Quality Evaluation of Dietary Supplements for Weight Loss Based on Garcinia cambogia
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Samples
2.3. Reference Fruit Extracts
2.4. Gas Chromatography–Mass Spectrometry
2.5. High-Performance Liquid Chromatography Analysis
2.6. Statistical Analysis
3. Results and Discussion
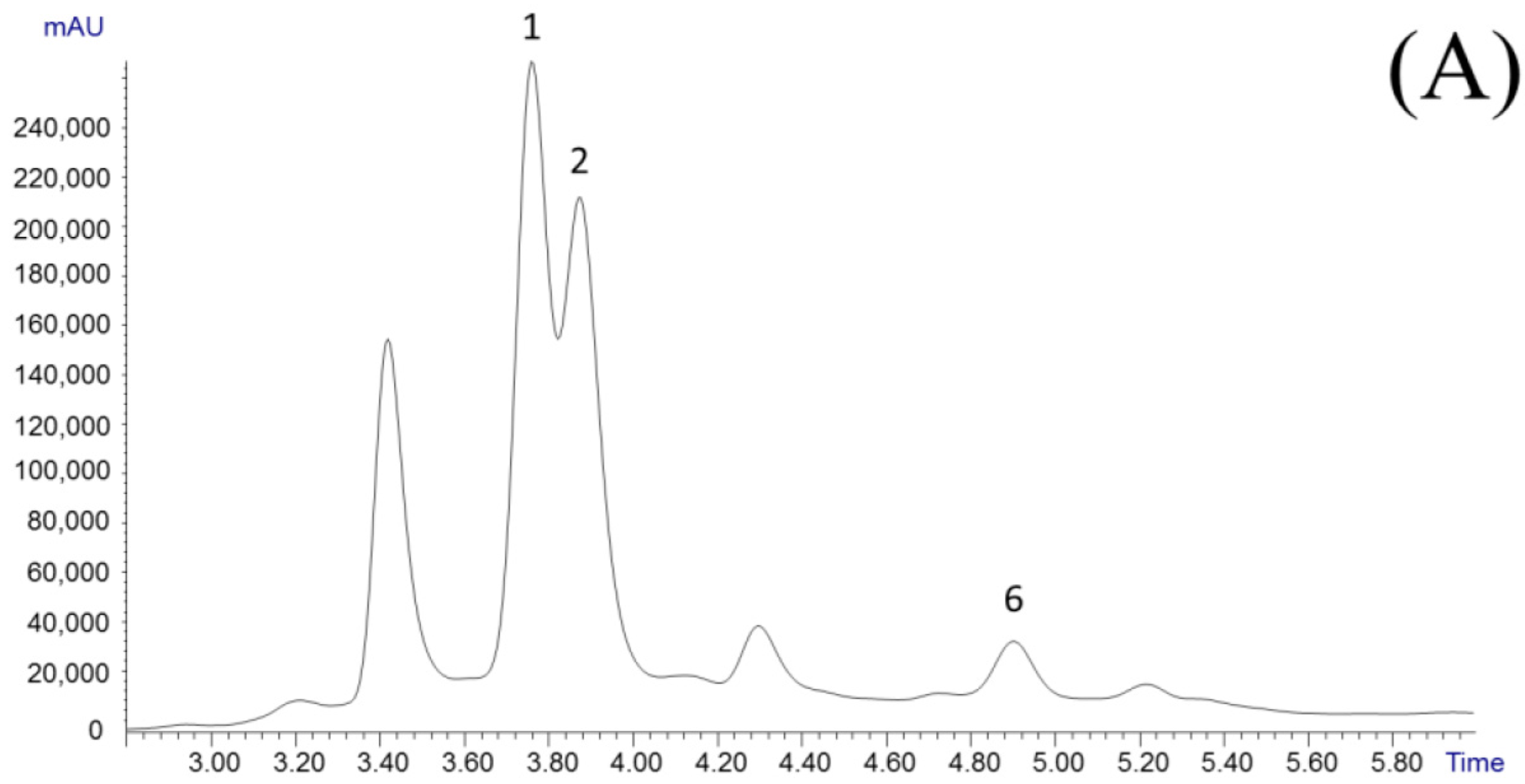
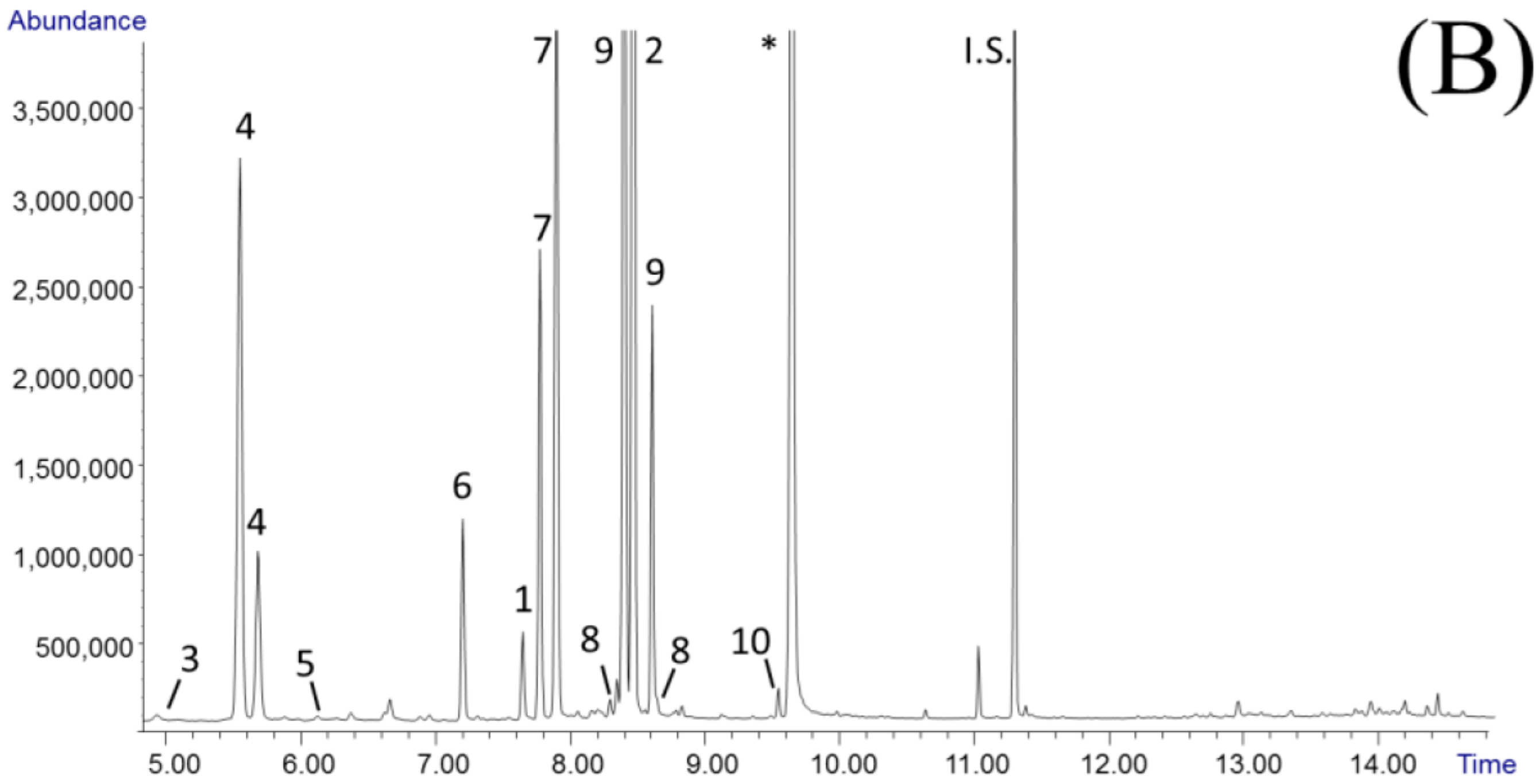
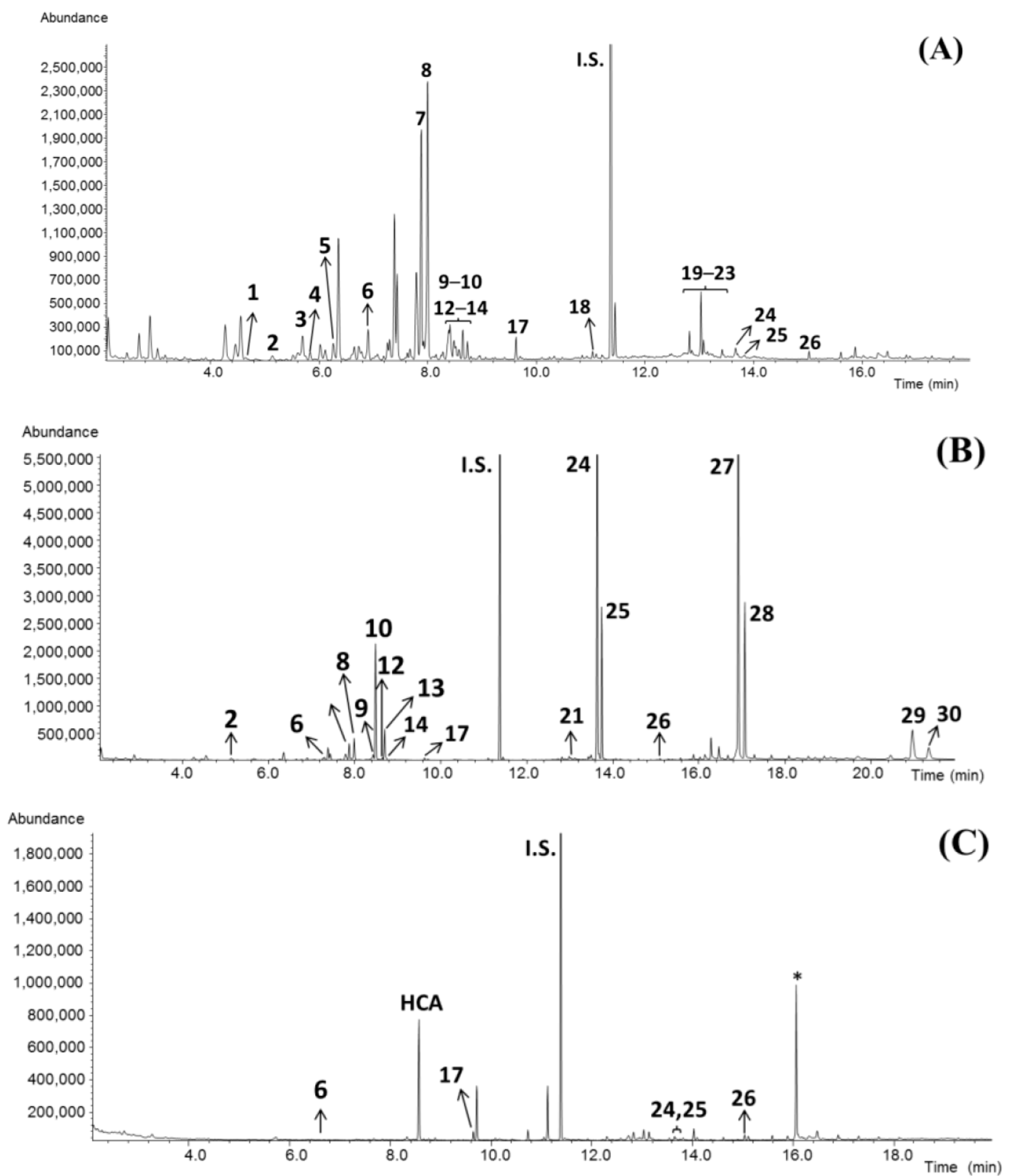
3.1. Analysis of Garcinia Fruit Rind Extracts
3.2. Analysis of Garcinia Cambogia Food Supplements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 November 2020).
- Bellaizac, J.; Chito, D.M.; Rada-Mendoza, M. Commercial Dietary Supplements for overweight control: Natural sources of easy access, mechanisms of action and adverse effects. Rev. Esp. Nutr. Hum. Diet. 2022, 26, 1–48. [Google Scholar] [CrossRef]
- Chanmee, W.; Chaicharoenpong, C.; Petsom, A. Lipase Inhibitor from Fruits of Solanum stramonifolium Jacq. Food Nutr. Sci. 2013, 4, 554–558. [Google Scholar] [CrossRef][Green Version]
- Yun, J.W. Possible anti-obesity therapeutics from nature? A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Shouval, D. Hepatotoxicity of herbal and dietary supplements: An update. Arch. Toxicol. 2015, 89, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Santo, B.L.S.D.E.; Santana, L.F.; Junior, W.H.K.; Araújo, F.D.O.D.; Bogo, D.; Freitas, K.D.C.; Guimarães, R.D.C.A.; Hiane, P.A.; Pott, A.; Filiú, W.F.D.O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef]
