Anti-Obesity Effects of Aqueous Extracts of Sunbanghwalmyung-Eum in High-Fat- and High-Cholesterol-Diet-Induced Obese C57BL/6J Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of SBH Extract and HPLC Analysis of SBH
2.3. Animal Experiment
2.4. Plasma Biochemical Parameter Analysis
2.5. OGTT
2.6. H&E Staining
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Quantification of the Eight Marker Compounds in SBH
3.2. SBH Reduced HFHCD-Induced Body Weight Gain
3.3. SBH Reduced HFHCD-Induced Liver Disorder Factor and Lipid Parameters
3.4. SBH Suppressed HFHCD-Induced Glucose Tolerance and Plasma Biomarkers of Diabetes
3.5. SBH Reversed HFHCD-Induced Regulation of Signaling Molecules
3.6. SBH Reversed HFHCD-Induced Inhibition of AMPK and HSL Phosphorylation
3.7. SBH Reduced HFHCD-Induced Expression of Inflammatory Cytokines
4. Discussion
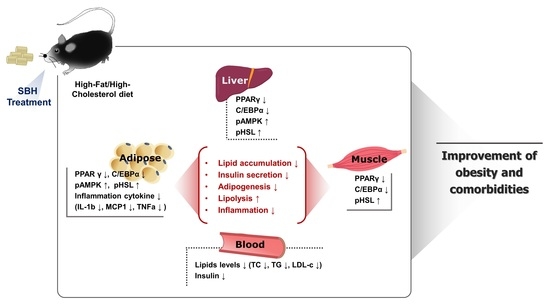
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Al Rifai, M.; Silverman, M.G.; Nasir, K.; Budoff, M.J.; Blankstein, R.; Szklo, M.; Katz, R.; Blumenthal, R.S.; Blaha, M.J. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015, 239, 629–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Kruijsdijk, R.C.; van der Wall, E.; Visseren, F.L. Obesity and Cancer: The Role of Dysfunctional Adipose Tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, S.J.; White, A.D.; Bijland, S.; Rutherford, C.; Graham, D.; Richter, E.A.; Viollet, B.; Touyz, R.M.; Palmer, T.M.; Salt, I.P. Activation of AMP-activated protein kinase rapidly suppresses multiple pro-inflammatory pathways in adipocytes including IL-1 receptor-associated kinase-4 phosphorylation. Mol. Cell. Endocrinol. 2016, 440, 44–56. [Google Scholar] [CrossRef]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Madsen, M.S.; Siersbæk, R.; Boergesen, M.; Nielsen, R.; Mandrup, S.; Bashour, K.T.; Tsai, J.; Shen, K.; Lee, J.-H.; Sun, E.; et al. Peroxisome Proliferator-Activated Receptor γ and C/EBPα Synergistically Activate Key Metabolic Adipocyte Genes by Assisted Loading. Mol. Cell. Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef] [Green Version]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. North Am. 2016, 45, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Föger, B. Lipid lowering therapy in type 2 diabetes. Wien. Med. Wochenschr. 2011, 161, 289–296. [Google Scholar] [CrossRef]
- Desai, C.S.; Martin, S.S.; Blumenthal, R.S. Non-cardiovascular effects associated with statins. BMJ 2014, 349, g3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-L.; Lin, L.-C.; Tung, Y.-T.; Ho, S.-T.; Chen, Y.-L.; Lin, C.-C.; Wu, J.-H. Rhododendron oldhamii leaf extract improves fatty liver syndrome by increasing lipid oxidation and decreasing the lipogenesis pathway in mice. Int. J. Med Sci. 2017, 14, 862–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J. Donguibogam, 3rd ed.; Daesung Publisher: Korea, Seoul, 1999. [Google Scholar]
