlavata, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Reynet, C.; Kahn, C.R. Increased expression of mitochondrial-encoded genes in skeletal muscle of humans with diabetes mellitus. J. Clin. Invest. 1995, 95, 1383–1388. [Google Scholar] [CrossRef] [Green Version]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef]
- Lahera, V.; de Las Heras, N.; López-Farré, A.; Manucha, W.; Ferder, L. Role of Mitochondrial Dysfunction in Hypertension and Obesity. Curr. Hypertens. Rep. 2017, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef] [Green Version]
- Choo, H.J.; Kim, J.H.; Kwon, O.B.; Lee, C.S.; Mun, J.Y.; Han, S.S.; Yoon, Y.S.; Yoon, G.; Choi, K.M.; Ko, Y.G. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 2006, 49, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Choi, C.S.; Birkenfeld, A.L.; Alves, T.C.; Jornayvaz, F.R.; Jurczak, M.J.; Zhang, D.; Woo, D.K.; Shadel, G.S.; Ladiges, W.; et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010, 12, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Steckhan, N.; Hohmann, C.D.; Kessler, C.; Dobos, G.; Michalsen, A.; Cramer, H. Effects of different dietary approaches on inflammatory markers in patients with metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016, 32, 338–348. [Google Scholar] [CrossRef]
- Keane, D.; Kelly, S.; Healy, N.P.; McArdle, M.A.; Holohan, K.; Roche, H.M. Diet and metabolic syndrome: An overview. Curr. Vasc. Pharmacol. 2013, 11, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Drake, I.; Sonestedt, E.; Ericson, U.; Wallström, P.; Orho-Melander, M. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br. J. Nutr. 2018, 119, 1168–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan Diet Health Benefits in Metabolic Syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef]
- Martinez, K.B.; Leone, V.; Chang, E.B. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 2017, 8, 130–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirmiran, P.; Bahadoran, Z.; Vakili, A.Z.; Azizi, F. Western dietary pattern increases risk of cardiovascular disease in Iranian adults: A prospective population-based study. Appl. Physiol. Nutr. Metab. 2017, 42, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.; Harris, M. Key elements of plant-based diets associated with reduced risk of metabolic syndrome. Curr. Diabetes Rep. 2014, 14, 524. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association-Recommended Diet in Coronary Artery Disease Trial. J. Am. Heart Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef] [Green Version]
- Magriplis, E.; Chourdakis, M. Special Issue “Mediterranean Diet and Metabolic Diseases”. Nutrients 2021, 13, 2680. [Google Scholar] [CrossRef]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef] [Green Version]
- Carbone, F.; Ciaula, A.D.; Pagano, S.; Minetti, S.; Ansaldo, A.M.; Ferrara, D.; Belfiore, A.; Elia, E.; Pugliese, S.; Ostilio Palmieri, V.; et al. Anti-ApoA-1 IgGs predict resistance to waist circumference reduction after Mediterranean diet. Eur. J. Clin. Invest. 2021, 51, e13410. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-López, L.; Santiago-Díaz, G.; Nava-Hernández, J.; Muñoz-Torres, A.V.; Medina-Bravo, P.; Torres-Tamayo, M. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatrics 2014, 14, 175. [Google Scholar] [CrossRef] [Green Version]