- Preuss, H.G.; Bagchi, D.; Bagchi, M.; Rao, C.; Satyanarayana, S.; Dey, D.K. Efficacy of a novel, natural extract of (-)-hydroxycitric acid (HCA-SX) and a combination of HCA-SX, niacin-bound chromium and Gymnema sylvestre extract in weight management in human volunteers: A pilot study. Nutr. Res. 2004, 24, 45–58. [Google Scholar] [CrossRef]
- Soni, M.; Burdock, G.; Preuss, H.; Stohs, S.; Ohia, S.; Bagchi, D. Safety assessment of (-)-hydroxycitric acid and Super CitriMax®, a novel calcium/potassium salt. Food Chem. Toxicol. 2004, 42, 1513–1529. [Google Scholar] [CrossRef]
- Fassina, P.; Adami, F.S.; Zani, V.T.; Machado, I.C.K.; Garavaglia, J.; Grave, M.T.Q.; Ramos, R.; Dal Bosco, S.M. The effect of Garcinia cambogia as coadjuvant in the weight loss process. Nutr. Hosp. 2015, 32, 2400–2408. [Google Scholar] [CrossRef]
- Crescioli, G.; Lombardi, N.; Bettiol, A.; Marconi, E.; Risaliti, F.; Bertoni, M.; Ippolito, F.M.; Maggini, V.; Gallo, E.; Firenzuoli, F.; et al. Acute liver injury following Garcinia cambogia weight-loss supplementation: Case series and literature review. Intern. Emerg. Med. 2018, 13, 857–872. [Google Scholar] [CrossRef]
- Golzarand, M.; Omidian, M.; Toolabi, K. Effect of Garcinia cambogia supplement on obesity indices: A systematic review and dose-response meta-analysis. Complement. Ther. Med. 2020, 52, 102451. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Allison, D.B.; Vasselli, J.R.; Pietrobelli, A.; Greenfield, D.; Nunez, C. Garcinia cambogia (Hydroxycitric Acid) as a Potential Antiobesity Agent. JAMA 1998, 280, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.; Kamel, H.H.; Eltawab, M.A.A. The relation of high fat diet, metabolic disturbances and brain oxidative dysfunction: Modulation by hydroxy citric