- Nie, B.; Li, X.; Wei, Y.; Chen, M.; Zhou, J.; Lou, L.; Dong, B.; Wu, A.; Zhang, D.; Zhu, L.; et al. Xianfanghuomin-gyin, a Chinese Compound Medicine, Modulates the Proliferation and Differentiation of T Lymphocyte in a Colla-gen-Induced Arthritis Mouse Model. Evid.-Based Complement. Alternat. Med. 2016, 2016, 6356871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-T.; Lee, C.-E.; Son, J.H.; Lee, I.-C.; Lee, J.-Y.; Park, T.-S.; Jang, M.-J.; Song, M.-A.; Jee, S.-Y.; An, B.-J. Cytotoxicity and Physio-logical Activity of SunbangHwalmyung-Um. Korea J. Herbol. 2005, 20, 51–58. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Park, J.Y. A Histological Study on the Visual Cell Layer of the Endemic Korean Species Liobagrus mediadiposalis (Pisces: Amblycipitidae). Appl. Microsc. 2017, 47, 238–241. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.M.; Choi, Y.H.; Yoon, J.J.; Lee, Y.J.; Cho, K.W.; Kang, D.G.; Lee, H.S. Oleanolic acid modulates the renin-angiotensin system and cardiac natriuretic hormone concomitantly with volume and pressure balance in rats. Eur. J. Pharmacol. 2017, 809, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, E.-J.; Chen, L.; Stein, A.P.; Reuhl, K.R.; Yang, C.S. Effects of Phenethyl Isothiocyanate on Acetaminophen Metabolism and Hepatotoxicity in Mice. Toxicol. Appl. Pharmacol. 1997, 144, 306–314. [Google Scholar] [CrossRef]
- Mathiesen, D.S.; Bagger, J.I.; Bergmann, N.C.; Lund, A.; Christensen, M.B.; Vilsbøll, T.; Knop, F.K. The Effects of Dual GLP-1/GIP Receptor Agonism on Glucagon Secretion—A Review. Int. J. Mol. Sci. 2019, 20, 4092. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-M.; Lee, Y.-S.; Kim, W.; Kim, S.J.; Shin, K.-O.; Yu, J.-Y.; Lee, M.K.; Lee, Y.-M.; Hong, J.T.; Yun, Y.-P.; et al. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J. Nutr. Biochem. 2013, 25, 201–207. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. WHO Newsroom Fact Sheets; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Turpin, S.M.; Ryall, J.G.; Southgate, R.; Darby, I.; Hevener, A.L.; Febbraio, M.A.; Kemp, B.E.; Lynch, G.S.; Watt, M.J. Examination of ‘lipotoxicity’ in skeletal muscle of high-fat fed and ob/ob mice. J. Physiol. 2009, 587, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Khalilpourfarshbafi, M.; Gholami, K.; Murugan, D.D.; Sattar, M.Z.A.; Abdullah, N.A. Differential effects of dietary flavonoids on adipogenesis. Eur. J. Nutr. 2018, 58, 5–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, M.-Y.; Kim, H.-L.; Park, J.; An, H.-J.; Kim, S.-H.; Kim, S.-J.; So, H.-S.; Park, R.; Um, J.-Y.; Hong, S.-H. Rubi Fructus (Rubus coreanus) Inhibits Differentiation to Adipocytes in 3T3-L1 Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 475386. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. Minireview: The AMP-Activated Protein Kinase Cascade: The Key Sensor of Cellular Energy Status. Endocrinology 2003, 144, 5179–5183. [Google Scholar] [CrossRef]