- Sayon-Orea, C.; Razquin, C.; Bullo, M.; Corella, D.; Fito, M.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; Warnberg, J.; Martinez, J.A.; et al. Effect of a Nutritional and Behavioral Intervention on Energy-Reduced Mediterranean Diet Adherence Among Patients with Metabolic Syndrome: Interim Analysis of the PREDIMED-Plus Randomized Clinical Trial. JAMA 2019, 322, 1486–1499. [Google Scholar] [CrossRef]
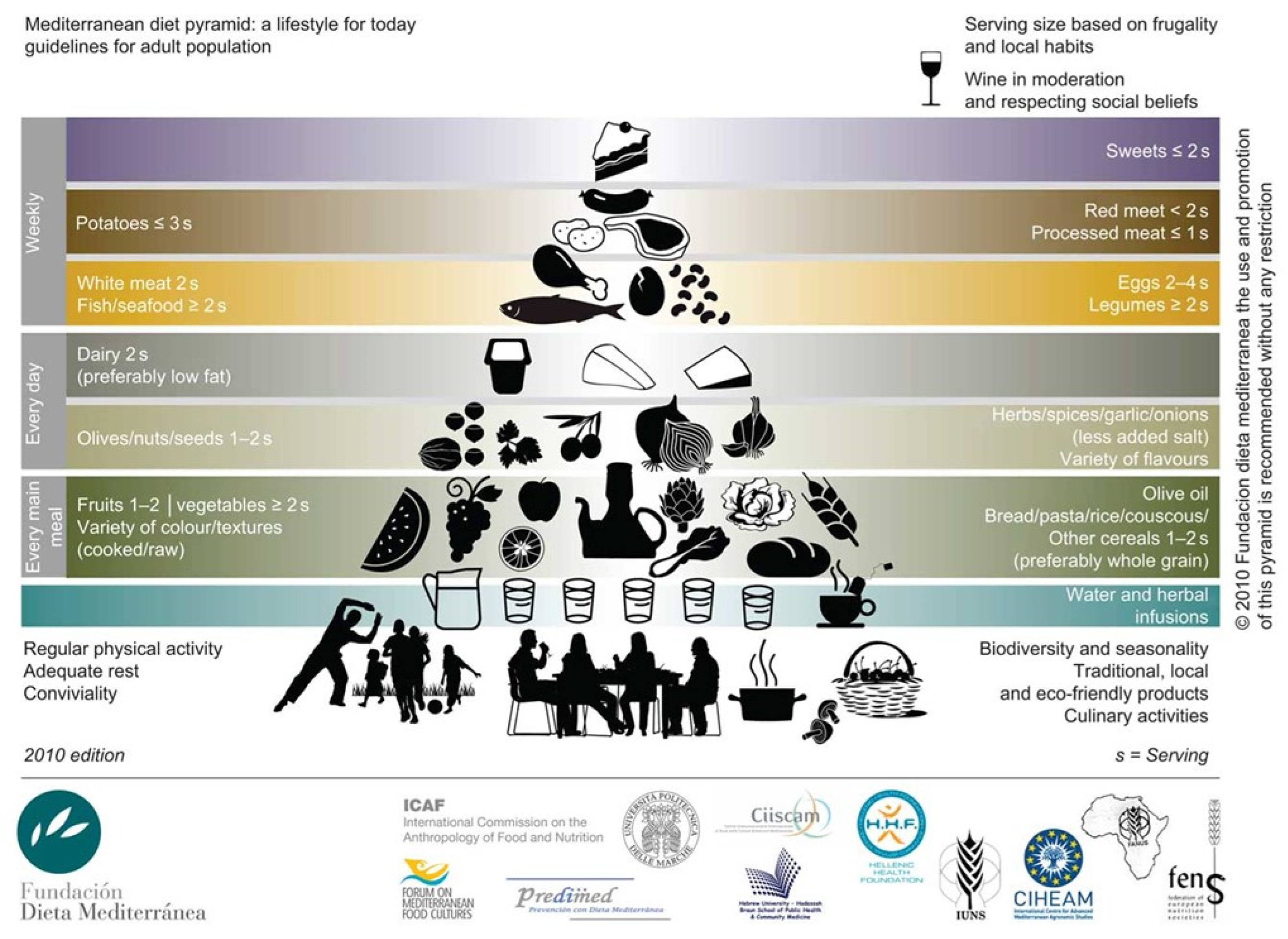
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P.; et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2014, 37 (Suppl. S1), S120–S143. [Google Scholar] [CrossRef] [Green Version]
- Portincasa, P.; Wang, D.Q.H. Il valore di una dieta “prudente” nell’individuo adulto. Considerazioni per vivere meglio e forse piu’ a lungo. In Atti e Relazioni Accademia Pugliese delle Scienze; Adda Editrice: Bari, Italy, 2022; Volume LVII, pp. 85–108. [Google Scholar]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Luchsinger, J.A.; Schupf, N.; Brickman, A.M.; Cosentino, S.; Tang, M.X.; Stern, Y. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009, 302, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Buckland, G.; Agudo, A.; Lujan, L.; Jakszyn, P.; Bueno-de-Mesquita, H.B.; Palli, D.; Boeing, H.; Carneiro, F.; Krogh, V.; Sacerdote, C.; et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am. J. Clin. Nutr. 2010, 91, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckland, G.; Travier, N.; Cottet, V.; Gonzalez, C.A.; Lujan-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H.; et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 2013, 132, 2918–2927. [Google Scholar] [CrossRef]
- La Vecchia, C. Association between Mediterranean dietary patterns and cancer risk. Nutr. Rev. 2009, 67 (Suppl. S1), S126–S129. [Google Scholar] [CrossRef] [Green Version]
- Panagiotakos, D.B.; Polystipioti, A.; Papairakleous, N.; Polychronopoulos, E. Long-term adoption of a Mediterranean diet is associated with a better health status in elderly people; a cross-sectional survey in Cyprus. Asia Pac. J. Clin. Nutr. 2007, 16, 331–337. [Google Scholar]
- Nunez-Cordoba, J.M.; Valencia-Serrano, F.; Toledo, E.; Alonso, A.; Martinez-Gonzalez, M.A. The Mediterranean diet and incidence of hypertension: The Seguimiento Universidad de Navarra (SUN) Study. Am. J. Epidemiol. 2009, 169, 339–346. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; de la Fuente-Arrillaga, C.; Nunez-Cordoba, J.M.; Basterra-Gortari, F.J.; Beunza, J.J.; Vazquez, Z.; Benito, S.; Tortosa, A.; Bes-Rastrollo, M. Adherence to Mediterranean diet and risk of developing diabetes: Prospective cohort study. BMJ 2008, 336, 1348–1351. [Google Scholar] [CrossRef] [Green Version]
- Schroder, H.; Marrugat, J.; Vila, J.; Covas, M.I.; Elosua, R. Adherence to the traditional mediterranean diet is inversely associated with body mass index and obesity in a spanish population. J. Nutr. 2004, 134, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Tektonidis, T.G.; Akesson, A.; Gigante, B.; Wolk, A.; Larsson, S.C. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: A population-based cohort study. Atherosclerosis 2015, 243, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Li, T.Y.; Fung, T.T.; Li, S.; Willett, W.C.; Rimm, E.B.; Hu, F.B. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am. J. Clin. Nutr. 2014, 99, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.S.; Golden, S.H.; Anderson, C.; Bray, G.A.; Burke, L.E.; de Boer, I.H.; Deedwania, P.; Eckel, R.H.; Ershow, A.G.; Fradkin, J.; et al. Update on Prevention of Cardiovascular Disease in Adults with Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2015, 38, 1777–1803. [Google Scholar] [CrossRef] [Green Version]