acid. Lipids Health Dis. 2011, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Andueza, N.; Giner, R.; Portillo, M. Risks Associated with the Use of Garcinia as a Nutritional Complement to Lose Weight. Nutrients 2021, 13, 450. [Google Scholar] [CrossRef]
- Lobb, A. Hepatoxicity associated with weight-loss supplements: A case for better post-marketing surveillance. World J. Gastroenterol. 2009, 15, 1786–1787. [Google Scholar] [CrossRef]
- Ordeig, A.M.; García, N.B. Hepatotoxicity caused by Garcinia cambogia. Gastroenterol. Hepatol. 2020, 43, 134–135. [Google Scholar] [CrossRef]
- Fibigr, J.; Šatínský, D.; Solich, P. Current trends in the analysis and quality control of food supplements based on plant extracts. Anal. Chim. Acta 2018, 1036, 1–15. [Google Scholar] [CrossRef]
- Mena-García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. A multi-analytical strategy for evaluation of quality and authenticity of artichoke food supplements for overweight control. J. Chromatogr. A 2021, 1647, 462102. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Sakariah, K. Determination of organic acids in Garcinia cambogia (Desr.) by high-performance liquid chromatography. J. Chromatogr. A 1998, 806, 337–339. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Sakariah, K.K. Determination of (-) Hydroxycitric Acid in Commercial Samples of Garcinia cambogia Extract by Liquid Chromatography with Ultraviolet Detection. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 915–923. [Google Scholar] [CrossRef]