- Landsberg, L.; Aronne, L.J.; Beilin, L.J.; Burke, V.; Igel, L.I.; Lloyd-Jones, D.; Sowers, J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment-A position paper of the The Obesity Society and the American Society of Hypertension. Obesity 2012, 21, 8–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, L.; Sui, Q.; Lin, F.; Jiang, W.; Chen, J.; Lu, W.; Gao, Q. Facile synthesis of acacetin and its derivatives. Bioorganic Med. Chem. Lett. 2016, 26, 3577–3580. [Google Scholar] [CrossRef]








| Primary Antibody | Dilution | Catalogue No. | Company | Secondary Antibody | Dilution | Catalogue No. | Company |
|---|---|---|---|---|---|---|---|
| AMPKα | 1:1000 | sc-5298 | Santa | Goat-anti-rabbit | 1:5000 | ADI-SAB-300 | Enzo |
| C/EBPα | 1:1000 | sc-365318 | Santa | Goat-anti-mouse | 1:5000 | ADI-SAB-100 | Enzo |
| HSL | 1:500 | ab45422 | Abcam | Goat-anti-rabbit | 1:5000 | ADI-SAB-300 | Enzo |
| IL-1β | 1:1000 | 12242s | Cell Signaling | Goat-anti-mouse | 1:5000 | ADI-SAB-100 | Enzo |
| MCP1 | 1:1000 | ab25124 | Abcam | Goat-anti-rabbit | 1:5000 | ADI-SAB-300 | Enzo |
| PPARγ | 1:500 | 2430s | Cell Signaling | Goat-anti-rabbit | 1:5000 | ADI-SAB-300 | Enzo |
| pAMPKα | 1:500 | 2535s | Cell Signaling | Goat-anti-rabbit | 1:5000 | ADI-SAB-300 | Enzo |
| pHSL | 1:500 | 4139s | Cell Signaling | Goat-anti-rabbit | 1:5000 | ADI-SAB-300 | Enzo |
| TNFα | 1:1000 | sc-52746 | Santa | Goat-anti-mouse | 1:5000 | ADI-SAB-100 | Enzo |
| GAPDH | 1:1000 | Sc-32233 | Santa | Goat-anti-mouse | 1:5000 | ADI-SAB-100 | Enzo |
| Tissue Type | Nor | HFHC | Sim | SBH |
|---|---|---|---|---|
| Liver | 1.288 ± 0.060 | 2.621 ± 0.284 ### | 1.633 ± 0.079 *** | 1.945 ± 0.083 * |
| Adipose tissue | 0.618 ± 0.037 | 2.601 ± 0.158 ### | 1.483 ± 0.061 ** | 1.694 ± 0.115 0.057 |
| Kidney | 0.158 ± 0.026 | 0.181 ± 0.010 | 0.167 ± 0.021 | 0.182 ± 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-L.; Ahn, Y.M.; Lee, S.M.; Seo, C.-S.; Park, S.-H.; Bang, O.-S.; Jung, J. Anti-Obesity Effects of Aqueous Extracts of Sunbanghwalmyung-Eum in High-Fat- and High-Cholesterol-Diet-Induced Obese C57BL/6J Mice. Nutrients 2022, 14, 2929. https://doi.org/10.3390/nu14142929
Kim H-L, Ahn YM, Lee SM, Seo C-S, Park S-H, Bang O-S, Jung J. Anti-Obesity Effects of Aqueous Extracts of Sunbanghwalmyung-Eum in High-Fat- and High-Cholesterol-Diet-Induced Obese C57BL/6J Mice. Nutrients. 2022; 14(14):2929. https://doi.org/10.3390/nu14142929
Chicago/Turabian StyleKim, Hye-Lin, You Mee Ahn, So Min Lee, Chang-Seob Seo, Seong-Hwan Park, Ok-Sun Bang, and Jeeyoun Jung. 2022. "Anti-Obesity Effects of Aqueous Extracts of Sunbanghwalmyung-Eum in High-Fat- and High-Cholesterol-Diet-Induced Obese C57BL/6J Mice" Nutrients 14, no. 14: 2929. https://doi.org/10.3390/nu14142929
APA StyleKim, H.-L., Ahn, Y. M., Lee, S. M., Seo, C.-S., Park, S.-H., Bang, O.-S., & Jung, J. (2022). Anti-Obesity Effects of Aqueous Extracts of Sunbanghwalmyung-Eum in High-Fat- and High-Cholesterol-Diet-Induced Obese C57BL/6J Mice. Nutrients, 14(14), 2929. https://doi.org/10.3390/nu14142929