- Zito, F.P.; Polese, B.; Vozzella, L.; Gala, A.; Genovese, D.; Verlezza, V.; Medugno, F.; Santini, A.; Barrea, L.; Cargiolli, M.; et al. Good adherence to mediterranean diet can prevent gastrointestinal symptoms: A survey from Southern Italy. World J. Gastrointest. Pharm. 2016, 7, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Hayek, S.; Khalil, N.; Serale, N.; Vergani, L.; Calasso, M.; De Angelis, M.; Portincasa, P. Role of Sumac (Rhus coriaria L.) in the management of metabolic syndrome and related disorders: Focus on NAFLD-atherosclerosis interplay. J. Funct. Foods 2021, 87, 104811. [Google Scholar] [CrossRef]
- Khalil, M.; Rita Caponio, G.; Diab, F.; Shanmugam, H.; Di Ciaula, A.; Khalifeh, H.; Vergani, L.; Calasso, M.; De Angelis, M.; Portincasa, P. Unraveling the beneficial effects of herbal Lebanese mixture “Za’atar”. History, studies, and properties of a potential healthy food ingredient. J. Funct. Foods 2022, 90, 104993. [Google Scholar] [CrossRef]
- Khalil, M.; Khalifeh, H.; Baldini, F.; Serale, N.; Parodi, A.; Voci, A.; Vergani, L.; Daher, A. Antitumor Activity of Ethanolic Extract from Thymbra Spicata L. aerial Parts: Effects on Cell Viability and Proliferation, Apoptosis Induction, STAT3, and NF-kB Signaling. Nutr. Cancer 2021, 73, 1193–1206. [Google Scholar] [CrossRef]
- Khalil, M.; Bazzi, A.; Zeineddine, D.; Jomaa, W.; Daher, A.; Awada, R. Repressive effect of Rhus coriaria L. fruit extracts on microglial cells-mediated inflammatory and oxidative stress responses. J. Ethnopharmacol. 2021, 269, 113748. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Farooqui, T.; Panza, F.; Frisardi, V. Metabolic syndrome as a risk factor for neurological disorders. Cell. Mol. Life Sci. CMLS 2012, 69, 741–762. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Freeman, B.A.; Baker, P.R.; Schopfer, F.J.; Woodcock, S.R.; Napolitano, A.; d’Ischia, M. Nitro-fatty acid formation and signaling. J. Biol. Chem. 2008, 283, 15515–15519. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, A.A. Phytochemicals, Signal Transduction, and Neurological Disorders; Springer: New York, NY, USA, 2012. [Google Scholar]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch. Neurol. 2006, 63, 1709–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Quezada, I.; Roman-Vinas, B.; Serra-Majem, L. The Mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Persuitte, G.; Olendzki, B.C.; Wedick, N.M.; Zhang, Z.; Merriam, P.A.; Fang, H.; Carmody, J.; Olendzki, G.F.; Ma, Y. Dietary magnesium intake improves insulin resistance among non-diabetic individuals with metabolic syndrome participating in a dietary trial. Nutrients 2013, 5, 3910–3919. [Google Scholar] [CrossRef]
- Lu, J.; Gu, Y.; Guo, M.; Chen, P.; Wang, H.; Yu, X. Serum Magnesium Concentration Is Inversely Associated with Albuminuria and Retinopathy among Patients with Diabetes. J. Diabetes Res. 2016, 2016, 1260141. [Google Scholar] [CrossRef]
- Paletas, K.; Athanasiadou, E.; Sarigianni, M.; Paschos, P.; Kalogirou, A.; Hassapidou, M.; Tsapas, A. The protective role of the Mediterranean diet on the prevalence of metabolic syndrome in a population of Greek obese subjects. J. Am. Coll. Nutr. 2010, 29, 41–45. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef]