- Jena, B.S.; Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Chemistry and Biochemistry of (-)-Hydroxycitric Acid from Garcinia. J. Agric. Food Chem. 2002, 50, 10–22. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention (USP). Powdered Garcinia Hydroxycitrate Extract. In USP35 NF30, 2012: U. S. Pharmacopoeia National Formulary, 35th ed.; United States Pharmacopeia: Rockville, MD, USA, 2011; pp. 1305–1306. [Google Scholar]
- Loe, Y.-C.C.; Bergeron, N.; Rodriguez, N.; Schwarz, J.-M. Gas Chromatography/Mass Spectrometry Method to Quantify Blood Hydroxycitrate Concentration. Anal. Biochem. 2001, 292, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Seethapathy, G.S.; Tadesse, M.; Urumarudappa, S.K.J.; Gunaga, S.V.; Vasudeva, R.; Malterud, K.E.; Shaanker, R.U.; De Boer, H.J.; Ravikanth, G.; Wangensteen, H. Authentication of Garcinia fruits and food supplements using DNA barcoding and NMR spectroscopy. Sci. Rep. 2018, 8, 10561. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.; Jena, B.S.; Sakariah, K.K. Improved Liquid Chromatographic Method for Determination of Organic Acids in Leaves, Pulp, Fruits, and Rinds of Garcinia. J. AOAC Int. 2003, 86, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Mallik, S.B.; Rao, G.V.; Reddy, G.C.; Row, T.N.G. Garcinia lactone. Acta Crystallogr. Sect. E Crystallogr. Commun. 2007, 63, o3869. [Google Scholar] [CrossRef]
- Petersson, G. Mass spectrometry of aldonic and deoxyaldonic acids as trimethylsilyl derivatives. Tetrahedron 1970, 26, 3413–3428. [Google Scholar] [CrossRef]
- Malip, M.; Masri, M. Establishment of Protocol for Carbohydrate Analysis and Monitoring Seasonal Variations between Carbohydrates and Nitrogen Levels in Garcinia mangostana (Mangosteen). Acta Hortic. 2006, 727, 569–574. [Google Scholar] [CrossRef]
- Subhashini, N.J.P.; Nagarajan, G.R.; Kavimani, S. In vitro antioxidant and anticholinesterase activities of Garcinia cambogia. Int. J. Pharm. Pharmaceut. Sci. 2011, 3, 129–132. [Google Scholar]
- Chuah, L.O.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Alitheen, N.B. In Vitro and In Vivo Toxicity of Garciniaor Hydroxycitric Acid: A Review. Evid. Based Complement. Altern. Med. 2012, 2012, 197920. [Google Scholar] [CrossRef]
- Sanz, M.L.; Villamiel, M.; Martínez-Castro, I. Inositols and carbohydrates in different fresh fruit juices. Food Chem. 2004, 87, 325–328. [Google Scholar] [CrossRef]
- Megías-Pérez, R.; Hahn, C.; Ruiz-Matute, A.I.; Behrends, B.; Albach, D.C.; Kuhnert, N. Changes in low molecular weight carbohydrates in kale during development and acclimation to cold temperatures determined by chromatographic techniques coupled to mass spectrometry. Food Res. Int. 2020, 127, 108727. [Google Scholar] [CrossRef]
- Mena-García, A.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.; Sanz, M. Exploitation of artichoke byproducts to obtain bioactive extracts enriched in inositols and caffeoylquinic acids by Microwave Assisted Extraction. J. Chromatogr. A 2020, 1613, 460703. [Google Scholar] [CrossRef] [PubMed]
- Ávila, E.L.; Rodríguez, M.C.; Velásquez, H.J.C. Influence of Maltodextrin and Spray Drying Process Conditions on Sugarcane Juice Powder Quality. Rev. Fac. Nac. Agron. Medellín 2015, 68, 7509–7520. [Google Scholar] [CrossRef]
- FDA. Food Additive Status List. 2021. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list#ftnM (accessed on 1 January 2022).