- Farooqui, A.A. Essential Fatty Acid Metabolism in Metabolic Syndrome and Neurological Disorders. In Metabolic Syndrome; Springer: New York, NY, USA, 2013; pp. 67–101. [Google Scholar]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Richard, C.; Couture, P.; Desroches, S.; Lamarche, B. Effect of the Mediterranean diet with and without weight loss on markers of inflammation in men with metabolic syndrome. Obesity 2013, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Georgousopoulou, E.N.; Kastorini, C.M.; Milionis, H.J.; Ntziou, E.; Kostapanos, M.S.; Nikolaou, V.; Vemmos, K.N.; Goudevenos, J.A.; Panagiotakos, D.B. Association between mediterranean diet and non-fatal cardiovascular events, in the context of anxiety and depression disorders: A case/case-control study. Hell. J. Cardiol. 2014, 55, 24–31. [Google Scholar]
- Couto, E.; Boffetta, P.; Lagiou, P.; Ferrari, P.; Buckland, G.; Overvad, K.; Dahm, C.C.; Tjonneland, A.; Olsen, A.; Clavel-Chapelon, F.; et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer 2011, 104, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvado, J.; Bullo, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salamone, F.; Li Volti, G.; Titta, L.; Puzzo, L.; Barbagallo, I.; La Delia, F.; Zelber-Sagi, S.; Malaguarnera, M.; Pelicci, P.G.; Giorgio, M.; et al. Moro orange juice prevents fatty liver in mice. World J. Gastroenterol. 2012, 18, 3862–3868. [Google Scholar] [CrossRef]
- Garcia-Marcos, L.; Castro-Rodriguez, J.A.; Weinmayr, G.; Panagiotakos, D.B.; Priftis, K.N.; Nagel, G. Influence of Mediterranean diet on asthma in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2013, 24, 330–338. [Google Scholar] [CrossRef]
- Salas-Salvado, J.; Bullo, M.; Babio, N.; Martinez-Gonzalez, M.A.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Aros, F.; et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.Y.; Yiu, K.H.; Li, S.W.; Lee, S.; Tam, S.; Lau, C.P.; Tse, H.F. Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with Type 2 diabetes mellitus. Diabet Med. 2010, 27, 54–60. [Google Scholar] [CrossRef]
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The emerging role of nutrition in Parkinson’s disease. Front Aging Neurosci 2014, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gonzalez, M.A.; Bes-Rastrollo, M.; Serra-Majem, L.; Lairon, D.; Estruch, R.; Trichopoulou, A. Mediterranean food pattern and the primary prevention of chronic disease: Recent developments. Nutr. Rev. 2009, 67 (Suppl. S1), S111–S116. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Hsieh, P.L.; Ou, H.C.; Cheng, Y.H.; Chu, P.M. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1700928. [Google Scholar] [CrossRef] [PubMed]
- Duluc, L.; Jacques, C.; Soleti, R.; Andriantsitohaina, R.; Simard, G. Delphinidin inhibits VEGF induced-mitochondrial biogenesis and Akt activation in endothelial cells. Int. J. Biochem. Cell Biol. 2014, 53, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zou, Q.; Suo, Y.; Tan, X.; Yuan, T.; Liu, Z.; Liu, X. Lycopene ameliorates systemic inflammation-induced synaptic dysfunction via improving insulin resistance and mitochondrial dysfunction in the liver-brain axis. Food Funct. 2019, 10, 2125–2137. [Google Scholar] [CrossRef]