- Saito, K.; Tomita, F. Difructose Anhydrides: Their Mass-Production and Physiological Functions. Biosci. Biotechnol. Biochem. 2000, 64, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Manley-Harris, M.; Richards, G.N. Di-d-fructose dianhydrides and related oligomers from thermal treatments of inulin and sucrose. Carbohydr. Res. 1996, 287, 183–202. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Soria, A.C.; Martínez-Castro, I.; Sanz, M.L. A New Methodology Based on GC−MS To Detect Honey Adulteration with Commercial Syrups. J. Agric. Food Chem. 2007, 55, 7264–7269. [Google Scholar] [CrossRef]
- Sanz, M.L.; Sanz, J.; Martinez-Castro, I. Presence of some cyclitols in honey. Food Chem. 2004, 84, 133–135. [Google Scholar] [CrossRef]
- Carrero-Carralero, C.; Mansukhani, D.; Ruiz-Matute, A.I.; Martínez-Castro, I.; Ramos, L.; Sanz, M.L. Extraction and characterization of low molecular weight bioactive carbohydrates from mung bean (Vigna radiata). Food Chem. 2018, 266, 146–154. [Google Scholar] [CrossRef]
| Samples | ||||||
|---|---|---|---|---|---|---|
| Compound (mg g−1) | ID | GC1 | GC2 | GC3 | GC4 | GC5 |
| (-)-HCAL # | 1 | 108 (5) | 93 (18) | 115 (20) | 140 (31) | 99 (22) |
| (-)-HCA # | 2 | 87.7 (0.6) | 77 (13) | 107 (21) | 121 (29) | 89 (14) |
| Arabinonic acid γ lactone $ | 3 | 0.28 (0.03) | 0.26 (0.01) | - | - | 0.45 (0.02) |
| Arabinose $ | 4 | 22 (4) | 22. 40 (0.02) | 15 (1) | 20 (1) | 11.5 (0.8) |
| Pentitol $ | 5 | 0.20 (0.08) | tr | tr | tr | tr |
| Citric acid $ | 6 | 6 (1) | 4.8 (0.4) | 18 (2) | 10.2 (0.3) | 5.5 (0.6) |
| Fructose (F) $ | 7 | 25 (3) | 20.4 (0.6) | 31 (4) | 8.02 (0.03) | 3.5 (0.1) |
| Galactose $ | 8 | 0.37 (0.06) | 0.34 (0.03) | 0.3 (0.1) | 0.82 (0.02) | 1.34 (0.06) |
| Glucose (G) $ | 9 | 38 (4) | 27.01 (0.02) | 33 (4) | 7.2 (0.2) | 3.9 (0.3) |
| myo-inositol $ | 10 | 0.43 (0.02) | 0.1336 (0.0004) | 0.15 (0.02) | 0.16 (0.02) | 0.106 (0.001) |
| G/F | - | 1.54 (0.02) | 1.32 (0.04) | 1.070 (0.005) | 0.86 (0.02) | 1.13 (0.06) |
| (-)-HCA (%, w/w) | ||
|---|---|---|
| FSOC | Experimental * | Declared |
| GCFS1A | 21.35 (2.01) b | 47 a |
| GCFS1B | 19.80 (0.98) b | 47 a |
| GCFS2 | 33.70 (1.08) a | 34 a 47 a |
| GCFS3 | 52.12 (2.54) a | |
| GCFS4 | 30.31 (7.47) a | 36 a |
| GCFS5 | 56.00 (1.62) a | 46 b |
| GCFS6 | 54.60 (7.42) a | 56 a |
| GCFS7 | 42.86 (2.09) a | 41 a |
| GCFS8 | 49.81 (2.71) a | 51 a |
| GCFS9 | 50.27 (3.81) a | 48 a |
| GCFS10 | 46.62 (2.38) a | 48 a |
| GCFS11 | 10.45 (0.28) a | 12 a |
| GCFS12 | 46.42 (1.42) a | 48 a |
| GCFS13 | 6.57 (0.62) | - |
| GCFS14 | 4.29 (0.84) | - |
| GCFS15 | 60.13 (1.05) a | 55 b |