- Feng, C.; Luo, T.; Zhang, S.; Liu, K.; Zhang, Y.; Luo, Y.; Ge, P. Lycopene protects human SHSY5Y neuroblastoma cells against hydrogen peroxideinduced death via inhibition of oxidative stress and mitochondriaassociated apoptotic pathways. Mol. Med. Rep. 2016, 13, 4205–4214. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Y.; Hao, Y.; Wang, Z.; Yang, Z.; Wang, Z.; Wang, J. 5-Heptadecylresorcinol attenuates oxidative damage and mitochondria-mediated apoptosis through activation of the SIRT3/FOXO3a signaling pathway in neurocytes. Food Funct. 2020, 11, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, N.; Desquiret-Dumas, V.; Leman, G.; Chupin, S.; Baron, S.; Nivet-Antoine, V.; Vessieres, E.; Ayer, A.; Henrion, D.; Lenaers, G.; et al. Resveratrol Directly Binds to Mitochondrial Complex I and Increases Oxidative Stress in Brain Mitochondria of Aged Mice. PLoS ONE 2015, 10, e0144290. [Google Scholar] [CrossRef] [Green Version]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Zhang, S.; Wu, J.; Sun, X.; Shen, Z.; Dong, J.; Huang, J. Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1a Signaling Pathway by Ginger (Zingiber officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol. J. Food Sci. 2019, 84, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ternero, C.; Werner, C.M.; Nickel, A.G.; Herrera, M.D.; Motilva, M.J.; Bohm, M.; Alvarez de Sotomayor, M.; Laufs, U. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 2017, 48, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, L.; Wang, Y.; Zhang, G.; Cheng, Y.; Feng, Z.; Bai, X.; Liu, J. High ratio of omega-3/omega-6 polyunsaturated fatty acids targets mTORC1 to prevent high-fat diet-induced metabolic syndrome and mitochondrial dysfunction in mice. J. Nutr. Biochem. 2020, 79, 108330. [Google Scholar] [CrossRef]
- Sanchez-Calvo, B.; Cassina, A.; Mastrogiovanni, M.; Santos, M.; Trias, E.; Kelley, E.E.; Rubbo, H.; Trostchansky, A. Olive oil-derived nitro-fatty acids: Protection of mitochondrial function in non-alcoholic fatty liver disease. J. Nutr. Biochem. 2021, 94, 108646. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Li, L.; Espe, M.; Lu, K.L.; Rahimnejad, S. Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef]
- Keshtzar, E.; Khodayar, M.J.; Javadipour, M.; Ghaffari, M.A.; Bolduc, D.L.; Rezaei, M. Ellagic acid protects against arsenic toxicity in isolated rat mitochondria possibly through the maintaining of complex II. Hum. Exp. Toxicol. 2016, 35, 1060–1072. [Google Scholar] [CrossRef]
- Zamani, F.; Samiei, F.; Mousavi, Z.; Azari, M.R.; Seydi, E.; Pourahmad, J. Apigenin ameliorates oxidative stress and mitochondrial damage induced by multiwall carbon nanotubes in rat kidney mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zhang, J.; Zheng, Z.; Wang, Z. Antioxidant Apigenin Relieves Age-Related Muscle Atrophy by Inhibiting Oxidative Stress and Hyperactive Mitophagy and Apoptosis in Skeletal Muscle of Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2081–2088. [Google Scholar] [CrossRef]
- Daussin, F.N.; Cuillerier, A.; Touron, J.; Bensaid, S.; Melo, B.; Al Rewashdy, A.; Vasam, G.; Menzies, K.J.; Harper, M.E.; Heyman, E.; et al. Dietary Cocoa Flavanols Enhance Mitochondrial Function in Skeletal Muscle and Modify Whole-Body Metabolism in Healthy Mice. Nutrients 2021, 13, 3466. [Google Scholar] [CrossRef]
- Okada, L.; Oliveira, C.P.; Stefano, J.T.; Nogueira, M.A.; Silva, I.; Cordeiro, F.B.; Alves, V.A.F.; Torrinhas, R.S.; Carrilho, F.J.; Puri, P.; et al. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH—Proteomic and lipidomic insight. Clin. Nutr. 2018, 37, 1474–1484. [Google Scholar] [CrossRef]
- Anderson, E.J.; Thayne, K.A.; Harris, M.; Shaikh, S.R.; Darden, T.M.; Lark, D.S.; Williams, J.M.; Chitwood, W.R.; Kypson, A.P.; Rodriguez, E. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARgamma activation? Antioxid. Redox Signal 2014, 21, 1156–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, J.; Smith, G.I.; Kelly, S.C.; Julliand, S.; Reeds, D.N.; Mittendorfer, B. Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Physiol. Rep. 2016, 4, e12785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capo, X.; Martorell, M.; Sureda, A.; Llompart, I.; Tur, J.A.; Pons, A. Diet supplementation with DHA-enriched food in football players during training season enhances the mitochondrial antioxidant capabilities in blood mononuclear cells. Eur. J. Nutr. 2015, 54, 35–49. [Google Scholar] [CrossRef] [PubMed]
- de Ligt, M.; Bruls, Y.M.H.; Hansen, J.; Habets, M.F.; Havekes, B.; Nascimento, E.B.M.; Moonen-Kornips, E.; Schaart, G.; Schrauwen-Hinderling, V.B.; van Marken Lichtenbelt, W.; et al. Resveratrol improves ex vivo mitochondrial function but does not affect insulin sensitivity or brown adipose tissue in first degree relatives of patients with type 2 diabetes. Mol. Metab. 2018, 12, 39–47. [Google Scholar] [CrossRef]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.S.; Golden, A.; O’Broin, P.; Sinclair, D.A.; Bonkowski, M.S.; Coleville, A.J.; Powell, D.; et al. Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.; van Boekschoten, M.; de Groot, P.; Bendik, I.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joy, J.M.; Vogel, R.M.; Moon, J.R.; Falcone, P.H.; Mosman, M.M.; Pietrzkowski, Z.; Reyes, T.; Kim, M.P. Ancient peat and apple extracts supplementation may improve strength and power adaptations in resistance trained men. BMC Complement Altern. Med. 2016, 16, 224. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, S.; Guha, M.; Kashina, A.; Avadhani, N.G. Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Nadtochiy, S.M.; Redman, E.K. Mediterranean diet and cardioprotection: The role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition 2011, 27, 733–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, W.C.; Khairallah, R.J.; Dabkowski, E.R. Update on lipids and mitochondrial function: Impact of dietary n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 122–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Western Diet | Vegan Diet | Mediterranean Diet | |
|---|---|---|---|
| Characteristics | High fat and sugar | High vegetable | Low meat |
| Low fat | High vegetable and olive oil | ||
| No meat | High plant-based foods | ||
| Main components | Red meat | Fiber | Fiber |
| (Saturated fat and cholesterol) | Grain | Antioxidants | |
| Refined grains | Cereals | Unsaturated fats | |
| Fructose beverage | Whole grain | ||
| Health consequences | Obesity | Healthy (if balanced) Deficiency of essential macro and micronutrients (if unbalanced) | Healthy |
| Insulin resistance | |||
| NAFLD | |||
| Diabetes | |||
| CVD | |||
| Mechanisms | ↑ Adipose tissue ↑ Circulating FFAs ↑ Hepatic lipid accumulation ↑ Triglycerides ↑ Cholesterol ↑ Fasting glucose ↑ De novo lipogenesis ↑ VLDL ↑ ER stress ↑ Lysosomal permeabilization ↓ Insulin sensitivity | ↓ Circulating FFAs ↓ Hepatic steatosis ↓ Lipolysis ↓ De novo lipogenesis ↑ Insulin sensitivity | ↓ Circulating FFAs ↓ Hepatic steatosis ↓ Triglycerides ↓ Cholesterol ↓ Inflammation ↓ Lipolysis ↓ De novo lipogenesis ↓ ROS ↓ CRP ↑ Insulin sensitivity ↓ Inflammatory markers |