| GCFS16 | 46.91 (6.49) b | 60 a |
| GCFS17 | 65.8 (2.1) a | 62 a |
| ID. | Compound | 1A | 1B | 2 | 3 | 4 & | 5 | 6 | 7 $ | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 # |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pentitol | - | - | - | 0.025 (0.006) | - | - | - | 0.03 (0.01) | - | - | - | - | - | - | - | - | - | tr |
| 2 | Arabitol | 0.040 (0.007) | 0.033 (0.003) | 0.018 (0.010) | 0.049 (0.003) | tr | 0.663 (0.001) | 0.075 (0.002) | 0.046 (0.002) | 0.0344 (0.0005) | - | 0.11 (0.05) | - | - | tr | 0.07 (0.02) | - | - | - |
| 3, 4 | Arabinose | - | - | 0.101 (0.006) | 0.22 (0.02) | 0.5 (0.2) | 0.184 (0.002) | 0.9 (0.2) | 0.5 (0.1) | - | - | - | 0.0191 (0.0003) | - | 0.02 (0.01) | - | - | - | 0.32 (0.04) |
| 5 | Pentitol | 0.048 (0.001) | 0.042 (0.001) | 0.055 (0.001) | 0.114 (0.002) | - | 0.0611 (0.0008) | 0.18 (0.04) | 0.17 (0.05) | - | - | - | - | - | - | - | - | 0.024 (0.003) | 0.076 (0.001) |
| 7, 8 | Fructose (F) | 0.0842 (0.0002) | 0.13 (0.06) | 0.08 (0.03) | 2.4 (0.1) | 1.48 (0.03) | 0.271 (0.003) | 1.9 (0.3) | 0.6 (0.2) | 0.335 (0.002) | - | - | 0.0145 (0.0001) | 0.0169 (0.0004) | 0.005 (0.003) | - | - | - | 0.034 (0.006) |
| 9, 14 | Galactose | tr * | 0.038 (0.007) | 0.08 (0.02) | 0.35 (0.03) | 0.44 (0.02) | 0.28 (0.04) | 0.15 (0.03) | 0.04 (0.01) | 0.072 (0.003) | - | - | - | - | 0.005 (0.002) | 0.022 (0.001) | - | - | 0.026 (0.003) |
| 10, 13 | Glucose (G) | 0.187 (0.043) | 0.172 (0.008) | 0.491 (0.004) | 0.11 (0.02) | 2.25 (0.08) | 0.55 (0.02) | 0.38 (0.10) | 0.075 (0.007) | 1.18 (0.02) | - | - | 0.015 (0.002) | 0.373 (0.004) | 0.021 (0.005) | 0.144 (0.006) | - | - | 0.017 (0.002) |
| 17 | myo-inositol | 0.025 (0.002) | 0.022 (0.001) | 0.040 (0.001) | 0.11 (0.02) | 0.086 (0.005) | 0.24 (0.02) | 0.064 (0.005) | 0.056 (0.002) | 0.02 (0.01) | 0.023 (0.005) | 0.04 (0.02) | 0.005 (0.001) | 0.0163 (0.0005) | 0.013 (0.004) | 0.022 (0.002) | tr | 0.023 (0.003) | 0.23 (0.03) |
| 18 | Galactosyl-glycerol | - | - | - | 0.018 (0.001) | - | 0.042 (0.007) | - | - | - | - | - | - | - | - | - | - | - | - |
| 19–23 | DFAs | - | - | 0.08 (0.03) | 0.51 (0.03) | 0.13 (0.04) | 0.20 (0.04) | 0.30 (0.05) | 0.15 (0.03) | 0.030 (0.004) | - | - | tr | - | 0.045 (0.005) | 0.05 (0.02) | - | - | - |
| 24, 25 | Maltose | 0.104 (0.002) | 0.0855 (0.0001) | 16.85 (0.01) | 0.063 (0.001) | 15.3 (1.3) | 2.2 (0.1) | - | tr | 6.9 (0.1) | tr | - | - | 5.3 (0.3) | 0.6 (0.1) | - | - | - | - |
| 26 | Digalactosyl-glycerol | 0.008 (0.001) | 0.007 (0.003) | 0.0108 (0.0004) | 0.0281 (0.0003) | 0.025 (0.003) | 0.02 (0.01) | 0.012 (0.008) | 0.006 (0.003) | 0.004 (0.002) | 0.025 (0.004) | tr | - | 0.016 (0.003) | - | - | - | - | 0.0113 (0.0003) |