| Effect on Mitochondria | ↑ mtROS | ↓ mtROS | ↓ mtROS |
| ↓ mitochondrial biogenesis | ↑ mitochondrial biogenesis | ↑ mitochondrial biogenesis | |
| ↓ mitochondrial respiration | ↑ mitochondrial respiration | ↑ mitochondrial respiration | |
| References | [188,190,191] | [189,192,193] | [194,195,196,197,198,199] |
| Compound | Study | Model | Effects | Reference |
|---|---|---|---|---|
| Chlorogenic Acid (CGA) | In vitro OxLDL-treated HUVECs | Oxidative Damage/Mitochondrial Dysfunction | ↑ SIRT1 expression ↓ OxLDL-impaired SIRT1 Level ↓ ROS ↑ SIRT1, AMPK, and PGC-1α pathway | [248] |
| Delphinidin | In vitro VEGF-treated HUVECs | Post-ischemic neovascularization | ↑ NRF1, Tfb2m, Tfam and PolG ↓ Abnormal increase in mitochondrial respiration, mtDNA content, and complex IV activity | [249] |
| Lycopene (LYC) | In vivo LPS-treated mice | Inflammation | ↑ SIRT1 ↑ PGC1α ↑ Cox5b, Cox7a1, Cox8b, and Cycs ↑ Complexes Ι, ΙΙ, ΙΙΙ, and ΙѴ | [250] |
| Lycopene (LYC) | In vitro H2O2-treated SH-SY5Y | Oxidative stress /Apoptosis | ↑ Depolarization ↑ Bcl2 ↓ Bax | [251] |
| 5-Heptadecylresorcinol (AR-C17) | In vitro H2O2-treated PC-12 | Apoptosis/Mitochondrial dysfunction | ↓ ROS ↑ Mitochondrial respiration ↑ ATP ↑ SIRT-3 ↑ FOXO3a ↓ H2O2-cell apoptosis | [252] |
| Resveratrol | In vivo Mice | Insulin resistance/Obesity | ↑ SIRT1 activity ↑ PGC-1α activity ↑ Mitochondrial activity ↑ Aerobic capacity | [253] |
| Resveratrol | In vitro solubilized complex I In vivo Mice | Aging | ↑ Complex I activity in vitro ↑ Complex I activity in young mice ↑ Oxidative stress in old mice | [227] |
| Resveratrol | In vivo HFD mice | Obesity/Ageing | ↑ SIRT1 enzymatic activity ↑ PGC-1α deacetylation and activity | [254] |
| Butyric acid | In vivo HFD mice | Metabolic syndrome | ↑ PGC-1α ↑ CPT1b ↑ COX-I ↑ PPAR-δ ↑ Fatty acid oxidation | [255] |
| Butyrate and its synthetic derivative FBA | In vivo mice In vitro HepG2 cells | Insulin resistance/Obesity | ↑ Oxygen consumption ↑ Citrate synthase activity ↓ H2O2 ↑ Aconitase activity ↑ Mfn1, Mfn2, Opa1 ↓ Drp1 and Fis1 | [256] |
| Ginger extract (GE)/ 6-gingerol | In vivo mice In vitro HepG2 cells | - | ↑ mtDNA ↑ OXPHOS ↑ATP ↑ Complex I and IV activity ↑ AMPK-PGC-1α signaling | [257] |
| Ferulic acid (FA) | In vivo HFD mice In vitro PBMC and EPC | Cardiovascular disease | ↑ Mitochondrial biogenesis markers ↓ Oxidative stress ↓ PBMC apoptosis ↑ PGC-1α | [258] |
| Different ω-3/ω-6 PUFAs ratios | In vivo mice | Metabolic syndrome | ↓ Metabolic risk factors ↓ p-mTOR ↑ Mitochondrial electron transport chain ↑ Tricarboxylic acid cycle ↑ Mitochondrial activities ↓ Fumaric acid ↓ Oxidative stress | [259] |
| Extra virgin olive oil (EVOO) and nitrite (NO3) | In vivo mice | NAFLD | ↑ HO-1 expression ↑ Complexes II and V ↑ NO2-OA ↓ Cholesterol ↓ LDL ↓ Endothelial dysfunction ↓ Blood pressure ↓ Thrombosis ↓ Hyperglycemia | [260] |
| Hydroxytyrosol (HT) | In vivo mice | Metabolic syndrome | ↓ Drp1 ↑ Complex Ι and II ↓ Complex V ↓ PARP | [261] |
| Hydroxytyrosol (HT) | In vivo HFD-Megalobrama amblycephala fish In vitro hepatocytes | Hepatic fat deposition | ↑ Citrate synthase activity ↑ ATP content ↑ Mitochondria number ↑ PGC-1α, PGC-1β, NRF1 and TFAM | [262] |
| Ellagic acid (EA) | In vivo chronic arsenic-rats | Diabetes/Cancer | ↓ ROS ↓ Mitochondrial damage ↑ Dehydrogenase complex ΙΙ-associated activity | [263]. |
| Apigenin (APG) | In vivo multiwall CNT (MWCNT)-exposed rats | Kidney toxicity | ↑ Succinate dehydrogenase ↓ ROS ↑ Mitochondrial membrane potential ↓ Mitochondrial swelling ↓ Release cytochrome | [264] |
| Apigenin (APG) | In vivo aged Mice | Muscle Atrophy | ↑ Basal oxygen consumption ↑ Complexes I, II, and IV activity ↑ ATP content ↑ PGC-1α, TFAM, and NRF-1 ↓ Cyt-C release to cytosol | [265] |
| Cocoa Flavanols | In vivo mice | Healthy and SIRT3-/-mice | ↑ Mitochondrial respiration ↑ AMPK phosphorylation ↑ Mitochondria mass ↑ NAD+/NADH ↑ Complex I and IV activity | [266] |
| Authors | Year | Sample Size | Gender M/F (Age) | Participants | Format, Dose | Duration of Study | Main Findings |
|---|---|---|---|---|---|---|---|
| Anderson et al. [268] | 2014 | 24 | 16/8 (63.1 ± 8.4; 65.8 ±9.9) | Elective cardiac surgery for patients | Oral consumption of EPA and DHA capsule, 3.4 g/day | 2–3 weeks | ↑ PPARγ ↑ Mitochondrial fatty acid oxidation ↑ TxnRd2 enzyme |
| Capo et al. [270] | 2014 | 15 | 15/0 (20.4 ± 0.5) | Exercise-induced oxidative stress | Beverage enriched with DHA | 2 months | ↑ Antioxidant activity ↓ ROS production ↑ DHA |
| Yoshino et al. [269] | 2016 | 20 | 60 to 85 | Large hypertrophic response | Consumption of 4 pills (1.86g EPA+ 1.50 g DHA) | 6 months | ↑ Respiratory electron transport activity ↑ Oxidative phosphorylation ↑ ECM organization ↑ UCP3 and UQCRC1 |
| Most et al. [273] | 2016 | 38 | 18/20 (38 ± 2) | Subjects with obesity | Consumption of 282 mg EGCG + 80 mg RES | 12 weeks | ↑ Complexes III and V ↑ Citric acid cycle ↑ Respiratory electron-transport chain ↑ Fat oxidation |
| Joy et al. [274] | 2016 | 25 | 25/0 (28 ± 5) | Resistance-trained subjects | Consumption of 150 mg (ancient peat and apple extract (TRT)) | 12 weeks | ↑ Mitochondrial ATP production ↓ ROS ↓ Oxidative stress |
| Pollack et al. [272] | 2017 | 30 | 19/11 (67 ± 7) | Older glucose-intolerant patients | Treated with 2−3 g Resveratrol/day | 6 weeks | ↑ Mitochondrial number ↑ Oxidative phosphorylation ↑ ENDOG ↑ P GC1α |
| Samara et al. [267] | 2018 | 60 | 18–75 years | Patients with NASH | Oral consumption of n − 3 PUFA capsules, 0.945 g/day | 6 months | ↑ ALA, EPA, glycerophospholipids ↓ Arachidonic acid ↑ FABPL ↑ PRDX6 ↓ PGRMC1 ↑ PEBP1, ApoJ ↑ FASTKD2 |
| de Ligt et al. [271] | 2018 | 13 | 13/0 (59.2 to 67.6) | Patients with overweight/T2DM | Consumption of 150 mg Resveratrol/day | 6 months | ↑ State 3 respiration ↓ Complex IV ↑ Mitochondrial function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, M.; Shanmugam, H.; Abdallah, H.; John Britto, J.S.; Galerati, I.; Gómez-Ambrosi, J.; Frühbeck, G.; Portincasa, P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients 2022, 14, 3112. https://doi.org/10.3390/nu14153112
Khalil M, Shanmugam H, Abdallah H, John Britto JS, Galerati I, Gómez-Ambrosi J, Frühbeck G, Portincasa P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients. 2022; 14(15):3112. https://doi.org/10.3390/nu14153112
Chicago/Turabian StyleKhalil, Mohamad, Harshitha Shanmugam, Hala Abdallah, Jerlin Stephy John Britto, Ilaria Galerati, Javier Gómez-Ambrosi, Gema Frühbeck, and Piero Portincasa. 2022. "The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome" Nutrients 14, no. 15: 3112. https://doi.org/10.3390/nu14153112
APA StyleKhalil, M., Shanmugam, H., Abdallah, H., John Britto, J. S., Galerati, I., Gómez-Ambrosi, J., Frühbeck, G., & Portincasa, P. (2022). The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients, 14(15), 3112. https://doi.org/10.3390/nu14153112