| 27, 28 | Maltotriose | 0.381 (0.008) | tr | 153.4 (15.4) | - | 14.2 (183) | 11.6 (0.4) | - | - | 41.9 (0.9) | - | - | - | 27.8 (4.4) | - | - | - | - | - |
| 29, 30 | Maltotetraose | - | - | 17.0 (2.2) | - | 2.3 (0.2) | 10.3 (0.1) | - | - | 33.6 (1.86) | - | - | - | 0.9 (0.1) | - | - | - | - | - |
| - | G/F | 2.2 (0.5) | 1.5 (0.6) | 7 (3) | 0.05 (0.01) | 1.53 (0.09) | 2.04 (0.06) | 0.20 (0.01) | 0.14 (0.03) | 3.50 (0.04) | - | - | 1.1 (0.1) | 22.1 (0.7) | 4 (1) | - | - | - | 0.5 (0.2) |
| ID. | Compound | 1A | 1B | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | Citric acid | 9.0 (0.2) | 6.9 (0.6) | 3.500 (0.001) | 8.0 (0.4) | 6.4 (0.2) | 5.6 (0.2) | 7.66 (0.04) | 5.47 (0.05) | 3.17 (0.06) | 1.09 (0. 05) | 4.1 (0.1) | 0.677 (0.008) | 1.47 (0.03) | 1.5 (0.6) | - | 4.1 (0.3) | 2.28 (0.10) | 1.06 (0.01) |
| 11 | Vitamin B6 | 0.177 (0.068) | 0.118 (0.016) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 12 | Gluconic acid | 0.020 (0.002) | 0.019 (0.006) | 0.124 (0.006) | 0.16 (0.03) | 21 (5) | 0.066 (0.008) | 0.063 (0.006) | 0.053 (0.006) | 0.03 (0.01) | - | 0.12 (0.05) | 0.01029 (0.00003) | - | 0.006 (0.004) | tr | tr | - | 0.031 (0.006) |
| 15 | Glucaric acid | - | - | - | - | - | 0.015 (0.004) | 0.0091 (0.0004) | 0.011 (0.002) | - | - | - | - | - | - | - | - | tr | - |
| 16 | Vitamin C | 6.02 (0.01) | 5.7 (0.1) | - | - | 72.2 (0.1) | - | - | - | - | - | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena-García, A.; Bellaizac-Riascos, A.J.; Rada-Mendoza, M.; Chito-Trujillo, D.M.; Ruiz-Matute, A.I.; Sanz, M.L. Quality Evaluation of Dietary Supplements for Weight Loss Based on Garcinia cambogia. Nutrients 2022, 14, 3077. https://doi.org/10.3390/nu14153077
Mena-García A, Bellaizac-Riascos AJ, Rada-Mendoza M, Chito-Trujillo DM, Ruiz-Matute AI, Sanz ML. Quality Evaluation of Dietary Supplements for Weight Loss Based on Garcinia cambogia. Nutrients. 2022; 14(15):3077. https://doi.org/10.3390/nu14153077
Chicago/Turabian StyleMena-García, Adal, Angie Julieth Bellaizac-Riascos, Maite Rada-Mendoza, Diana María Chito-Trujillo, Ana Isabel Ruiz-Matute, and María Luz Sanz. 2022. "Quality Evaluation of Dietary Supplements for Weight Loss Based on Garcinia cambogia" Nutrients 14, no. 15: 3077. https://doi.org/10.3390/nu14153077
APA StyleMena-García, A., Bellaizac-Riascos, A. J., Rada-Mendoza, M., Chito-Trujillo, D. M., Ruiz-Matute, A. I., & Sanz, M. L. (2022). Quality Evaluation of Dietary Supplements for Weight Loss Based on Garcinia cambogia. Nutrients, 14(15), 3077. https://doi.org/10.3390/nu14153077